Volume Changes in Gray Matter in Patients With Schizophrenia
Abstract
OBJECTIVE: Schizophrenia is generally characterized by a progressive decline in functioning. Although structural brain abnormalities, particularly decrements in gray matter volume, are considered important to the pathology of schizophrenia, it is not resolved whether the brain abnormalities become more prominent over time. METHOD: Magnetic resonance brain images from 159 patients with schizophrenia and 158 healthy comparison subjects between 16 and 70 years of age were compared. Using linear regression analysis, the authors analyzed the relationship between the volumes of the total brain, gray and white matter, cerebellum, and lateral and third ventricles with patient age. RESULTS: Total brain (–2.2%), cerebral gray matter (–3.3%), prefrontal gray matter (–4.4%), and prefrontal white matter (–3.5%) volumes were smaller, and lateral (27%) and third (30%) ventricle and peripheral CSF (11%) volumes were larger in schizophrenia patients. A significant group-by-age interaction for gray matter volume was found, as shown by a steeper regression slope between age and gray matter volume in patients (–3.43 ml/year) than in healthy comparison subjects (–2.74 ml/year). CONCLUSIONS: The smaller brains of the patients with schizophrenia can be explained by decreases in gray matter volume. Moreover, the finding that the smaller gray matter volume was more pronounced in older patients with schizophrenia may suggest progressive loss of cerebral gray matter in schizophrenia patients.
Schizophrenia is a serious chronic psychiatric disorder affecting approximately 1% of the population around the world and is usually characterized by a progressive decline in functioning, although its course may vary considerably (1, 2). Progression in various aspects of the illness has been found, including an increase in the severity of negative symptoms (3), overall cognitive decline as measured with the Clinical Dementia Rating Scale (4), and a decrease in abstract thinking (5). Other cognitive functions in patients showed changes that were comparable with those found in healthy comparison subjects (5).
The etiology of schizophrenia is unknown, but numerous findings from imaging studies strongly support the view that schizophrenia is a brain disease particularly involving decrements in gray matter volume (6–9). It has not been resolved whether the structural brain changes are static or increase over the course of the illness. Progressive decrements in volumes of total brain matter (10), the frontal lobe (11), and frontal and superior temporal gray matter (12) have been reported in patients with recent-onset (10, 11) and chronic (12) schizophrenia, with follow-up intervals of 2–5 years. In patients with childhood-onset schizophrenia, a fourfold decrease in cortical gray matter volume and an increase in ventricular volume were reported in a 2-year longitudinal study (13, 14). Some studies have suggested an increase in ventricular volume in chronically ill patients over time (12, 15–18), although usually only in those with a poor outcome of the disease (15–17, but see reference 12) or in a subgroup of patients (18). In other studies no changes in ventricular volume were found (19–23). Thus, the available evidence, although limited, suggests that progressive changes in brain volume may occur in schizophrenia patients (24). However, it remains unresolved whether brain changes in patients with schizophrenia progress across the entire course of the illness. Although ultimately this question will have to be answered in a longitudinal study, changes measured cross-sectionally can provide suggestive evidence in this regard.
We compared brain volumes across a patient group with a 55-year age span by means of magnetic resonance brain images of 159 patients with schizophrenia and 158 healthy comparison subjects. Our aim was to determine whether age-related changes in brain volumes are excessive in schizophrenia patients.
Method
Subjects
A total of 159 patients (112 men, 47 women) with schizophrenia or schizophreniform disorder and 158 healthy comparison subjects (106 men, 52 women) from the Utrecht Schizophrenia Project participated after written informed consent was obtained. These subjects have been described (25). All subjects were between 16 and 70 years of age. Subjects with a major medical or neurological illness, including past head trauma, hypertension, cardiac disease, diabetes mellitus, cerebrovascular disease, epilepsy, migraine, endocrine disorders, drug or alcohol dependence, or an IQ below 80 were excluded. The patients were recruited from various outpatient and inpatient clinics; treatment setting was unrelated to age. The correlation of outcome (defined by the logarithmic transformed ratio of the cumulative months of hospitalization and the cumulative months of illness since the appearance of symptoms) with age was not significant (r=0.01, p=0.88).
