Concordant Restoration of Ovarian Function and Mood in Perimenopausal Depression
Abstract
OBJECTIVE: Despite reports of estradiol’s therapeutic efficacy in perimenopausal depression, the relationship between ovarian function and mood in perimenopausal depression remains unclear. The purpose of this study was to examine changes in mood and pituitary-ovarian axis function in women exhibiting perimenopausal depression. METHOD: Depression ratings (from the Center for Epidemiologic Studies—Depression Scale [CES-D Scale]) and follicle-stimulating hormone (FSH) plasma levels of depressed perimenopausal women (N=110) attending a menopause clinic were obtained at baseline and after a 6-week screening period. RESULTS: Eighteen women experienced an improvement in depression (50% decline in CES-D Scale scores) at week 6, which was associated with a significant decrease in FSH plasma levels (baseline: mean=73.3 IU/liter [SD=42.0]; week 6: mean=42.2 IU/liter [SD=28.6]). Similarly, those subjects experiencing a 50% drop in FSH plasma levels had significant decreases in CES-D Scale scores (baseline: mean=23.3 [SD=6.8]; week 6: mean=18.1 [SD=10.9]). However, between women with CES-D Scale scores ≥15 and those with CES-D Scale scores <15, no significant differences in FSH levels were observed either at baseline (mean=65.5 IU/liter [SD=35.7] and 60.9 IU/liter [SD=34.9], respectively) or at week 6 (mean=56.2 IU/liter [SD=36.6] and 51.5 IU/liter [SD=34.2]). CONCLUSIONS: Mood variability in women with perimenopausal depression may reflect episodic alterations in ovarian function that are best detected by longitudinal study designs.
The perimenopausal phase is a time of considerable variability in reproductive function (1), which appears in some women to be associated with an increased susceptibility to develop depression. Elevated gonadotropin levels occur during the late perimenopausal phase, with plasma levels of follicle-stimulating hormone (FSH) increasing in association with impaired ovarian function and decreased estradiol secretion. In contrast to the postmenopausal phase, ovarian function may vary considerably in the perimenopausal phase, with restoration of normal menstrual cycle function observed as frequently as the onset of menopause (1). In addition, during the perimenopausal phase, levels of estradiol secretion may be reduced, normal, or even at times increased (2, 3). It is unclear, however, whether the variability in ovarian hormone secretion during the perimenopausal phase has any causal role in the development of depression.
Estradiol replacement has been identified as a successful treatment for perimenopausal depression (4, 5), but perimenopausal women with depression have no evidence of abnormal reproductive function relative to those without depression. Indeed, it is possible that the therapeutic efficacy of estradiol reflects the psychotropic properties of estrogen rather than its action to correct a hormonal deficiency or withdrawal state that predisposes to the development of depression. The role of changes in ovarian steroids in the development of perimenopausal depression can best be determined by longitudinal studies that document the coincident onset of both endocrine evidence of perimenopausal reproductive function and behavioral evidence of depression. Nonetheless, the oscillation between perimenopausal and premenopausal reproductive function provides an opportunity to establish the temporal coincidence of perimenopausal reproductive function and depression.
We observed several women who came to our clinic exhibiting depression and whose FSH plasma levels, obtained at each clinic visit, declined over a 6–8-week period of observation concurrent with spontaneous improvements in mood symptoms. We were interested in systematically examining whether this putative relationship between normalization of ovarian function and mood could be generalized. Thus, we serially evaluated over a 6-week screening phase mood scores and FSH plasma levels in a larger group of women who came to our clinic exhibiting perimenopausal depression.
