Greater Availability of Brain Dopamine Transporters in Major Depression Shown by [99mTc]TRODAT-1 SPECT Imaging
Abstract
OBJECTIVE: Studies of laboratory animals have shown that administration of antidepressants of all pharmacological classes produces changes in dopamine transporter binding affinity. These observations suggest that dopamine transporter function may play a critical role in the pathophysiology of depression. The present study was an examination of the availability of brain dopamine transporter sites in patients with major depression and in healthy comparison subjects. METHOD: Single photon emission computed tomographic (SPECT) brain scans were acquired for 15 drug-free depressed patients and 46 age- and gender-matched healthy comparison subjects by using [99mTc]TRODAT-1, a selective dopamine transporter imaging agent. Specific regions of interest in the basal ganglia and supratentorial areas of the brain were examined. Specific uptake values of dopamine transporter [99mTc]TRODAT-1 binding affinity were calculated from the SPECT scan data, and the values for the patients and healthy subjects were compared. RESULTS: The specific uptake values of [99mTc]TRODAT-1 binding were significantly higher in the right anterior putamen (23%), right posterior putamen (36%), left posterior putamen (18%), and left caudate nucleus (12%) of the patients than in the comparison subjects. These differences persisted when the data were further analyzed according to gender and age. CONCLUSIONS: Dopamine transporter affinity may be higher than normal in the basal ganglia of depressed patients. These findings suggest that dopamine function may be altered in depression and may also be a mechanism of antidepressant activity.
Evidence from studies of laboratory animals and humans suggests that dopamine may play key roles in the pathophysiology of depression (1, 2) and in the therapeutic action of antidepressants (3, 4).
For example, low levels of dopamine metabolites have been measured in the CSF of depressed patients (1). Reports of low levels of the dopamine metabolite dihydroxyphenylacetic acid in the CSF of depressed patients (5) and in the basal ganglia of postmortem brains of depressed suicide victims (6) lend additional support to a theory of dopamine abnormality in depression. Direct measurement of brain monoamine metabolites from the internal jugular vein of treatment-resistant depressed patients revealed low homovanillic acid levels that were highly correlated with illness severity (7).
Additional evidence of abnormal dopamine function in depression is suggested by observations of a high prevalence of comorbid depression in patients with Parkinson’s and Huntington’s disease (8, 9). In these disorders, affective symptoms may precede the onset of motor disturbances, suggesting that mood may be affected by even modest alterations in brain dopamine function and is not merely the result of a psychological reaction to progressive physical impairment (10).
Imaging studies have also demonstrated abnormalities in the basal ganglia, where dopamine is the predominant monoamine (11). Magnetic resonance imaging (MRI) signal intensities have been found to be higher in the basal ganglia of some depressed patients than in healthy comparison subjects (12), while low basal ganglia volume has been detected in depression by using computed tomography (CT) and MRI techniques (13, 14). Studies using single photon emission computed tomography (SPECT) have shown low regional cerebral blood flow (rCBF) in the basal ganglia, while other studies using positron emission tomography (PET) have identified low glucose metabolism in the basal ganglia of depressed patients. In an extensive review of rCBF and metabolic abnormalities in depressed patients, Soares and Mann (15) concluded that the most consistent imaging findings are low rCBF and glucose metabolism in the prefrontal cortex and basal ganglia. Recent reports of low metabolism in the ventral striatum (16) and high metabolism in the right dorsal head of the caudate nucleus (17) are consistent with the Soares and Mann interpretation (15).
Finally, experiments with laboratory animals have shown that long-term administration of antidepressants of various pharmacological classes increases activity of dopamine D2-receptor-like receptors (i.e., D2, D3, or D4) (4). These effects have been demonstrated with tricyclic antidepressants, selective serotonin reuptake inhibitors, monoamine oxidase inhibitors (MAOIs), α2 antagonists (e.g., mianserin), mixed reuptake inhibitors (e.g., venlafaxine), trazodone, nomifensine, and electroconvulsive therapy (18–21).