The presence or absence of psychopathology was established for all subjects by using the Comprehensive Assessment of Symptoms and History (26) and the Schedule for Affective Disorders and Schizophrenia (27) and was assessed by two independent raters. Diagnostic consensus was achieved in the presence of a psychiatrist (e.g., R.S.K.). All of the patients met the DSM-IV criteria for schizophrenia or schizophreniform disorder; those with schizophreniform disorder met the criteria for a diagnosis of schizophrenia after 1 year of illness. All of the healthy comparison subjects met the Research Diagnostic Criteria (28) for “never mentally ill” and had no first-degree family member with a mental illness. “Age at onset of illness” was defined as the first time the patients had sought medical or psychological help for their psychotic symptoms. All of the patients had received antipsychotic medication in the past, and all but four of the patients had received antipsychotics at the time of the magnetic resonance imaging (MRI) scan. Patient medication included typical and atypical (clozapine, risperidone, olanzapine, and sertindole) antipsychotics. The comparison subjects were matched with the patients for the socioeconomic status of their parents, which was expressed as the highest level of education completed by one of the parents. (See Table 1 for demographic characteristics.) The MRI scans from all subjects were evaluated by two independent clinical neuroradiologists. No gross abnormalities were reported in any of the subjects.
Brain Imaging
MRIs were acquired on a Philips NT (Best, the Netherlands) scanner operating at 1.5 T for all subjects. A three-dimensional fast field echo (TE=4.6 msec, TR=30 msec, flip angle=30°, field of view=256×256 mm2) scan with 160–180 contiguous coronal 1.2-mm slices and a T2-weighted dual echo–turbo spin echo (TE1=14 msec, TE2=80 msec, TR=6350 msec, flip angle=90°, field of view=256×256 mm2) scan with 120 contiguous coronal 1.6-mm slices of the whole head were used for the quantitative measurements. In addition, a T2-weighted dual echo–turbo spin echo (TE1=9 msec, TE2=100 msec, flip angle=90°, field of view=250×250 mm2) scan with 17 axial 5-mm slices and 1.2-mm gap of the whole head was acquired for clinical neurodiagnostic evaluation. Processing was done on the neuroimaging computer network of the Department of Psychiatry at the University Medical Center Utrecht. All images were coded to ensure investigator blindness to subject identification and diagnosis; scans were put into Talairach frames without scaling and corrected for inhomogeneities in the magnetic field.
Quantitative assessments of intracranial, total brain, gray and white matter of the cerebrum (total brain excluding the cerebellum and stem), lateral and third ventricles, and peripheral CSF volumes were performed on the basis of histogram analyses and series of mathematical morphological operators to connect all voxels of interest; they were validated previously (29). A plane intersecting the fourth ventricle and the aqueduct delimited the cerebellum. In lateral ventricle segmentation, automatic decision rules bridged connections that were not detectable and prevented “leaking” into the cisterns (30). Coronal slices clearly showing the anterior and posterior commissures delimited the third ventricle; the upper boundary was a plane intersecting the plexus choroideus ventriculi tertii that was perpendicular to the midsagittal slice. All images were checked after measurement and corrected manually if necessary by using Analyze (31). Segmentation of the frontal lobes was performed automatically by using the ANIMAL anatomical segmentation algorithm (32), which has been validated previously for volume measurement of the frontal lobe (33). The interrater reliability of the volume measurements determined by the intraclass correlation coefficient in 10 brains was at least 0.95. (See Table 1 for mean brain volumes.)
Statistical Analysis
Data were examined for outliers, extreme values, and the normality of the distribution. There was no need for transformation, except for lateral and third ventricle volumes, peripheral CSF volume, and outcome. These variables were normalized by using logarithmic transformations. Multiple linear least-squares regressions of total brain, gray and white matter of the cerebrum, frontal lobe, cerebellum, and lateral and third ventricles, and peripheral CSF volumes were performed. Group (schizophrenia patients or comparison subjects) and sex (men or women) entered the analysis as predictor variables, and intracranial volume and age were entered as covariates. Results from regression equations were expressed as unstandardized (raw) regression coefficients (b). Results from regression equations based on the logarithmically transformed variables could no longer be expressed as mean differences between groups. Instead, they were expressed as the ratio between groups as described in terms of their multiplication factor and 95% confidence interval (CI). The multiplication factor was calculated by exponentiation of b and its lower and upper confidence limits, in which b was the regression coefficient of the logarithmically transformed dependent variable for the group.