Method
Subjects
Subjects were women attending a menopause clinic who were between the ages of 40 and 55 and exhibiting depression occurring in association with a history of menstrual cycle irregularity of at least 6 months duration (but no longer than 1 year of amenorrhea). In addition, subjects for this study were selected if the first FSH plasma level obtained was >20 IU/liter (5). Women who came to the menopause clinic with adverse mood symptoms (N=315) were screened for inclusion in this study. Of these 315 subjects, 110 women met perimenopausal criteria (i.e., menstrual cycle irregularity/amenorrhea and FSH plasma levels ≥20 IU/liter). Subjects were self-referred in response to local advertisements or were referred by their physicians. All women were medication free during the study period. Written informed consent to measure plasma hormone levels and monitor mood symptoms was obtained from each woman before her participation. FSH plasma levels were measured and mood ratings (which included the Center for Epidemiologic Studies—Depression Scale [CES-D Scale] [6] score) were completed at each clinic visit (every 2 weeks for 6 weeks). FSH plasma levels were measured by AxSYM radioimmunoassay (Abbott Laboratories, Abbot Park, Ill.), as previously described (7). Interassay and intraassay coefficients of variation were 4% and 3%, respectively. In addition, daily ratings (a 6-point self-rating scale) of hot flushes were completed by all but six women and were used to assign the subjects to one of two groups: women with an average daily hot flush severity score of ≤2 (a score of 2 is minimal severity) and those with an average daily score >2.
Comparisons
In order to examine the relationship between changes in FSH plasma levels and CES-D Scale scores over the 6 weeks of this study, women were grouped for three comparison purposes. First, women were divided on the basis of the severity of depressive symptoms into two groups: those with CES-D Scale scores ≥15 and those with scores <15. FSH plasma levels were compared in women with high and low CES-D Scale scores at two time points (i.e., the beginning [baseline] and the end of the screening period [week 6]).
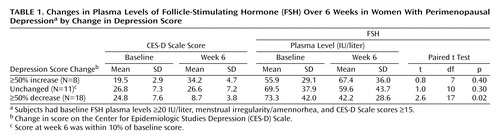
Second, women with CES-D Scale scores ≥15 (N=74) at baseline were assigned to one of three groups on the basis of the observed change in CES-D Scale scores from baseline to week 6: those whose scores decreased by 50% (N=18), those whose scores increased by 50% (N=8), and those whose scores were unchanged (N=11) (defined as a ≤10% change in CES-D Scale score over the study period). (The remaining 37 women met none of these grouping criteria.) FSH plasma levels were compared across time points within each group.
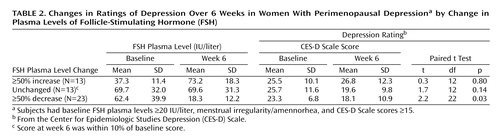
Third, women with CES-D Scale scores ≥15 at visit 1 were assigned to one of three groups on the basis of FSH plasma level changes from baseline to week 6: those whose levels increased by 50% (N=13), those whose levels decreased by 50% (N=23), and those whose levels were unchanged (N=13) (defined as a ≤10% change in FSH level over the study period). CES-D Scale scores were compared across time points within each group.
Statistical Analysis
Within-subject differences in FSH plasma levels and CES-D Scale scores across the study period (i.e., between baseline and week 6) were analyzed by using Bonferroni-adjusted paired t tests. Differences in the number of women reporting hot flushes between the two groups whose CES-D Scale scores either increased or decreased by 50% over the study period were analyzed by using Fisher’s exact test. Between-subject FSH plasma levels in women with and without depression were compared at the beginning and the end of screening with Students t tests. The relationship between CES-D Scale scores and FSH plasma levels at baseline and week 6 were examined by Pearson product-moment correlation coefficients. Statistical analysis was performed with the Systat 10.0 software program (SPSS, Inc., Chicago).
Results
The average age of the women in this study was 48.7 years (SD=3.7). FSH plasma levels dropped significantly over the study period (baseline: mean=64.0 IU/liter [SD=35.4]; week 6: mean=54.2 IU/liter [SD=35.5]) (t=2.8, df=109, p=0.007). These changes in FSH plasma levels were accompanied by a nonsignificant decrease in CES-D Scale scores (baseline: mean=19.7 [SD=10.8]; week 6: mean=17.8 [SD=0.6]) (t=1.9, df=109, p=0.06). Forty-seven subjects (43%) reported hot flushes and were confirmed to have an average hot flush severity score >2; 59 (54%) had no hot flushes (average score ≤2).