We hypothesized that dopamine function is altered in the basal ganglia of patients with depression. In this study, we used the technetium-labeled complex [99mTc][2[[2-[[[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]oct-2-yl]-methyl](2-mercaptoethyl)amino] ethyl] amino]ethane-thiolato(3-)-N2,N2′,S2,S2′]oxo-[1R-(exo-exo)] (22) ([99mTc] TRODAT-1) SPECT brain imaging to examine dopamine transporter activity in depressed patients and healthy comparison subjects.
Method
Patients
Outpatients at least 18 years of age from the University of Pennsylvania Depression Research Unit were enrolled in the study. All underwent a Structured Diagnostic Interview for DSM-IV (SCID) (23) and met the DSM-IV criteria for a major depressive episode. All patients had a score of at least 16 on the 17-item Hamilton Depression Rating Scale (24) at the time of the study procedure. All patients were in good physical health as determined by a physical examination and laboratory evaluation including a complete blood count, measurements of serum electrolytes, glucose, and hepatic enzymes, renal and thyroid panels, urinalysis, urine drug screen, and a 12-lead ECG. Women of child-bearing potential had negative results on pregnancy tests.
Patients were excluded from the study if they had a current DSM-IV axis I diagnosis other than major depression, a history of mania, a history of schizophrenia, alcohol or drug abuse within 3 months, or actively suicidal ideation.
The medical exclusion criteria included pregnancy or breast-feeding among women, unstable medical conditions (e.g., angina, diabetes mellitus, hypertension), a history of transient ischemic attacks, intracranial hemorrhage, brain tumor, encephalitis, normal-pressure hydrocephalus, Parkinson’s (or other basal ganglia) disease, malignancy, and hepatic or renal disease. Patients taking chemotherapy, over-the-counter preparations (e.g., St. John’s wort), phenothiazines, barbiturates, mood stabilizers, or antidepressant medications were also excluded.
Before enrollment in the study, patients were required to have taken no antidepressants for at least 7 days, no MAOIs for at least 2 weeks, and no fluoxetine for at least 3 weeks (25).
Comparison Subjects
Age-matched nonpsychiatric healthy comparison subjects were recruited by investigators at the University of Pennsylvania Division of Nuclear Medicine to participate in [99mTc]TRODAT-1 studies. The comparison subjects each had a psychiatric evaluation with the SCID format (23) and had no DSM-IV axis I diagnosis. Each was given a complete physical and laboratory evaluation similar to that for the patients, and none had a significant medical illness or laboratory abnormality. All were free from psychotropic medication, and none had a history of central nervous system disease.
The comparison subjects were age- and gender-matched with the patient group and were consecutively selected from a pool of 101 healthy volunteers who had been studied under similar experimental conditions with [99mTc]TRODAT-1 SPECT as part of an established normative database of healthy subjects (26, 27).
Procedures
All subjects were provided with a detailed description of the study purpose and procedures in accordance with the ethical standards set forth by the institutional review board of the University of Pennsylvania. All subjects provided written informed consent before enrolling in the study.
Each subject was placed in a supine, resting position on the SPECT imaging table. A 22-gauge intravenous catheter needle was inserted into an antecubital vein, and a patent intravenous line was maintained with a physiological saline in a sterile cap. Three ECG chest leads were applied for continuous ECG recording. After 20 minutes, [99mTc]TRODAT-1, 740 MBq (20 mCi), was injected through the indwelling venous catheter. The individuals remained at rest for the next 25 minutes, while dynamic SPECT brain images were acquired.
Image Acquisition
Images of the brain were acquired with a triple-head gamma camera equipped with ultra-high-resolution fan-beam collimators (Picker 3000; Picker International, Cleveland). The acquisition variables included a continuous mode with 40 projection angles over a 120° arc to obtain data in a 128×128 matrix with a pixel width of 2.11 mm and a slice thickness of 3.56 mm. The center of rotation was 14 cm. The low-pass filter was based on a systematic analysis of variables that produced the best signal-to-noise characteristics in the images.