To evaluate interactions with age, similar multiple linear least-squares regressions were performed by adding the interaction between age and group as a predictor variable. Results were expressed as a regression slope (b), indicating the changes in volume differences between the patients with schizophrenia and the healthy comparison subjects in the older subjects relative to the younger subjects. In case of a significant main or interaction effect for group, post hoc analyses were performed
1. For side (left or right) by adding handedness (right, left, or ambidexter) as a predictor variable.
2. By adding antipsychotic medication dose or outcome as a predictor variable to the regression analysis for the patients.
3. (For interaction effects with age only) by adding the standard deviation of the unstandardized residual of the dependent variable per age decade as a weight in weighted linear regression analysis to exclude the cohort-biasing effects of the cross-sectional design.
4. By adding time of the scan to the analysis to exclude artifacts due to time of measurement.
The SPSS 9.0 statistical package for Windows (SPSS, Inc., Chicago) was used for these analyses, with a two-tailed alpha level of 0.05.
Results
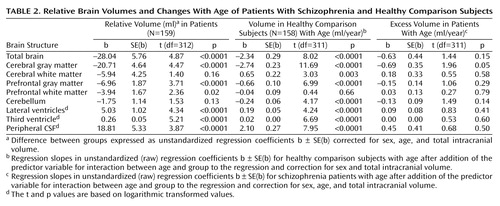
Table 1 presents the mean brain volumes for the patients with schizophrenia and the healthy comparison subjects. Table 2 and Figure 1 present the main results from the linear regression procedures.
Effects of Diagnosis
Total brain (regression coefficient [b]=–28.04 ml, SE=5.76; t=4.87, df=312, p<0.0001, representing –2.2% smaller total brain volume after correction for sex, age, and intracranial volume), cerebral gray matter (b=–20.71 ml, SD=4.64; t=4.47, df=312, p<0.0001; –3.3% smaller), prefrontal gray matter (b=–6.96 ml, SE=1.87; t=3.71, df=312, p<0.0001; –4.4% smaller), and prefrontal white matter (b=–3.94 ml, SE=1.67; t=2.36, df=312, p=0.02; –3.5% smaller) volumes were significantly smaller in the schizophrenia patients than in the comparison subjects. Lateral ventricle (multiplication factor exponent b=1.27, 95% CI=1.21–1.35; t=4.34, df=312, p<0.0001, representing 27% larger volume), third ventricle (exponent b=1.30, 95% CI=1.24–1.37; t=5.21, df=312, p<0.0001; 30% larger), and peripheral CSF (exponent b=1.11, 95% CI=1.08–1.14; t=3.87, df=312, p<0.0001; 11% larger) volumes were significantly larger in the schizophrenic patients than in the healthy comparison subjects. White matter and cerebellar volumes did not differ between the patients and the healthy comparison subjects. There were no effects of side on any of these findings. Medication at the time of the scan did not explain the volume changes in the patients.
Main Effects of Age
For the healthy comparison subjects, volumes of the total brain, cerebral and prefrontal gray matter, and cerebellum were significantly smaller in the older subjects. Volumes of the white matter, lateral and third ventricles, and peripheral CSF were significantly larger in the older subjects.
Effects of Diagnosis and Age
There was a significant group-by-age interaction for gray matter volume due to a steeper regression slope between age and gray matter volume in the patients with schizophrenia (regression slope [b]=–0.69 ml/year, SE=0.35; t=1.96, df=311, p=0.05, representing a difference in increase in volume of 0.69 ml/year and thus a total volume decrease of –3.43 ml/year) than in the healthy comparison subjects (b=–2.74 ml/year, SE=0.23; t=11.69, df=311, p<0.0001) (Figure 1). The few outliers in Figure 1 did not influence the results.
