An MRI Study of Basal Ganglia Volumes in First-Episode Schizophrenia Patients Treated With Risperidone
Abstract
OBJECTIVE: The basal ganglia may contribute to extrapyramidal movement disorders, affective disturbances, and cognitive deficits in schizophrenia. Basal ganglia volumes are putatively affected by antipsychotic medications. The purpose of this study was to determine the long-term effects of risperidone treatment in a cohort of first-episode patients with schizophrenia. METHOD: The subjects were 30 patients with first-episode schizophrenia, 12 patients chronically treated with typical antipsychotics, and 23 healthy comparison subjects. They were scanned by magnetic resonance imaging at baseline. The first-episode patients received 1 year of continuous risperidone treatment, after which they and the comparison subjects were rescanned. Caudate, putamen, and globus pallidus volumes were determined from coronal images. RESULTS: The baseline caudate, putamen, and globus pallidus volumes were significantly larger in the chronically treated patients than in the untreated first-episode subjects and comparison subjects. These volumes did not differ between the first-episode patients and healthy comparison subjects. Basal ganglia volumes were unchanged after 1 year of exposure to risperidone in the first-episode subjects. Extrapyramidal movement disorders were present in the majority of chronically treated patients and more than one-third of the never-medicated first-episode patients at baseline. CONCLUSIONS: This group of first-episode patients did not exhibit abnormalities of basal ganglia volumes, nor were basal ganglia volumes affected by exposure to risperidone. Movement disorders were observed in both first-episode and chronically treated patients, suggesting effects of both illness and medications.
Diseases that affect the basal ganglia, such as Huntington’s disease and Parkinson’s disease, are associated with affective disturbances and psychosis (1, 2). Involuntary movements, affective disturbance, and catatonia in schizophrenia are phenotypically similar to symptoms found in basal ganglia disorders, suggesting the possibility of basal ganglia pathology in schizophrenia. Postmortem studies suggest both abnormally high and abnormally low basal ganglia volumes in schizophrenia (3, 4), and some (5, 6) but not all (7) magnetic resonance imaging (MRI) studies have shown low caudate volumes in antipsychotic-naive patients.
Several longitudinal MRI studies of basal ganglia volumes in schizophrenia during treatment with typical or atypical antipsychotics have been described. Chronic exposure to typical antipsychotic medications was associated with basal ganglia enlargement in patients who were previously drug naive (8, 9), and volume decreased when patients were switched to clozapine or other atypical antipsychotic (8, 10, 11).
The effects of treatment with atypical antipsychotics on basal ganglia volumes in a drug-naive population are unclear. We performed a longitudinal study of basal ganglia volumes in patients with first-episode schizophrenia, measuring volumes before and after treatment with risperidone. Comparison groups included patients treated chronically with typical antipsychotics and healthy subjects. The hypotheses were that 1) basal ganglia volumes in first-episode patients would be smaller than those in healthy subjects, 2) basal ganglia volumes in patients treated chronically with typical antipsychotics would be larger than the baseline values for the first-episode patients, and 3) there would be a decrease in volumes following risperidone treatment.
Method
Subjects
Demographic characteristics of the 30 first-episode, 12 chronic, and 23 healthy subjects appear in Table 1. The ratings of socioeconomic status were based on the Canadian National Occupational Classification system (12). The ratings of parental socioeconomic status reflected the higher-rated parent. Diagnoses were made according to the DSM-IV criteria, with information obtained from the Structured Clinical Interview for DSM-IV. All first-episode and chronically treated patients were diagnosed with schizophrenia, except for one first-episode subject diagnosed with schizoaffective disorder.
First-episode patients were included if lifetime exposure to antipsychotic medication did not exceed 8 weeks of treatment in the year of the baseline scan or 4 weeks of continuous treatment immediately before scanning. The first-episode patients’ total lifetime exposure to antipsychotics was less than 12 weeks (14). The chronically treated patients had received 12 or more weeks of continuous treatment with typical antipsychotic medication immediately before the baseline scan. The dose of antipsychotic medication was converted to chlorpromazine equivalents (13). The mean dose for the chronically treated patients was 276.1 mg/day of chlorpromazine units. The 23 healthy comparison subjects were recruited from hospital staff and the local community.
The exclusion criteria were a history of significant head injury or loss of consciousness exceeding 5 minutes, a history of facial or nasal trauma, a history of DSM-IV substance abuse or seizure disorder, and a family history of psychotic disorders. Written consent was obtained from all participants. The study was approved by the Dalhousie University Research Ethics Committee.