Manual demarcation of brain regions of interest was then performed. A set of standardized templates containing small and large regions of interest were fit on each scan. Within the x-y plane, small regions of interest in the template were smaller than the actual structures they represented in order to minimize resolution-induced problems with ill-defined edges. To reduce the effects of volume averaging in the axial direction, the small regions of interest were not placed on the slices that contained the uppermost and lowermost portions of the structures they represented. This tended to limit the small regions of interest to the central aspect of the structures they represented. In contrast, boundaries for the whole brain were drawn by hand on slices located 12 mm above the highest slice that included the basal ganglia.
The primary outcome measure was specific uptake 3 to 4 hours after [99mTc]TRODAT-1 administration, when the distribution of [99mTc]TRODAT-1 approached a transient near-equilibrium-like state that reflected the ratio k3/k4, which is related to [99mTc]TRODAT-1 binding potential.
Statistical Procedures
Intrarater reliability for analyses of the manually defined regions of interest was estimated by using intraclass correlation coefficients. The procedures were implemented with a commercial statistical package (StatS, Redmond, Wash., Think Point Software). This procedure resulted in a high correlation because the high-contrast images were particularly amenable to “punch biopsy” approaches to image analysis, while the “large” regions of interest were difficult to misplace. The reliability of the image analysis techniques was found to be high, with intraclass correlations consistently above 0.95, as expected for work with relatively high-contrast images (28).
Group comparisons of mean specific uptake values of [99mTc] TRODAT-1 binding for each region of interest were analyzed by using the unpaired Student t test without correction for multiple comparisons. Statistical significance was defined as p<0.05.
Results
Demographic Characteristics
We studied 15 patients (nine men, six women) with a mean age of 40 years (SD=12, range=25–59) with major depression: 10 had major depressive disorder, and five had type II bipolar disorder or major depression not otherwise specified. All had moderate to severe depression; their mean Hamilton scale score was 22 (SD=4, range=17–28) at the time of the procedure.
From the pool of healthy subjects, 46 (22 men, 24 women) were consecutively matched to the patients; their mean age was 40 years (SD=11, range=26–60). None of the comparison subjects met the DSM-IV criteria for major depression, and none had a Hamilton depression scale score above 6. All were drug free at the time of [99mTc]TRODAT-1 testing (except for some women of child-bearing potential who were taking oral contraceptives).
SPECT Imaging
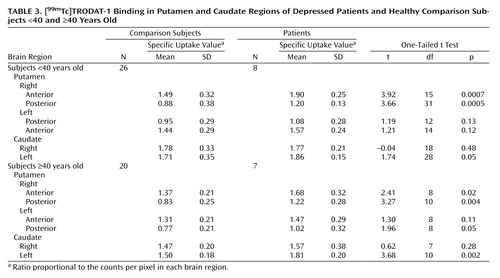
Specific uptake values representing [99mTc]TRODAT-1 binding to dopamine transporter sites in the caudate and putamen regions are shown in Table 1. The mean values were significantly higher in the depressed patients than in the comparison subjects for the right anterior putamen (23%) (Figure 1) and the right posterior putamen (36%). The values were also significantly higher in the depressed patients for the left posterior putamen (18%) and the left caudate region (12%) (Table 1). No differences between subject groups were observed for the left anterior putamen or for the right caudate region.
Gender-specific analyses continued to show higher specific uptake values in the right anterior putamen and right posterior putamen of depressed men and depressed women than in gender-matched comparison subjects (Table 2). The values for the left caudate region were higher in the depressed men than in the male comparison subjects but failed to show a significant difference in women.
Similarly, higher specific uptake values in the putamen and caudate regions in the depressed patients continued to be present when the data were analyzed according to age (Table 3).