At baseline, 74 women had CES-D Scale scores ≥15 (depressed), and 36 had CES-D Scale scores <15 (nondepressed). Mean FSH plasma levels were 65.5 IU/liter (SD=35.7) and 60.9 IU/liter (SD=34.9), respectively (t=0.6, df=108, p=0.53). At week 6, 62 subjects had CES-D Scale scores of ≥15, and 48 had CES-D Scale scores <15. Mean FSH plasma levels were 56.2 IU/liter (SD=36.6) and 51.5 IU/liter (SD=34.2), respectively (t=0.7, df=108, p=0.50). There were no significant correlations between CES-D Scale scores and FSH plasma levels at either time point.
Of the 18 depressed women whose CES-D Scale scores decreased by ≥50%, 16 experienced a decline in FSH plasma levels during the study period. FSH plasma levels in this group of 18 subjects showed a significant decrease (Table 1), and we observed an incremental decline in FSH levels at each of the four clinic visits that paralleled the improvements in CES-D Scale scores (Figure 1). In contrast, the women whose CES-D Scale scores increased had FSH plasma levels that increased nonsignificantly. For those subjects whose CES-D Scale scores did not change across the study, there was a nonsignificant decrease in FSH plasma levels. There was no difference in the number of women reporting hot flushes between the groups whose CES-D Scale scores decreased (N=6 of 18, 33%) and those whose CES-D Scale scores increased (N=3 of 8, 37%).
In the group whose FSH plasma levels decreased by ≥50%, 17 of the 23 women experienced a decline in CES-D Scale scores (of any magnitude) during the study period, and CES-D Scale scores in this group significantly decreased (Table 2). In contrast, for those women whose FSH plasma levels increased by ≥50%, CES-D Scale scores increased nonsignificantly. For the group whose FSH plasma levels did not change, there was a nonsignificant decrease in CES-D Scale scores.
Discussion
In this study, we identified a subgroup of women with perimenopausal depression whose mood symptoms remitted spontaneously in association with a significant decline in gonadotropin levels (and a presumed alteration in ovarian function).
Recent double-blind, placebo-controlled studies have demonstrated the antidepressant-like effects of estradiol treatment in perimenopausal depression (4, 5). These studies also document several characteristics of the relationship between estradiol replacement and mood in these women: 1) the mood-enhancing effects of estradiol in perimenopausal depression occur independent of the presence of hot flushes, 2) the therapeutic response to estradiol is not predicted by baseline or posttreatment plasma estradiol levels, 3) mood symptoms resolve rapidly with treatment (within 3 weeks or less), and 4) response to hormonal treatment is nonuniform despite women meeting similar diagnostic and endocrine criteria. In addition, basal hormone studies have suggested that perimenopausal depression is not a simple hormone deficiency, and basal plasma levels of FSH and estradiol do not distinguish perimenopausal women with depression from those without depression (8, 9).
Our data are consistent with the aforementioned observations. First, CES-D Scale scores improved in both women with and without hot flushes. Second, despite our observing a significant association between changes in FSH plasma levels and CES-D Scale scores when measured across the study period (i.e., between baseline and week 6), repeated cross-sectional measurements showed no relationship between FSH plasma levels and depression, i.e., the more depressed women (defined by CES-D Scale scores ≥15) could not be distinguished from those with lower depression scores on the basis of FSH plasma levels. Our data (Figure 1) also may suggest a more direct relationship between declining FSH levels and CES-D Scale scores, since both measures changed concurrently over the four visits occurring at 2-week intervals, consistent with the relatively brief time to therapeutic response observed in several therapeutic trials of estradiol in depression (4, 10, 11). Finally, the response to changes in CES-D Scale scores or FSH levels was not uniform: 16 (89%) of the 18 women whose CES-D Scale scores declined by ≥50% experienced a decline in FSH levels. Similarly, in perimenopausal depression, response rates to estradiol replacement were reported to range between 70% and 80%. In addition, women with other reproductive endocrine-related mood disorders have been shown to experience varying rates of response to identical hormonal intervention. In premenstrual syndrome, for example, approximately 60% of women respond to gonadotropin-releasing hormone-induced ovarian suppression (12, 13). Thus, a nonuniform response to an identical hormonal intervention is a common characteristic of reproductive endocrine-related mood disorders and does not invalidate a proposed relationship between changes in ovarian steroids and mood.
