Minor Physical Anomalies and Schizophrenia Spectrum Disorders: A Prospective Investigation
Abstract
OBJECTIVE: The authors prospectively assessed the relationship between minor physical anomalies identified in childhood and adult psychiatric outcome. METHOD: In 1972, minor physical anomalies were measured in a group of 265 Danish children ages 11–13. The examination was part of a larger study investigating early signs of schizophrenia spectrum disorders. Many of the subjects had a parent with schizophrenia, leaving them at high risk for developing a schizophrenia spectrum disorder. In 1991, adult psychiatric outcome data were obtained for 91.3% (N=242) of the original subjects, including 81 who were at high risk. RESULTS: Individuals with a high number of minor physical anomalies developed schizophrenia spectrum disorders significantly more often than they developed a no mental illness outcome. Further, individuals with a high number of minor physical anomalies tended to develop schizophrenia spectrum disorders more often than other psychopathology. Among individuals at genetic high risk, higher numbers of minor physical anomalies may interact with pre-existing vulnerabilities for schizophrenia to increase the likelihood of a schizophrenia spectrum disorder outcome. CONCLUSIONS: Minor physical anomalies may provide important clues to understanding schizophrenia spectrum disorders from a neurodevelopmental perspective. Minor physical anomalies appear to signal stressors relevant to schizophrenia spectrum development, especially in those at genetic risk for schizophrenia.
Minor physical anomalies are slight deviations in external physical characteristics (e.g., high-steepled palate, large or small distance between tear ducts, adherent earlobes). Kraepelin noted an association between minor physical anomalies and mental illness. In 1896, he pointed to “so-called signs of degeneracy…smallness or deformity of the skull, child-like habitus, missing teeth, deformed ears” (1). Researchers have found higher numbers of minor physical anomalies in individuals suffering from developmental disorders, including schizophrenia (2–4).
Researchers contend that minor physical anomalies represent external signs of prenatal development disruptions (5). The study of minor physical anomalies, therefore, may help identify potential etiological antecedents of schizophrenia. Hypotheses differ regarding the exact timing of minor physical anomaly development. Smith (6), however, has suggested that minor physical anomalies form during periods of abnormal ectodermal development (due to genetics or a teratogen such as maternal alcohol abuse or smoking during pregnancy). The central nervous system (CNS) also stems from the ectoderm; therefore, the presence of minor physical anomalies may signal abnormal development of the CNS (6, 7).
Research has shown a higher number of minor physical anomalies in disorders other than schizophrenia. Smith (6) has reported a higher numbers of minor physical anomalies in individuals with Down’s syndrome. Fogel and colleagues (8) reported an association between minor physical anomalies and hyperactivity. Murphy and Owen (1) reported higher numbers of minor physical anomalies in individuals with various other psychiatric conditions. Green and colleagues (9) found more minor physical anomalies in patients with schizophrenia than in patients with bipolar disorder. Lohr and Flynn (3) reported no difference between patients with schizophrenia and those with mood disorders; however, schizophrenia patients had significantly more minor physical anomalies than normal control subjects. In addition, higher numbers of minor physical anomalies have been reported in individuals with schizophrenia spectrum disorders (e.g., paranoid personality disorder, schizotypal personality disorder) than in normal control subjects (10).
Offspring of parents with schizophrenia are at greater risk of developing schizophrenia, which suggests a genetic liability for the disorder. Mednick and colleagues (11) hypothesized that genetic liability may result in a preprogrammed disruption of fetal neural development that increases risk for later development of schizophrenia. Further, Mednick and colleagues (11) suggested that an interaction between a genetic liability and an environmental stressor (e.g., birth complications, unstable rearing circumstances) increases the risk of schizophrenia. Green and colleagues (9) suggested that minor physical anomalies mark the occurrence of a stressor in vulnerable individuals, increasing the risk for schizophrenia.
Researchers cite potential scorer bias as a common problem in the assessment of minor physical anomalies (1, 12). Minor physical anomalies are assessed through a brief in-person examination. Even if examiners are technically “blind” to diagnosis, person-to-person contact can often offer examiners clues to diagnosis. This is especially true in studies where nonpatient populations are being compared with severely disturbed patients (1). Assessing subjects prospectively, before the onset of symptoms, and following their psychiatric status over time overcomes this methodological limitation.