Discussion
We performed SPECT brain imaging with [99mTc]TRODAT-1, a selective dopamine transporter ligand, to study the availability of dopamine transporters in the basal ganglia region of depressed patients. We observed higher specific uptake values for [99mTc]TRODAT-1 binding in several subregions of the putamen and caudate nuclei of depressed patients than in comparison subjects. Significantly greater [99mTc]TRODAT-1 binding was observed in the right anterior (23%) and right posterior (36%) putamen, with a more modest difference in the left posterior putamen (18%). A higher specific uptake value was observed in the left (12%), but not the right, caudate region.
Although several SPECT studies have previously investigated dopamine uptake sites in depression, we believe that the present study is the first to examine the dopamine uptake site in depression by using the [99mTc]TRODAT-1 radioligand. Laasonen-Balk et al. (29) measured striatal dopamine transporter sites in 15 drug-naive depressed patients and 18 healthy comparison subjects by using [123I]β-CIT, an iodinated radioligand that binds to both dopamine and serotonin transporter sites. They found significantly higher striatal [123I]β-CIT binding to “presumed” dopamine transporter sites in the depressed patients. In contrast, Malison et al. (30) reported no significant difference in [123I]β-CIT binding to striatal dopamine uptake sites between 15 depressed patients and 15 healthy comparison subjects.
The greater dopamine transporter availability observed in the present study could be the result of a decrease in synaptic levels of dopamine or a diminished dopaminergic “tone” in depression. In this regard, the dopamine transporter plays a critical role in regulating synaptic dopamine concentrations by rapidly taking up extracellular dopamine into presynaptic terminals following its release. The dopamine uptake site is the primary determinant of extracellular dopamine concentrations and maintains the balance between dopamine homeostasis and function (31).
It is also possible that our observation of greater dopamine transporter availability in depression reflects a compensatory mechanism resulting from increased dopaminergic “tone” in depression. For example, D2 receptor agonists increase dopamine transporter turnover (32). Since the activity of the dopamine transporter is regulated by the endogenous dopamine “tone,” it may also represent a useful biomarker for studying changes in central dopamine activity in depression.
The greater dopamine transporter specific uptake values observed by Laasonen-Balk et al. (29) could be related to the psychomotor disturbances associated with depression. The caudate and putamen have direct effects on psychomotor activity (33), and some investigators have suggested that dopamine may be involved in the pathogenesis of psychomotor slowing in melancholic depression (34).
Altered dopamine transporter binding could also be related to the presence of mood changes in depression. Transient dysphoria resulting from tryptophan depletion has been found to produce reductions in striatal rCBF and glucose metabolism. Bremner et al. (35) observed low glucose metabolism in the caudate and putamen by using PET, while Smith et al. (36) observed low rCBF in the left, but not the right, caudate region with PET. The finding supports our observation of high dopamine transporter specific uptake values in the left caudate region in depression. Similarly, others have reported that transient sadness is associated with increased rCBF in the caudate and putamen (37).
The presence of striatal dopamine dysregulation could affect cortical and limbic lobe function as well. In this model, the caudate nucleus is part of a complex system in which specific cortical pathways project to the striatum and then to the globus pallidus, substantia nigra, and finally, back to the cortical areas (9, 38). In addition, the striatum receives neuronal inputs from other brain regions, such as the thalamus and amygdala. Several of these pathways involve neuronal circuits that modulate mood and emotional behavior. As a result, a dysfunction of dopamine in the striatum could impair the corticostriatal circuits disrupting limbic input back to the cortex in depression (39, 40).
Finally, our observation of differences in [99mTc]TRODAT-1 binding between the right and left sides of the striatum may result from chirality in dopamine receptor density, which appears to be higher in the right than in the left striatum (41).