There were no effects for side on gray matter volume. No other significant interaction effects for schizophrenia and age were found. Outcome and antipsychotic medication at the time of the scan did not explain the regression of gray matter volume with age. The standard deviation of the unstandardized residual of gray matter volume per age decade and time of the scan did not influence the interaction effect.
Discussion
This cross-sectional study compared the brain morphology of 159 patients with schizophrenia and 158 healthy comparison subjects across a 55-year age range. The main finding was the more pronounced decrease in gray matter volume in the older patients with schizophrenia. Moreover, irrespective of age, volumes of the total brain and of the cerebral and prefrontal gray matter were smaller, while volumes of the lateral and third ventricles and peripheral CSF were larger in the schizophrenic patients than in the healthy comparison subjects. The effects could not be explained by outcome, antipsychotic medication at the time of the scan, variations of gray matter volume with age, or time-of-measurement effects, except for the greater lateral and third ventricle volumes in the schizophrenic patients, which were associated with poor outcome.
Our results are consistent with those of longitudinal studies that reported progressive decreases in frontal and superior temporal gray matter volumes in patients with chronic schizophrenia (12), as well as in adolescents with childhood-onset schizophrenia (13). Our findings also appear to be in agreement with the cognitive and functional decline reported in schizophrenia patients who were chronically ill (4). Moreover, the findings are consistent with an increase in the severity of negative symptoms (3) and a decrease in abstract thinking (5) in older, as compared to younger, patients with schizophrenia.
Irrespective of age, cerebral and prefrontal gray matter volumes were smaller in the patients with schizophrenia. This finding replicates those of earlier studies reporting smaller volumes of cerebral gray matter in neuroleptic-naive patients (34–36) and in patients with chronic schizophrenia (6, 37–41) that were unrelated to age at onset (42). The smaller volumes of the total brain and frontal lobe and the larger volumes of the lateral and third ventricles and peripheral CSF are consistent with the majority of the results from earlier reports. Indeed, the extent of the volume differences was quite compatible with the differences reported in a recent meta-analysis of volumetric MRI studies in patients with schizophrenia (7).
The effects of age on total brain, gray matter, cerebellar, and lateral and third ventricular volumes found in the healthy subjects in this study are consistent with previous findings (43–48). They imply that with advancing age, brain volumes decrease and CSF volumes increase. The increased white matter volume found in the older subjects in this study—although small at 0.65 ml/year—was somewhat unexpected because previously no changes (43, 44, 47) or decreases in volume with age (49) were reported in adults. However, recently, white matter volume increases up until the fourth decade (50) have been reported. Since we included subjects who were 16 years and older, our finding was probably due to the inclusion of younger subjects. Indeed, when two separate lines were fitted into the regression analysis (51), the regression slope for white matter volume and age was steeper in the younger (age ≤25 years) than in the older subjects, although neither reached statistical significance.
One may speculate as to the nature of the more pronounced decrease in gray matter volume in the older patients with schizophrenia. A degenerative process is possible; one could hypothesize that the reported decreases in neuropil (52, 53), synaptophysin immunoreactivity (54), and dendritic spine density (55), as well as cell loss (56, 57), in patients with schizophrenia are progressive during the course of the illness. Progressive synaptic degeneration has been reported in the left thalamus in schizophrenia patients in a postmortem study (58). Moreover, it could be argued that processes that are related to normal aging of the brain may go awry in schizophrenia patients. Other, neurochemical, abnormalities that have been proposed in schizophrenia patients may also be implicated (24). Obviously, it cannot be ignored that an unhealthy lifestyle in schizophrenia patients contributed to our findings; higher rates of smoking and nicotine addiction (59) as well as poor nutrition (60) have been reported.
This study was limited in several respects; those should be taken into consideration when interpreting its findings. First, its design was not longitudinal. Therefore, the age-related findings were potentially confounded by cohort or time-of-measurement effects (61). Although effects from cohort or time of measurement cannot be excluded completely, we analyzed some of the possible confounders to assess these effects as much as possible. Outcome and age at onset of the disease did not influence the gray matter changes with age. Changes in gray matter variance with age and time of the scan did not affect the results. All volume measures were corrected for intracranial volume, which remains stable with age. Second, medication may have influenced our findings in patients (62). Although antipsychotic medication taken at the time of the scan was not related to the excessive decrease in gray matter volume with age, the effects of cumulative years of medication treatment cannot be ruled out. However, no clear effects of antipsychotics on brain morphology have been found in earlier studies (24).