The present investigation attempted to replicate research indicating higher numbers of minor physical anomalies among individuals with schizophrenia spectrum disorders through a prospective assessment. We used the more inclusive diagnostic grouping schizophrenia spectrum disorders, as opposed to schizophrenia only, to increase sample size for analyses. To our knowledge, these longitudinal data provide the first investigation of minor physical anomalies assessed before severe symptom onset. Premorbid examination significantly reduces the possibility of rater bias.
It was hypothesized that the number of minor physical anomalies (assessed at 11–13 years of age) would differentiate between adult diagnostic groups and would interact with genetic risk status in determining schizophrenia spectrum disorder outcome. That is, among individuals compromised by genetic risk for schizophrenia, high numbers of minor physical anomalies would be associated with higher rates of schizophrenia spectrum disorder.
Method
Subjects
Subjects were drawn from a Danish birth cohort consisting of all children born between September 1, 1959, and December 31, 1961, at Rigshospitalet in Copenhagen (13). In 1972, a sample of 265 children from this cohort was intensively examined (14). All children whose mothers or fathers had a psychiatric hospital diagnosis of schizophrenia constituted the first group (N=90). A group of matched comparison subjects consisted of 93 children who had parents with psychiatric records other than schizophrenia. The remaining 82 subjects were matched comparison subjects with no parental records of psychiatric hospitalization. Both control groups were well-matched with the high-risk birth cohort for gender ratio, mother’s marital status at the time of conception, pregnancy number, social class, mother’s height and weight, and mother’s and father’s age (14).
Measurement of Minor Physical Anomalies
Minor physical anomalies were recorded in 1972 during an intensive examination. Subjects were 11–13 years of age. An experienced Danish pediatric neurologist examined each child for the presence of minor physical anomalies (listed in Appendix 1) as part of an extensive neurological assessment. The minor physical anomaly assessment was guided by the methods established by Waldrop and Halverson (15).
The number of minor physical anomalies has been found to be stable over time (15). We used this number in our analyses. Unfortunately for the purposes of reliability, only one neurologist rated the minor physical anomalies in this group. He was, however, well trained in their assessment. In addition, several sources report high interrater reliability for the total number of minor physical anomalies (5, 12, 16).
It should be noted that minor physical anomaly assessment was performed before any obvious signs of psychopathology (ages 11–13) and, therefore, completely blind to diagnostic outcome. To our knowledge, this is the first study to investigate prospectively the association between minor physical anomalies and schizophrenia spectrum disorders. Further, the pediatric neurologist was not informed of the diagnostic status of the parents of the children.
Diagnostic Assessment
In 1992, when the subjects were between 31–33 years of age, their psychiatric status was ascertained. A psychiatrist administered two structured clinical psychiatric interviews, the Structured Clinical Interview for DSM-III-R (SCID) (17) and the psychosis section of the Present State Examination (18). These interviews yielded DSM-III-R diagnoses. In addition, Danish psychiatric hospital records of subjects were examined. A detailed coding scheme yielded DSM-III-R diagnoses. On the basis of interview or hospital records, we obtained adult diagnostic outcomes for 242 of the 265 subjects (a 91.3% successful follow-up rate after 20 years; the remaining 23 subjects were neither interviewed nor had hospital records). Of these 242 subjects, 81 had a parent with schizophrenia, 87 had a parent with another disorder, and 74 had parents with no psychiatric hospitalization. A senior Danish psychiatrist (F.S.) supervised all the interviews and diagnoses. After complete description of the study to the subjects, written informed consent was obtained.
Sixteen patients with schizophrenia were identified, and 10 others were diagnosed with a schizophrenia spectrum disorder (any psychosis or delusional disorder: N=5; schizotypal personality disorder: N=3; paranoid personality disorder: N=2). No subject had a diagnosis of affective psychosis. On the basis of the finding of Weinstein et al. (10) of higher numbers of minor physical anomalies in schizotypal individuals, as well as to increase study group size, we grouped all individuals with a schizophrenia spectrum disorder (including schizophrenia) together. Of the 26 subjects with a schizophrenia spectrum disorder, 13 were diagnosed through interview only, and seven through hospital records only; six were diagnosed through both hospital records and interview.