Several caveats should be considered in the interpretation of our findings. For example, women were studied without regard to the phase of the menstrual cycle. This factor may influence dopamine transporter number and [99mTc]TRODAT-1 binding (42). In addition, diagnostic heterogeneity, differences in illness length and episode duration, and degree of psychosocial stress may have influenced the differences in [99mTc]TRODAT-1 binding between the patients and comparison subjects. Finally, differences in physical well-being, activity level, disruption of normal sleep, and circadian rhythms may have influenced [99mTc]TRODAT-1 binding in the patients.
In conclusion, by using the highly selective dopamine transporter radioligand [99mTc]TRODAT-1 and SPECT imaging to examine the availability of striatal dopamine transporter sites in 15 depressed patients and 46 healthy subjects, we found significantly higher specific uptake values of [99mTc]TRODAT-1 in the right anterior and right posterior putamen and in the left posterior putamen and left caudate regions of the depressed patients.
 |
 |
 |
Received Jan. 9, 2002; revision received April 2, 2003; accepted April 8, 2003. From the Depression Research Unit, Department of Psychiatry, and the Division of Nuclear Medicine, Department of Radiology, University of Pennsylvania Medical Center, Philadelphia. Address reprint requests to Dr. Amsterdam, Depression Research Unit, University Science Center, 3rd Floor, 3535 Market St., Philadelphia, PA 19104; [email protected] (e-mail). Funded by grant AG-17524 from the National Institute on Aging, grant DA-09469 from the National Institute on Drug Abuse, grant NS-18509 from the National Institute of Neurological and Communicative Disorders and Stroke, and the Jack Warsaw Fund for Research in Biological Psychiatry, Depression Research Unit, University of Pennsylvania Medical Center. The authors thank Karl Plossl, Ph.D., for radiochemistry assistance.
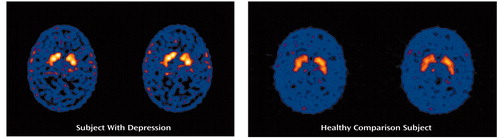
Figure 1. [99mTc]TRODAT-1 Binding in the Right Anterior Putamen in a Depressed Patient and a Healthy Comparison Subject Studied With SPECT Imaging
1. Willner P: Dopaminergic mechanisms in depression and mania, in Psychopharmacology: The Fourth Generation of Progress. Edited by Bloom FE, Kupfer DJ. New York, Raven Press, 1995, pp 921–931Google Scholar
2. Kapur S, Mann JJ: Role of the dopaminergic system in depression. Biol Psychiatry 1992; 32:1–17Crossref, Medline, Google Scholar
3. D’Aquila PS, Collu M, Cessa GL, Serra G: The role of dopamine in the mechanism of action of antidepressant drugs. Eur J Pharmacol 2000; 405:365–373Crossref, Medline, Google Scholar
4. Willner P: The mesolimbic dopamine system as a target for rapid antidepressant action. Int Clin Psychopharmacol 1997; 12(suppl 3):S7-S14Google Scholar
5. Roy A, Pickar D, Linnoila M, Doran AR, Ninan P, Paul SM: Cerebrospinal fluid monoamine and monoamine metabolite concentrations in melancholia. Psychiatry Res 1985; 15:281–292Crossref, Medline, Google Scholar
6. Bowden C, Cheetham SC, Lowther S, Katona CLE, Crompton MR, Horton RW: Reduced dopamine turnover in the basal ganglia of depressed suicides. Brain Res 1997; 769:135–140Crossref, Medline, Google Scholar
7. Lambert G, Johansson M, Agren H, Friberg P: Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: evidence in support of the catecholamine hypothesis of mood disorders. Arch Gen Psychiatry 2000; 57:787–793Crossref, Medline, Google Scholar
8. Cummings JL: Depression and Parkinson’s disease: a review. Am J Psychiatry 1992; 149:443–454Link, Google Scholar