Treatment and Clinical Measures
Ratings of psychiatric and extrapyramidal signs and symptoms were completed by research clinicians. Afterward, 24 of the first-episode patients were treated with risperidone (dose range=1–6 mg/day, mean=2.7 mg/day). Risperidone was the first atypical antipsychotic available in Canada for use with first-episode patients, and Canadian practice guidelines recommended using atypical antipsychotics for this patient group. Risperidone was routinely offered as first-line treatment during the time of enrollment in this study (1995–1998). Of the six patients not treated with risperidone, four refused all treatment, one was treated with olanzapine, and one participated in a controlled trial. Of the 24 patients taking risperidone, 15 were treated continuously for a minimum of 6 months, and these patients were used to study the effects of risperidone on basal ganglia structures. At baseline, one first-episode subject and two of the chronic subjects were receiving concomitant medications (lorazepam, 3 mg/day; benztropine, 2.0 mg/day; and fluvoxamine, 300 mg/day, respectively). At follow-up, one first-episode patient was receiving concomitant medication (sertraline, 25 mg/day). Of the nine patients treated with risperidone initially but excluded from the follow-up study, four did not receive a second MRI (two moved, two refused scan) and five were not taking risperidone at the time of the second scan (three changed medication, two later refused medication).
Extrapyramidal signs and symptoms were rated at baseline, at the time of the follow-up scan, and whenever medication was changed. The ratings were obtained by using the Extrapyramidal Symptom Rating Scale (15). Interrater reliability, as reflected by intraclass correlation coefficients (ICCs), was high (ICC=0.84). Psychiatric symptoms were assessed by using the Positive and Negative Syndrome Scale (16). Ratings were made at baseline, with changes in medications, and at follow-up.
MRI Measurements
The subjects were scanned with a Siemens Magneton Vision 1.5-T MRI scanner. A coronal inversion recovery sequence was obtained for each subject. The inversion recovery sequence was obtained as follows: TR/TE=2000/20 msec, field of view=200 cm, and matrix size=168×256, pixel size=1.04×0.78 mm. A total of 18 slices, 4 mm thick with a 1-mm interslice gap, were available. Follow-up scans were acquired a mean of 1.06 years after baseline (SD=0.41). Repeat scans were not available for the chronically treated group. Digitized slices were measured by using a Macintosh G3/350 Mz computer. Additional T2-weighted axial images of the entire brain were acquired to facilitate assessment of total intracranial volume. The axial T2-weighted images were highly sensitive to focal pathology and provided good demarcation of the CSF-containing spaces. Inversion recovery sequences (coronal T1) were obtained for anatomical detail and superior differentiation of gray and white tissue. The white-to-gray pixel intensity ratio in these scans was 1.42, compared with a value of 0.89 obtained by using a spoiled gradient recall acquisition sequence obtained on a 1.5-T GE scanner of similar quality.
Basal ganglia measurements were made by a single rater (D.J.L.) blind to diagnosis, time of scan, and gender. Total intracranial volumes were measured by two raters (D.J.L., V.M.G.). The films were visually inspected for motion artifact, tilt, rotation, and slice matching at follow-up. One subject was excluded because of head tilt. Slice-to-slice matching of the baseline and follow-up scans was assessed by comparison of the placements of the anterior commissure slice and of the first frontal horn slice. This approach was used in a serial scan follow-up study of lesions in multiple sclerosis (17).
All regions of interest were manually selected with interactive shareware (NIH Image, version 1.61 ppc) (18). The basal ganglia measures began two slices anterior and ended two slices posterior to the anterior commissure slice in every subject (five slices in each). The slices were determined a priori on the basis of the clarity of edge detection and unequivocal presentation of basal ganglia regions according to Duvernoy’s MRI atlas (19). All basal ganglia measurements were the mean of four independent trials.
The caudate, putamen, and globus pallidus were manually selected on each slice. The most anterior slice showed the head of the caudate-putamen complex; however, these nuclei could not be separated at this level. The total bilateral caudate-putamen complex on this slice was therefore divided into two halves. The nucleus accumbens was included in the total caudate volume. Intrarater reliability was assessed in a randomly selected subset of 10 scans (caudate, ICC=0.99; putamen, ICC=0.97; globus pallidus, ICC=0.96; intracranial volume, ICC=0.99).
Data Analysis
Continuous demographic variables were analyzed by omnibus analysis of variance (ANOVA). A single ANOVA was performed with age entered as a dependent measure and group (first-episode, chronic, healthy) entered as a main effect. Differences in gender, socioeconomic status, and ethnicity were analyzed by using chi-square goodness-of-fit tests. Measures of total intracranial volume and baseline regional brain volumes were analyzed by an omnibus analysis of covariance (ANCOVA). For analysis of total intracranial volume, group and gender were entered as main effects, with age entered as a covariate. For regional brain volumes, total (left plus right) caudate, putamen, and globus pallidus volumes were entered as dependent measures, with group entered as a main effect and total intracranial volume entered as a covariate. Post hoc analyses of significant main effects were performed by using Bonferroni inequality/Dunn’s tests for comparisons of groups (with significance set to p<0.05)and paired t tests for comparisons of baseline and follow-up volumes. Scores on the Positive and Negative Syndrome Scale and the Extrapyramidal Symptom Rating Scale at week 12 of the medication trial were used as outcome measures. Medication doses and extrapyramidal symptom scores in the first-episode and chronic patients were compared by using unpaired Student t tests. The difference between baseline and follow-up extrapyramidal symptom scores for the risperidone-treated patients was assessed by a paired t test.