Seventy subjects had diagnoses other than a schizophrenia spectrum disorder: nonpsychotic mood or anxiety disorder (N=19), nonpsychotic alcohol or drug abuse (N=33), a minor axis I disorder not requiring hospitalization (N=1), borderline personality disorder (N=5), schizoid personality disorder (N=1), antisocial personality disorder (N=1), other personality disorder (N=6), and personality disorder not otherwise specified (N=4). Only two of these subjects had hospital diagnoses (one for drug abuse, and one for alcohol abuse). The remaining 146 subjects were judged to have no mental illness.
Statistical Analysis
We used a chi-square analysis (two-tailed; two-by-three table) to compare the number of minor physical anomalies among subjects with a schizophrenia spectrum disorder, those with other psychopathology, and subjects with no mental illness across the entire cohort. We considered subjects at or above the median in number of minor physical anomalies to be in the high-anomaly group. After we controlled for type I error by using a Bonferroni correction, follow-up chi-square tests (two-tailed, including a report of Fisher’s exact test) compared the subjects with schizophrenia spectrum disorders with those who had other psychopathology and with those who had no mental illness. To investigate the effect of an interaction between minor physical anomalies and genetic risk on schizophrenia spectrum disorder outcome, we compared—among the high-risk subjects only—the number of minor physical anomalies between the schizophrenia spectrum disorder group and a combined other psychopathology/no mental illness group. We used chi-square analysis (two-tailed; two-by-two table; Fisher’s exact test reported as well).
Results
Association With Diagnostic Outcome
The number of minor physical anomalies ranged from zero to eight, with a median of three. Following the practices of Fogel and colleagues (8), we split subjects at the median. Those equal to or above the median were considered as having a high number of minor physical anomalies (N=131); those below the median were considered as having a low number of anomalies (N=111). Table 1 shows the adult psychiatric outcome for subjects with high and low numbers of minor physical anomalies.
Our first hypothesis was that the number of minor physical anomalies would differentiate between diagnostic groups. We ran a two (score: high versus low score) by three (diagnosis: schizophrenia spectrum disorder versus other psychopathology versus no mental illness) chi-square analysis to test this hypothesis. The number of minor physical anomalies discriminated between diagnostic groups (χ2=7.48, df=2, p<0.03).
Guided by our first hypothesis, we ran individual 2×2 chi-square analyses to determine if the number of minor physical anomalies differentiated between the subjects with schizophrenia spectrum disorders and the other two groups. As seen in Table 1, after Bonferroni correction for type I errors (alpha=0.025), a higher number of minor physical anomalies was significantly associated with a schizophrenia spectrum disorder outcome relative to an outcome of no mental illness (odds ratio=3.52, Wald 95% confidence interval [CI]=1.34–9.27; p=0.006, Fisher’s exact test). A higher number of minor physical anomalies did not significantly differentiate between an outcome of schizophrenia spectrum disorder and other psychopathology (odds ratio=2.50, Wald 95% CI=0.89–6.99; p<0.10, Fisher’s exact test). Although the finding was not significant at the p=0.025 level, the schizophrenia spectrum disorder subjects tended to have a high number of minor physical anomalies more often than did subjects with nonpsychotic disorders. The smaller number of subjects in the other psychopathology group (N=70) relative to the no mental illness group (N=146) reduced power and may have accounted for the lack of a statistically significant difference between the subjects with other psychopathology and those with a schizophrenia spectrum disorder. The odds ratio for the comparison showed that subjects with schizophrenia spectrum disorders were 2.5 times more likely than those with other psychopathology to have a high number of minor physical anomalies.
All findings reported by using the inclusive schizophrenia spectrum disorder grouping exhibited a similar pattern when either “schizophrenia only” or “spectrum only” subjects were considered.
Association With Genetic Risk
We also investigated a possible interactive effect of minor physical anomalies with high-risk status for schizophrenia spectrum disorder. We proposed that among high-risk individuals, a higher number of minor physical anomalies would signify a stressor contributing to schizophrenia spectrum disorders. We therefore hypothesized that high-risk individuals with a high number of minor physical anomalies would be more likely to have a schizophrenia spectrum disorder outcome than other outcomes.