9. Rogers MA, Bradshaw JL, Pantelis C, Phillips JG: Frontostriatal deficits in unipolar major depression. Brain Res Bull 1998; 47:297–310Crossref, Medline, Google Scholar
10. McHugh PR: The neuropsychiatry of basal ganglia disorders: a triadic syndrome and its explanation. Neuropsychiatry Neuropsychol Behav Neurol 1989; 2:239–247Google Scholar
11. Acton PD, Mozley PD: Single photon emission tomography imaging in parkinsonian disorders: a review. Behav Neurol 1999; 12:11–27Crossref, Google Scholar
12. Lenze E, Cross D, McKeel D, Neuman RJ, Sheline YI: White matter hyperintensities and gray matter lesions in physically healthy depressed subjects. Am J Psychiatry 1999; 156:1602–1607Link, Google Scholar
13. Krishnan KR, McDonald WM, Escalona PR, Doraiswamy PM, Na C, Husain MM, Figiel GS, Boyko OB, Ellinwood EH, Nemeroff CB: Magnetic resonance imaging of the caudate nuclei in depression: preliminary observations. Arch Gen Psychiatry 1992; 49:553–557Crossref, Medline, Google Scholar
14. Parashos IA, Tupler LA, Blitchington T, Krishnan KR: Magnetic-resonance morphometry in patients with major depression. Psychiatry Res Neuroimaging 1998; 84:7–15Crossref, Medline, Google Scholar
15. Soares JC, Mann JJ: The functional neuroanatomy of mood disorders. J Psychiatr Res 1997; 31:393–432Crossref, Medline, Google Scholar
16. Kennedy SH, Evans KR, Krüger S, Mayberg HS, Meyer JH, McCann S, Arifuzzman AI, Houle S, Vaccarino FJ: Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry 2001; 158:899–905Link, Google Scholar
17. Brody AL, Saxena S, Stoessel P, Gillies LA, Fairbanks LA, Alborzian S, Phelps ME, Huang SC, Wu HM, Ho ML, Ho MK, Au SC, Maidment K, Baxter LR: Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Arch Gen Psychiatry 2001; 58:631–640Crossref, Medline, Google Scholar
18. Arnt J, Hyttel J, Overo KF: Prolonged treatment with the specific 5-HT-uptake inhibitor citalopram: effect on dopaminergic and serotonergic functions. Pol J Pharmacol Pharm 1984; 36:221–230Medline, Google Scholar
19. Maj J, Rogoz Z: Pharmacological effects of venlafaxine, a new antidepressant, given repeatedly, on the alpha(1)-adrenergic, dopamine and serotonin systems. J Neural Transm 1999; 106:197–211Crossref, Medline, Google Scholar
20. Maj J, Dziedzicka-Wasylewska M, Rogoz R, Rogoz Z: Effect of antidepressant drugs administered repeatedly on the dopamine D-3 receptors in the rat brain. Eur J Pharmacol 1998; 351:31–37Crossref, Medline, Google Scholar
21. Plaznik A, Kostowski W: The effects of antidepressants and electroconvulsive shocks on the functioning of the mesolimbic dopaminergic system: a behavioral study. Eur J Pharmacol 1987; 135:389–396Crossref, Medline, Google Scholar
22. Kung MP, Stevenson DA, Plossl K, Meegalla SK, Beckwith A, Essman WD, Mu M, Lucki I, Kung HF: [99mTc]TRODAT-1: a novel technetium-99m complex as a dopamine transporter imaging agent. Eur J Nucl Med 1997; 24:372–380Medline, Google Scholar
23. First MB, Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-IV—Patient Edition (SCID-P). Washington, DC, American Psychiatric Press, 1994Google Scholar
24. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
25. Brunswick DJ, Amsterdam JD, Fawcett J, Quitkin FM, Reimherr FW, Rosenbaum JF, Beasley CM Jr: Fluoxetine and norfluoxetine plasma levels after discontinuing fluoxetine therapy. J Clin Psychopharmacol 2001; 21:616–618Crossref, Medline, Google Scholar