Increased FSH plasma levels were not consistently associated with worsening CES-D Scale scores, nor were increased CES-D Scale scores associated with corresponding elevations in FSH plasma levels, whereas a more uniform relationship was observed between mood and FSH plasma level when either measure decreased. These data suggest a threshold or ceiling of FSH plasma levels below which differences may influence mood and above which there is little association between changes in measures of FSH and mood. Insufficient numbers of perimenopausal women with low CES-D Scale scores and low FSH plasma levels presented to our clinic to examine the effects of increasing FSH levels on CES-D Scale scores.
Our finding that, in some women, measures of ovarian function change concurrently with perimenopausal depressive symptom severity provides support for a direct interaction between reproductive endocrine function and mood. Nevertheless, the driver of this association is unclear. Indeed, a possible bidirectional relationship between mood states and ovarian function may pertain. Spontaneous improvement in ovarian function may have mediated the remission of adverse mood symptoms, consistent with estradiol’s efficacy in perimenopausal depression (4, 5). Alternatively, a spontaneous improvement in mood may lead to reduced stress and a return to normalization of both hypothalamic-pituitary-adrenal and hypothalamic-pituitary-ovarian function (14–16). Finally, although there is little evidence that gonadotropins influence human behavior, it is theoretically possible that changes in FSH or luteinizing hormone may have direct effects on CNS function and mood (17, 18).
Our observations also may shed light on a methodologic confound in clinical trials in perimenopausal women. It is possible that spontaneous improvements in mood may contribute to apparent therapeutic or placebo responses, as was observed in a recent estradiol trial (4). Variability in reproductive function during the perimenopausal phase has been well documented (1–3) and is expected in studies of perimenopausal women. Thus, alterations in both ovarian function and mood may represent an important confound in treatment studies of depression employing perimenopausal subjects, thus necessitating the concurrent monitoring of both mood and measures of ovarian function.
In summary, these findings suggest a relationship between changes in ovarian function and mood in some perimenopausal women, with improvements in ovarian function being associated with remission of depressive symptoms. Longitudinal studies of women in the midlife are needed to further explore this association, but these data suggest that reproductive endocrine function should be evaluated in middle-aged depressed women with recent-onset depression.
 |
 |
Received Oct. 8, 2002; revision received March 19, 2003; accepted March 31, 2003. From the Behavioral Endocrinology Branch, National Institute of Mental Health; and the Clinical Center Nursing Department, National Institutes of Health, Bethesda, Md. Address reprint requests to Dr. Schmidt, NIMH, Bldg. 10, Room 3N238, 10 Center Dr., MSC 1276, Bethesda, MD 20892-1276.
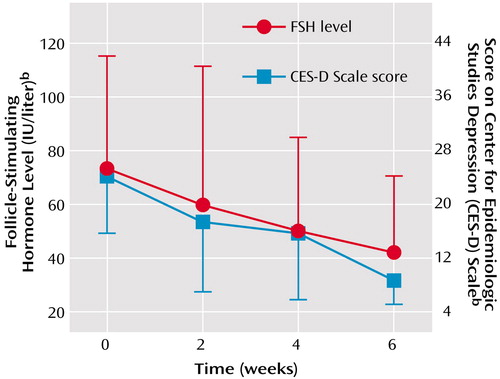
Figure 1. Concordant Decreases Over 6 Weeks in Plasma Levels of Follicle-Stimulating Hormone (FSH) and Depression in 18 Women With Perimenopausal Depressiona
aSubjects had baseline FSH plasma levels ≥20 IU/liter, menstrual irregularity/amennorhea, and CES-D Scale scores ≥15. The 18 women providing data were those whose CES-D Scale score decreased by ≥50% over the 6-week study.