This study confirmed that the smaller brains of patients with schizophrenia could be explained by smaller gray matter volumes. Moreover, the more pronounced decreases in gray matter volume in the older patients with schizophrenia may suggest a progressive loss of cerebral gray matter with schizophrenia. However, the effects of cumulative antipsychotic medication and an unhealthy lifestyle due to higher rates of smoking and nicotine addition as well as poor nutrition in the schizophrenia patients must be considered as alternative explanations.
 |
 |
Presented in part at the 39th annual meeting of the American College of Neuropsychopharmacology, San Juan, Puerto Rico, Dec. 10–14, 2000, and at the International Congress on Schizophrenia Research, Whistler, B.C., Canada, April 28–May 2, 2001. Received Jan. 19, 2001; revision received Aug. 30, 2001; accepted Sept. 5, 2001. From Department of Psychiatry, University Medical Center Utrecht; and the Center for Biostatistics, Utrecht University, the Netherlands. Address reprint requests to Dr. Hulshoff Pol, Department of Psychiatry, A01.126, University Medical Center Utrecht, 3584 CX Utrecht, the Netherlands; [email protected] (e-mail). Supported by a grant (7F99.[2].37) from HersenStichting Nederland (Dutch Brain Foundation).
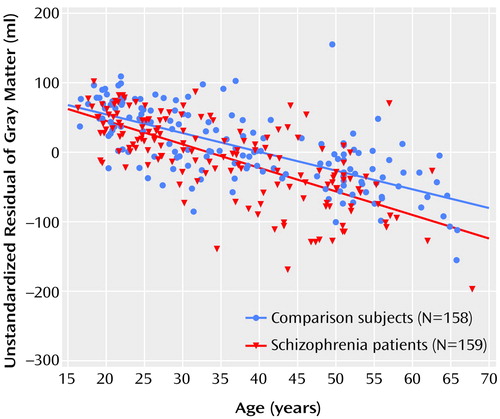
Figure 1. Relation of Cerebral Gray Matter Volume to Age for Schizophrenia Patients and Healthy Comparison Subjectsa
aUnstandardized residual reflects the difference between observed gray matter volume in milliliters and expected (grand average) volume on the basis of regression of the gray matter volume by sex and intracranial volume. The lines indicate the regression slopes of gray matter for age for the patients with schizophrenia (red) and for the healthy comparison subjects (blue) after correction for sex and total intracranial volume.
1. Schultz SK, Andreasen NC: Schizophrenia. Lancet 1999; 353:1425-1430Crossref, Medline, Google Scholar
2. Lieberman JA: Is schizophrenia a neurodegenerative disorder? a clinical and neurobiological perspective. Biol Psychiatry 1999; 46:729-739Crossref, Medline, Google Scholar
3. Davidson M, Harvey PD, Powchik P, Parrella M, White L, Knobler HY, Losonczy MF, Keefe RSE, Katz S, Frecska E: Severity of symptoms in chronically institutionalized geriatric schizophrenic patients. Am J Psychiatry 1995; 152:197-207Link, Google Scholar
4. Harvey PD, Silverman JM, Mohs RC, Parrella M, White L, Pwechik P, Davidson M, Davis KL: Cognitive decline in late-life schizophrenia: a longitudinal study of geriatric chronically hospitalized patients. Biol Psychiatry 1999; 45:32-40Crossref, Medline, Google Scholar
5. Fucetola R, Seidman LJ, Kremen WS, Faraone SV, Goldstein JM, Tsuang MT: Age and neuropsychologic function in schizophrenia: a decline in executive abilities beyond that observed in healthy volunteers. Biol Psychiatry 2000; 48:137-146Crossref, Medline, Google Scholar
6. Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A: Widespread cerebral gray matter volume deficits in schizophrenia. Arch Gen Psychiatry 1992; 49:195-205Crossref, Medline, Google Scholar
7. Wright IC, Rabe-Hesketh S, Woodruff PWR, David AS, Murray RM, Bullmore ET: Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry 2000; 157:16-25Link, Google Scholar
8. Lawrie SM, Abukmeil SS: Brain abnormality in schizophrenia: a systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry 1998; 172:110-120Crossref, Medline, Google Scholar
9. McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME: MRI anatomy of schizophrenia. Biol Psychiatry 1999; 45:1099-1119Crossref, Medline, Google Scholar
10. DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff A, Grimson R: Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res 1997; 74:129-140Crossref, Medline, Google Scholar
11. Gur RE, Cowell P, Turetsky BI, Gallacher F, Cannon T, Bilker W, Gur RC: A follow-up magnetic resonance imaging study of schizophrenia. Arch Gen Psychiatry 1998; 55:145-152Crossref, Medline, Google Scholar
12. Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A: Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry 2001; 58:148-157Crossref, Medline, Google Scholar
13. Rapoport JL, Giedd J, Kumra S, Jacobsen L, Smith A, Lee P, Nelson J, Hamburger S: Childhood-onset schizophrenia: progressive ventricular change during adolescence. Arch Gen Psychiatry 1997; 54:897-903Crossref, Medline, Google Scholar
14. Rapoport JL, Giedd JN, Blumenthal J, Hamburger S, Jeffries N, Fernandez T, Nicolson R, Bedwell J, Lenane M, Zijdenbos A, Paus T, Evans A: Progressive cortical change during adolescence in childhood-onset schizophrenia. Arch Gen Psychiatry 1999; 56:649-654Crossref, Medline, Google Scholar
15. Davis KL, Buchsbaum MS, Shihabuddin L, Spiegel-Cohen L, Metzger M, Frecska E, Keefe RS, Powchik P: Ventricular enlargement in poor-outcome schizophrenia. Biol Psychiatry 1998; 43:783-793Crossref, Medline, Google Scholar
16. Woods BT, Yurgelun-Todd D, Benes FM, Frankenburg FR, Pope HG Jr, McSpannen J: Progressive ventricular enlargement in schizophrenia: comparison to bipolar affective disorder and correlation with clinical course. Biol Psychiatry 1990; 27:341-352Crossref, Medline, Google Scholar
17. Kemali D, Maj M, Galderisi S, Milici N, Salvati A: Ventricle-to-brain ratio in schizophrenia: a controlled follow-up study. Biol Psychiatry 1989; 26:753-756Crossref, Medline, Google Scholar
18. Nair TR, Christensen JD, Kingsbury SJ, Kumar NG, Terry WM, Garver DL: Progression of cerebroventricular enlargement and the subtyping of schizophrenia. Psychiatry Res Neuroimaging 1997; 74:141-150Crossref, Medline, Google Scholar
19. Nasrallah HA, Olson SC, McCalley-Witters M, Chapman S, Jacoby CG: Cerebral ventricular enlargement in schizophrenia: a preliminary follow-up study. Arch Gen Psychiatry 1986; 43:157-159Crossref, Medline, Google Scholar
20. Illowsky B, Juliano DM, Bigelow L, Weinberger DR: Stability of CT scan findings in schizophrenia: results of an 8 year follow-up study. J Neurol Neurosurg Psychiatry 1988; 51:209-213Crossref, Medline, Google Scholar
21. Vita A, Sacchetti E, Valvasori G, Cazzulo CL: Brain morphology in schizophrenia: a 2- to 5-year CT scan follow-up study. Acta Psychiatr Scand 1988; 78:618-621Crossref, Medline, Google Scholar
22. Sponheim RS, Iacono WG, Beiser M: Stability of ventricular size after the onset of psychosis in schizophrenia. Psychiatry Res Neuroimaging 1991; 40:21-29Crossref, Medline, Google Scholar
23. DeLisi LE, Stritzke P, Riordan H, Holan V, Boccio A, Kushner M, McClelland J, Van Eyl O, Anand A: The timing of brain morphological changes in schizophrenia and their relationship to clinical outcome. Biol Psychiatry 1992; 31:214-254Medline, Google Scholar
24. DeLisi LE: Regional brain volume change over the life-time course of schizophrenia. J Psychiatr Res 1999; 33:535-541Crossref, Medline, Google Scholar
25. Hulshoff Pol HE, Schnack HG, Mandl RCW, van Haren NEM, Koning H, Collins DL, Evans AC, Kahn RS: Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry 2001; 58:1118-1125Crossref, Medline, Google Scholar