For these analyses, we selected only high-risk individuals (Table 1). This constraint limited the number of subjects (N=81). To increase the number of subjects in the comparison group, we combined the subjects with no mental illness and those with other psychopathology (N=64); these subjects did not significantly differ from each other on levels of minor physical anomalies (χ2=0.025, df=1, p<0.88; p=1.00, Fisher’s exact test). We conducted a chi-square test among high-risk subjects to assess whether minor physical anomalies would differentiate between those with schizophrenia spectrum disorders and those with either other psychopathology or no mental illness. As seen in Table 1, the results of the test were significant (odds ratio=3.29, Wald 95% CI=1.04–10.44; p<0.06, Fisher’s exact test). Among high-risk individuals, the number of minor physical anomalies differentiated between the schizophrenia spectrum disorder group and the other psychopathology/no mental illness group.
Our results suggest that among individuals at genetic risk for schizophrenia, a higher number of minor physical anomalies increased the likelihood of a schizophrenia spectrum disorder outcome. There was an interaction between a high number of minor physical anomalies and having a parent with schizophrenia for developing a schizophrenia spectrum disorder. These results support the hypothesis that minor physical anomalies signify a stressor relevant to schizophrenia spectrum disorders in individuals at genetic risk for schizophrenia.
It is also possible to interpret these results differently. High-risk status increases risk for neural developmental disruptions. Only some of the high-risk subjects have a genetically transmitted predisposition. Minor physical anomalies may mark those high-risk subjects who suffered a disruption in fetal neural development.
Similar analyses looking only at subjects with psychiatrically undiagnosed (“normal”) parents to obtain a purely “low-risk” group interested us. Unfortunately, the small number of subjects (especially among the schizophrenia spectrum disorder group [N=2]) limited the viability of these analyses.
Discussion
This investigation addressed 1) the association of minor physical anomalies and schizophrenia spectrum disorders and 2) the relevance of minor physical anomalies among individuals at genetic risk for schizophrenia.
A higher number of minor physical anomalies was related to a greater frequency of schizophrenia spectrum disorder outcome. This finding suggests a relation between minor physical anomalies and development of a schizophrenia spectrum disorder within the context of this study group. The presence of minor physical anomalies seemed specific to schizophrenia spectrum disorders. Subjects with schizophrenia spectrum disorders more often had a higher number of minor physical anomalies than did the subjects with no mental illness. Further, the schizophrenia spectrum disorder subjects tended to have a higher number of minor physical anomalies more often than did subjects with other psychopathology. Perhaps insults causing minor physical anomalies also cause neurodevelopmental disruptions that specifically predispose individuals to schizophrenia spectrum disorders.
A “two-hit” model has been proposed to explain the origins of schizophrenia (11). The first hit consists of a disruption of neural development. This may result from a genetic liability or an environmental teratogen (e.g., maternal influenza infection [19]). The second hit is environmental (e.g., obstetrical complications, poor family rearing circumstances [20]). Experiencing both hits increases likelihood for the development of schizophrenia.
In an approach similar to this “two-hit” model, Green et al. (9) argued that minor physical anomalies “can interact with other genetic and nongenetic factors to produce the symptoms of the illness” (p. 439). Our data seem to support this hypothesis. Minor physical anomalies interact with genetic risk to increase the likelihood of a schizophrenia spectrum disorder outcome.
Genetically liable (high-risk) individuals with a higher number of minor physical anomalies were in the schizophrenia spectrum disorder group more often than in the group with either other psychopathology or no mental illness. Additionally, of high-risk subjects with a high number of minor physical anomalies, 30.8% (N=12 of 39) developed a schizophrenia spectrum disorder, whereas only 11.9% (N=5 of 42) of high-risk subjects with a low number of minor physical anomalies developed a schizophrenia spectrum disorder.
Further, Green et al. (9) suggested that traits not elevated in first-degree relatives are not part of a genetic liability. They noted a relative lack of minor physical anomalies among first-degree relatives (siblings) of schizophrenia patients. Similarly, in our study group, offspring of a parent with schizophrenia (first-degree relatives) did not display a higher number of minor physical anomalies relative to offspring of parents with no psychiatric diagnoses (low-risk) (χ2=0.005, df=1, p<0.95; p=1.00, Fisher’s exact test). Therefore, our results suggest that minor physical anomalies may signify the occurrence of a nongenetic stressor.