Error bars represent standard deviations.
1. Kaufert PA, Gilbert P, Tate R: Defining menopausal status: the impact of longitudinal data. Maturitas 1987; 9:217–226Crossref, Medline, Google Scholar
2. Santoro N, Brown JR, Adel T, Skurnick JH: Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab 1996; 81:1495–1501Medline, Google Scholar
3. Burger HG, Dudley EC, Hopper JL, Shelley JM, Green A, Smith A, Dennerstein L, Morse C: The endocrinology of the menopausal transition: a cross-sectional study of a population-based sample. J Clin Endocrinol Metab 1995; 80:3537–3545Medline, Google Scholar
4. Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, Rubinow DR: Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol 2000; 183:414–420Crossref, Medline, Google Scholar
5. Soares CD, Almeida OP, Joffe H, Cohen LS: Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001; 58:529–534Crossref, Medline, Google Scholar
6. Radloff LS: The CES-D Scale: a self-report depression scale for research in the general population. J Applied Psychol Measurement 1977; 1:385–401Crossref, Google Scholar
7. Odell WD, Rayford PL, Ross GT: Simple, partially automated method for radioimmunoassay of human thyroid-stimulating, growth, luteinizing and follicle stimulating hormones. J Lab Clin Med 1967; 70:973–980Medline, Google Scholar
8. Schmidt PJ, Murphy JH, Haq N, Danaceau MA, Simpson St Clair L: Basal plasma hormone levels in depressed perimenopausal women. Psychoneuroendocrinology 2002; 27:907–920Crossref, Medline, Google Scholar
9. Saletu B, Brandstatter N, Metka M, Stamenkovic M, Anderer P, Semlitsch HV, Heytmanek G, Huber J, Grunberger J, Linzmayer L, Kurz C, Decker K, Binder G, Knogler W, Koll B: Hormonal, syndromal and EEG mapping studies in menopausal syndrome patients with and without depression as compared with controls. Maturitas 1996; 23:91–105Crossref, Medline, Google Scholar
10. Gregoire AJP, Kumar R, Everitt B, Henderson AF, Studd JWW: Transdermal oestrogen for treatment of severe postnatal depression. Lancet 1996; 347:930–933Crossref, Medline, Google Scholar
11. Ahokas A, Kaukoranta J, Wahlbeck K, Aito M: Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17β-estradiol: a preliminary study. J Clin Psychiatry 2001; 62:332–336Crossref, Medline, Google Scholar
12. Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR: Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med 1998; 338:209–216Crossref, Medline, Google Scholar
13. Freeman EW, Sondheimer SJ, Rickels K: Gonadotropin-releasing hormone agonist in the treatment of premenstrual symptoms with and without ongoing dysphoria: a controlled study. Psychopharmacol Bull 1997; 33:303–309Medline, Google Scholar
14. Marcus MD, Loucks TL, Berga SL: Psychological correlates of functional hypothalamic amenorrhea. Fertil Steril 2001; 76:310–316Crossref, Medline, Google Scholar
15. Young E, Korszun A: Psychoneuroendocrinology of depression: hypothalamic-pituitary-gonadal axis. Psychiatr Clin North Am 1998; 21:309–323Crossref, Medline, Google Scholar
16. Chrousos GP, Torpy DJ, Gold PW: Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med 1998; 129:229–240Crossref, Medline, Google Scholar
17. Short RA, Bowen RL, O’Brien PC, Graff-Radford NR: Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc 2001; 76:906–909Crossref, Medline, Google Scholar
18. Lei ZM, Rao CV: Neural actions of luteinizing hormone and human chorionic gonadotropin. Semin Reprod Med 2001; 19:103–109Crossref, Medline, Google Scholar