26. Mozley PD, Schneider JS, Acton PD, Plossl K, Stern MB, Siderowf A, Leopold NA, Li PY, Alavi A, Kung HF: Binding of [Tc-99m]TRODAT-1 to dopamine transporters in patients with Parkinson’s disease and in healthy volunteers. J Nucl Med 2000; 41:584–589Medline, Google Scholar
27. Mozley LH, Gur RC, Mozley PD, Gur RE: Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry 2001; 158:1492–1499Link, Google Scholar
28. Acton PD, Meyer PT, Mozley PD, Plossl K, Kung HF: Simplified quantification of dopamine transporters in humans using [Tc-99m]TRODAT-1 and single-photon emission tomography. Eur J Nucl Med 2000; 27:1714–1718Crossref, Medline, Google Scholar
29. Laasonen-Balk T, Kuikka J, Viinamaki H, Husso-Saastamoinen M, Lehtonen J, Tiihonen J: Striatal dopamine transporter density in major depression. Psychopharmacology (Berl) 1999; 144:282–285Crossref, Medline, Google Scholar
30. Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, Sanacora G, Owens MJ, Nemeroff CB, Rajeevan N, Baldwin RM, Seibyl JP, Innis RB, Charney DS: Reduced brain serotonin transporter availability in major depression as measured by [123I]-2β-carbomethoxy-3β-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry 1998; 44:1090–1098Crossref, Medline, Google Scholar
31. Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG: Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA 1998; 95:4029–4034Crossref, Medline, Google Scholar
32. Kimmel HL, Joyce AR, Carroll FI, Kuhar MJ: Dopamine D1 and D2 receptors influence dopamine transporter synthesis and degradation in the rat. J Pharmacol Exp Ther 2001; 298:129–140Medline, Google Scholar
33. Caligiuri MP, Ellwanger J: Motor and cognitive aspects of motor retardation in depression. J Affect Disord 2000; 57:83–93Crossref, Medline, Google Scholar
34. Sachdev P, Aniss AM: Slowness of movement in melancholic depression. Biol Psychiatry 1994; 35:253–262Crossref, Medline, Google Scholar
35. Bremner JD, Innis RB, Salomon RM, Staib LH, Ng CK, Miller HL, Bronen RA, Krystal JH, Duncan J, Rich D, Price LH, Malison R, Dey H, Soufer R, Charney DS: Positron emission tomography measurement of cerebral metabolic correlates of tryptophan depletion-induced depressive relapse. Arch Gen Psychiatry 1997; 54:364–374Crossref, Medline, Google Scholar
36. Smith KA, Morris JS, Friston KJ, Cowen PJ, Dolan RJ: Brain mechanisms associated with depressive relapse and associated cognitive impairment following acute tryptophan depletion. Br J Psychiatry 1999; 174:525–529Crossref, Medline, Google Scholar
37. George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM: Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry 1995; 152:341–351Link, Google Scholar
38. DeLong MR, Georgopoulos AP, Crutcher MD, Mitchell SJ, Richardson RT, Alexander GE: Functional organization of the basal ganglia: contributions of single-cell recording studies, in Functions of the Basal Ganglia: CIBA Foundation Symposium 107. Edited by Evered D, O’Connor M. London, Pitman, 1984, pp 64–82Google Scholar
39. Byrum CE, Ahearn EP, Krishnan KR: A neuroanatomic model for depression. Prog Neuropsychopharmacol 1999; 23:175–193Crossref, Google Scholar
40. Drevets WC: Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res 2000; 126:413–431Crossref, Medline, Google Scholar
41. Larisch R, Meyer W, Klimke A, Kehren F, Vosberg H, Muller-Gartner HW: Left-right asymmetry of striatal dopamine D-2 receptors. Nucl Med Commun 1998; 19:781–787Crossref, Medline, Google Scholar
42. Lammers CH, D’Souza U, Qin ZH, Lee SH, Yajima S, Mouradian MM: Regulation of striatal dopamine receptors by estrogen. Synapse 1999; 34:222–227Crossref, Medline, Google Scholar