The formation of minor physical anomalies during the early stages of fetal development sets minor physical anomalies apart from other proposed “second hits.” Second hits such as birth complications and poor family rearing occur either at birth or later in life. Minor physical anomalies most likely result from prenatal disturbances experienced in the first or second trimester of fetal development. It is unlikely that minor physical anomalies and birth complications, or minor physical anomalies and family rearing, engender similar factors leading to schizophrenia spectrum disorders. Nonetheless, when individuals with a genetic liability for schizophrenia experience any of these stressors, risk for schizophrenia spectrum disorders increases. Minor physical anomalies may mark the occurrence of a prenatal “second hit.” The mechanisms behind the relation between genetic vulnerability and early stressors such as minor physical anomalies leading to development of schizophrenia spectrum disorder warrants further investigation.
Other Possible Interpretations
Minor physical anomalies may relate to an outcome of schizophrenia spectrum disorder in those at high genetic risk for another reason. High-risk status may increase risk for neural developmental disruption. It is possible that minor physical anomalies relate to schizophrenia outcome in the high-risk group because the minor physical anomalies mark those high-risk subjects who actually suffered a developmental disruption.
We found no significant difference between high-risk and low-risk groups. We interpret this as evidence against the hypothesis that genetic risk will increase neural disruption responsible for minor physical anomalies. The number of individuals in the high-risk group afflicted with a genetic predisposition, however, was less than 100% (possibly closer to 8%–16%). The power of the statistical test may be insufficient to detect a significant difference between high-risk and low-risk subjects, since many high-risk subjects did not have a genetic predisposition. Thus, we cannot completely rule out the possibility that genetic risk increases neural disruption leading to minor physical anomalies.
Study Strengths
All measures for this study were prospective; data were gathered 20–33 years before diagnosis. This method of data collection greatly reduces the likelihood of the adult clinical picture influencing measurement of prenatal and early childhood events. A previous major criticism of investigations of minor physical anomalies was that the assessment of minor physical anomalies in adults with schizophrenia is not completely blind to diagnostic status (12). We circumvented this problem by assessing subjects at age 11–13, before any major signs of mental illness, and well before the critical age of risk. To our knowledge, this is the first study to examine minor physical anomalies before severe symptom onset.
Minor physical anomalies assessed in adolescence predicted a schizophrenia spectrum disorder outcome. The occurrence of minor physical anomalies may be a valuable tool (along with other measures) for recognizing individuals at higher risk for schizophrenia spectrum disorders. Minor physical anomaly assessment is easy and reliable. Early identification of vulnerable individuals may be invaluable in targeting those who may benefit from primary prevention programs.
Study Limitations
The low number of schizophrenia spectrum disorder outcomes in this investigation limits statistical power and the ability to draw strong conclusions. Future research investigating the genetic and environmental mechanisms responsible for the formation of minor physical anomalies may offer insight into the role that minor physical anomalies play in schizophrenia spectrum disorders. Research suggests (but has yet to establish) that minor physical anomalies act in conjunction with a pre-existing liability for schizophrenia spectrum disorders (9). Studies investigating the mechanisms behind the relationship between minor physical anomalies and these disorders may add to the understanding of schizophrenia spectrum etiology.
 |
Received Oct. 31, 2000; revisions received March 21 and Aug. 6, 2001; accepted Aug. 20, 2001. From the Institute of Preventive Medicine, Copenhagen; and the Social Science Research Institute, University of Southern California. Address reprint requests to Mr. Schiffman, Social Science Research Institute, University of Southern California, University Park, Los Angeles, CA 90089-0375. Supported by an NIMH Research Scientist Award (MH-00619) to Dr. Mednick and NIMH grant MH-37692.
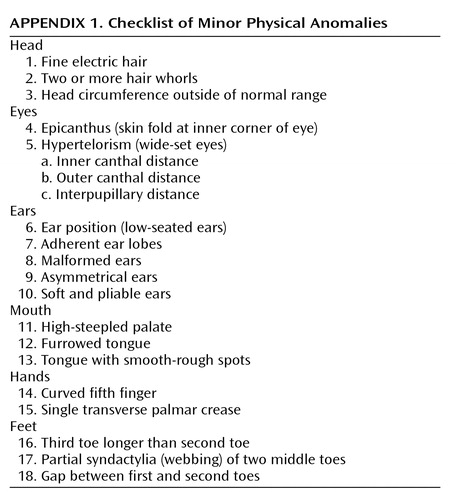 |
Appendix 1.