26. Andreasen NC, Flaum M, Arndt S: The Comprehensive Assessment of Symptoms and History (CASH): an instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry 1992; 49:615-623Crossref, Medline, Google Scholar
27. Endicott J, Spitzer RL: A diagnostic interview: the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry 1978; 35:837-844Crossref, Medline, Google Scholar
28. Pfohl B, Blum N, Zimmerman M: Structured Interview for DSM-IV Personality: SIDP-IV. Iowa City, University of Iowa, Department of Psychiatry, 1995Google Scholar
29. Schnack HG, Hulshoff Pol HE, Baaré WFC, Staal WG, Viergever MA, Kahn RS: Automated separation of gray and white matter from MR images of the human brain. Neuroimage 2001; 13:230-237Crossref, Medline, Google Scholar
30. Schnack HG, Hulshoff Pol HE, Baaré WFC, Viergever MA, Kahn RS: Automatic segmentation of the ventricular system from MR images of the human brain. Neuroimage 2001; 14:95-104Crossref, Medline, Google Scholar
31. Robb RA: Three-Dimensional Biomedical Imaging: Principles and Practice. New York, VCH, 1995Google Scholar
32. Collins DL, Holmes CJ, Peters TM, Evans AC: Automatic 3-D model-based neuroanatomical segmentation. Hum Brain Mapp 1996; 4:190-208Google Scholar
33. Mandl RCW, Hulshoff Pol HE, Collins DL, Ramsey NF, Baaré WFC, Staal WG, Kahn RS: Automatic volume measurement in schizophrenia: nonlinear or linear transformation? (abstract). Neuroimage 1999; 6:112Google Scholar
34. Zipursky RB, Lambe EK, Kapur S, Mikulis DJ: Cerebral gray matter deficits in first episode psychosis. Arch Gen Psychiatry 1998; 55:540-546Crossref, Medline, Google Scholar
35. Lim KO, Tew W, Kushner M, Chow K, Matsumoto B, DeLisi LE: Cortical gray matter volume deficit in patients with first-episode schizophrenia. Am J Psychiatry 1996; 153:1548-1553Link, Google Scholar
36. Gur RE, Turetsky BI, Bilker WB, Gur RC: Reduced gray matter volume in schizophrenia. Arch Gen Psychiatry 1999; 56:905-911Crossref, Medline, Google Scholar
37. Staal WG, Hulshoff Pol HE, Schnack HG, Hoogendoorn MLC, Jellema K, Kahn RS: Structural brain abnormalities in patients with schizophrenia and their healthy siblings. Am J Psychiatry 2000; 157:416-421Link, Google Scholar
38. Baaré WFC, Hulshoff Pol HE, Hijman R, Mali WP, Viergever MA, Kahn RS: Volumetric analysis of frontal lobe regions in schizophrenia: relation to cognitive function and symptomatology. Biol Psychiatry 1999; 45:1597-1605Crossref, Medline, Google Scholar
39. Cannon TD, vanErp TGM, Huttunen M, Lonnqvist J, Salonen O, Valanne L, Poutanen V, Standertskjold-Nordenstam C, Gur RE, Yan M: Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry 1998; 55:1084-1091Crossref, Medline, Google Scholar
40. Lim KO, Sullivan EV, Zipursky RB, Pfefferbaum A: Cortical gray matter volume deficits in schizophrenia: a replication. Schizophr Res 1996; 20:157-164Crossref, Medline, Google Scholar
41. Schlaepfer TE, Harris GJ, Tien AY, Peng LW, Lee S, Federman EB, Chase GA, Barta PE, Pearlson GD: Decreased regional cortical gray matter volume in schizophrenia. Am J Psychiatry 1994; 151:842-848Link, Google Scholar
42. Lim KO, Harris D, Beal M, Hoff AL, Minn K, Csernansky JG, Faustman WO, Marsh L, Sullivan EV, Pfefferbaum A: Gray matter deficits in young onset schizophrenia are independent of age of onset. Biol Psychiatry 1996; 40:4-13Crossref, Medline, Google Scholar
43. Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO: A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol 1994; 51:874-887Crossref, Medline, Google Scholar
44. Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR: Cerebral structure on MRI, part I: localization of age-related changes. Biol Psychiatry 1991; 29:55-67Crossref, Medline, Google Scholar
45. Murphy DGM, DeCarli C, Schapiro MB, Rapoport SI, Horwitz B: Age-related differences in volumes of subcortical nuclei, brain matter, and cerebrospinal fluid in healthy men as measured with magnetic resonance imaging. Arch Neurol 1992; 49:839-845Crossref, Medline, Google Scholar
46. Coffey CE, Wilkinson WE, Parashos IA, Soady SAR, Sullivan RJ, Patterson JL: Quantitative cerebral anatomy of the aging human brain: a cross-sectional study using magnetic resonance imaging. Neurology 1992; 42:527-536Crossref, Medline, Google Scholar
47. Lim KO, Zipursky RB, Watts MC, Pfefferbaum A: Decreased gray matter in normal aging: an in vivo magnetic resonance study. J Gerontol 1992; 47:B26-B30Google Scholar
48. Murphy DGM, DeCarli C, McIntosh AR, Daly E, Mentis MJ, Pietrini P: Szczepanik J, Schapiro MB, Grady CL, Horwitz B, Rapoport SI: Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry 1996; 53:585-594Crossref, Medline, Google Scholar
49. Guttmann CR, Jolesz FA, Kikinis R, Kikkiany RJ, Moss MB, Sandor T, Albert MS: White matter changes with normal aging. Neurology 1998; 50:972-978Crossref, Medline, Google Scholar
50. Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA: Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology 2000; 216:672-682Crossref, Medline, Google Scholar
51. Draper N, Smith H: Applied Regression Analysis, 3rd ed. New York, John Wiley & Sons, 1998Google Scholar
52. Selemon LD, Rajkowska G, Goldman-Rakic PS: Abnormally high neuronal density in the schizophrenic cortex: a morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry 1995; 52:805-818Crossref, Medline, Google Scholar
53. Selemon LD, Raikowska G, Goldman-Rakic PS: Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method. J Comp Neurol 1998; 392:402-412Crossref, Medline, Google Scholar
54. Glantz LA, Lewis DA: Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia: regional and diagnostic specificity. Arch Gen Psychiatry 1997; 54:943-952Crossref, Medline, Google Scholar
55. Glantz LA, Lewis DA: Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 2000; 57:65-73Crossref, Medline, Google Scholar
56. Benes FM, McSparren J, Bird ED, San Giovanni JP, Vincent SL: Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry 1991; 48:996-1001Crossref, Medline, Google Scholar
57. Pakkenberg B: Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psychiatry 1990; 47:1023-1028Crossref, Medline, Google Scholar
58. Blennow K: Synaptic degeneration in thalamus in schizophrenia. Lancet 1996; 348:692-693Crossref, Medline, Google Scholar
59. Kelly C, McCreadie RG: Smoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Am J Psychiatry 1999; 156:1751-1757Link, Google Scholar
60. Brown S, Birtwistle J, Roe L, Thompson C: The unhealthy lifestyle of people with schizophrenia. Psychol Med 1999; 29:697-701Crossref, Medline, Google Scholar
61. Kraemer HC, Yesavage JA, Taylor JL, Kupfer D: How can we learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry 2000; 157:163-171Link, Google Scholar
62. Madsen AL, Keidling N, Karle A, Esbjerg S, Hemmingsen R: Neuroleptics in progressive structural brain abnormalities in psychiatric illness (letter). Lancet 1998; 352:784-785Crossref, Medline, Google Scholar