1. Murphy KC, Owen MJ: Minor physical anomalies and their relationship to the aetiology of schizophrenia. Br J Psychiatry 1996; 168:139-142Crossref, Medline, Google Scholar
2. Ismail B, Cantor-Graae E, McNeil TF: Minor physical anomalies in schizophrenic patients and their siblings. Am J Psychiatry 1998; 155:1695-1702Link, Google Scholar
3. Lohr JB, Flynn K: Minor physical anomalies in schizophrenia and mood disorders. Schizophr Bull 1993; 19:551-556Crossref, Medline, Google Scholar
4. Green MF, Satz P, Gaier DJ, Ganzell S, Kharabi F: Minor physical anomalies in schizophrenia. Schizophr Bull 1989; 15:91-99Crossref, Medline, Google Scholar
5. Ismail B, Cantor-Graae E, McNeil TF: Minor physical anomalies in schizophrenia: cognitive, neurological and other clinical correlates. J Psychiatr Res 2000; 34:45-56Crossref, Medline, Google Scholar
6. Smith DW: Recognizable Patterns of Human Malformation: Genetic, Embryologic and Clinical Aspects. Philadelphia, WB Saunders, 1976Google Scholar
7. Guy JD, Majorski LV, Wallace CJ, Guy MP: The incidence of minor physical anomalies in adult male schizophrenics. Schizophr Bull 1983; 9:571-582Crossref, Medline, Google Scholar
8. Fogel CA, Mednick SA, Mednick B, Michelsen N: Minor physical anomalies and hyperactivity. Acta Psychiatr Scand 1986; 72:551-556Crossref, Google Scholar
9. Green MF, Satz P, Christenson C: Minor physical anomalies in schizophrenia patients, bipolar patients, and their siblings. Schizophr Bull 1994; 20:433-440Crossref, Medline, Google Scholar
10. Weinstein DD, Diforio D, Schiffman J, Walker E, Bonsall R: Minor physical anomalies, dermatoglyphic asymmetries, and cortisol levels in adolescents with schizotypal personality disorder. Am J Psychiatry 1999; 156:617-623Abstract, Google Scholar
11. Mednick SA, Watson JB, Huttunen M, Cannon TD, Katila H, Machon R, Mednick B, Hollister M, Parnas J, Schulsinger F, Sajaniemi N, Voldsgaard P, Pyhala R, Gutkind D, Wang X: A two-hit working model of the etiology of schizophrenia, in Origins and Development of Schizophrenia: Advances in Experimental Psychopathology. Edited by Lenzenweger M, Dworkin RH. Washington, DC, American Psychological Association, 1998, pp 27-66Google Scholar
12. Green MF: Schizophrenia from a Neurocognitive Perspective: Probing the Impenetrable Darkness. San Diego, Academic Press, 1998Google Scholar
13. Zachau-Chistiansen B, Ross EM: Babies: Human Development During the First Year. New York, Wiley, 1975Google Scholar
14. Mednick S, Mura E, Schulsinger F, Mednick B: Perinatal conditions and infant development in children with schizophrenic parents. Soc Biol 1971; 18:5103-5113Google Scholar
15. Waldrop MF, Halverson CF: Minor physical anomalies and hyperactive behavior in young children, in Exceptional Infant: Studies in Abnormalities. Edited by Hellmuth J. New York, Brunner/Mazel, 1971, pp 342-380Google Scholar
16. Steg J, Rapoport J: Minor physical anomalies in normal, neurotic, learning disabled, and severely disturbed children. J Autism and Childhood Schizophrenia 1975; 5:299-307Crossref, Medline, Google Scholar
17. Spitzer RL, Williams JB, Gibbon M: User’s Guide for the Structured Clinical Interview for DSM-III-R. Washington, DC, American Psychiatric Press, 1990Google Scholar
18. Wing JK, Cooper JE, Sartorious N: The Measurement and Classification of Psychiatric Symptoms. London, England, Cambridge University Press, 1974Google Scholar
19. Mednick SA, Huttunen M, Machon RA: Prenatal influenza infections and adult schizophrenia. Schizophr Bull 1994; 20:263-267Crossref, Medline, Google Scholar
20. Burman B, Mednick SA, Machon RA, Parnas J, Schulsinger F: Children at high risk for schizophrenia: parent and offspring perceptions of family relationships. J Abnorm Psychol 1987; 96:364-366Crossref, Medline, Google Scholar