Results
Demographic characteristics are given in Table 1. Age was significantly different between groups (F=5.91, df=2, 62, p=0.005), as post hoc analysis showed that the chronically treated patients were significantly older than the first-episode patients. Gender, ethnicity, and parental socioeconomic status did not differ significantly between groups (gender: χ2=1.81, df=2, p=0.40; ethnicity: χ2=1.27, df=2, p=0.53; parental socioeconomic status: χ2=6.24, df=4, p=0.18). Both the first-episode and chronic patients had lower socioeconomic status than the healthy subjects (χ2=43.22, df=6, p<0.0001).
Basal Ganglia Volumes
Individual volumes of the regions of interest at baseline are shown in Figure 1, and follow-up values appear in Figure 2 for the subjects with both baseline and follow-up values. The men had larger intracranial volumes than the women (F=37.63, df=1, 61, p=0.0001), but no significant differences were found between groups (F=0.42, df=2, 61, p=0.66), and no effect of age was found (F=0.41, df=1, 61, p=0.52). All subsequent regional ANCOVAs included total intracranial volume as a covariate.
A significant group effect on volume was observed for the caudate (F=3.18, df=2, 61, p<0.05), putamen (F=3.41, df=2, 61, p=0.04), and globus pallidus (F=11.87, df=2, 61, p=0.0001). Post hoc analyses revealed significantly larger caudate, putamen, and globus pallidus in the chronically treated patients than in the first-episode group (Figure 1). Post hoc analysis also revealed that the globus pallidus volumes of the chronically treated patients were significantly larger than those of the healthy comparison subjects. The caudate, putamen, and globus pallidus baseline volumes in the first-episode patients and healthy subjects did not differ significantly, nor did the volumes in the never-medicated first-episode patients and those in the first-episode patients exposed to less than 12 weeks of medication before scanning (p>0.10 for all regions).
Only the first-episode patients and healthy comparison subjects were included in the risperidone follow-up analyses (Figure 2). No significant group changes in total caudate, putamen, or globus pallidus volumes were observed at follow-up in either the first-episode patients or the healthy comparison subjects (p>0.20 for all comparisons).
Clinical Measures and Exploratory Analyses
Baseline scores on the Extrapyramidal Symptom Rating Scale were available for 23 never-treated first-episode patients and 11 chronically treated patients (Table 1). Ten of the never-treated first-episode patients had preexisting movement disorders, and 10 of the chronically treated patients had movement disorders at baseline. The chronically treated patients had significantly higher scores on the Extrapyramidal Symptom Rating Scale than the never-medicated patients (t=3.38, df=32, p=0.002). ANOVA did not reveal differences in baseline basal ganglia volumes between the never-medicated patients with extrapyramidal symptoms (i.e., those who had scores higher than 0 on the Extrapyramidal Symptom Rating Scale) and those without such symptoms (i.e., had scores of 0) (in all cases, F<1.50, df=1, 21, p>0.10). Fourteen first-episode patients treated with risperidone were rated for extrapyramidal symptoms again after 1 year of treatment (mean dose=2.7 mg/day, SD=1.4). Their total scores after treatment were not different from the initial scores (t=0.20, df=12, p=0.84). After treatment, the extrapyramidal symptom scores were lower in four patients (as in the patients in our prior study [20]), slightly higher in two patients, and unchanged in the remaining eight patients. Exploratory correlational analyses of extrapyramidal symptoms and baseline basal ganglia volumes in the chronically treated patients did not reveal statistically significant relationships between basal ganglia volumes and total length of antipsychotic exposure, total lifetime dose (converted to chlorpromazine units), or the product of exposure length and dose. However, a positive correlation between globus pallidus volume and total length of antipsychotic exposure approached significance in the chronically treated subjects (r=0.56, df=11, p=0.06).
Before the start of any antipsychotic drug treatment, scores on the Positive and Negative Syndrome Scale were collected for 24 first-episode patients. These scores were used in the subsequent analyses of symptom scores. As shown in Table 1, the first-episode patients had higher baseline scores on the Positive and Negative Syndrome Scale than the chronically medicated patients (t=–4.76, df=33, p<0.0001). Scores were also collected for 14 first-episode patients after 12 weeks of continuous risperidone treatment. Their scores were significantly reduced after 12 weeks of risperidone treatment (t=8.39, df=13, p<0.001). The 12-week scores on the Positive and Negative Syndrome Scale for the risperidone-treated patients did not differ significantly from the scores for the patients chronically treated with typical antipsychotics (t=1.10, df=23, p>0.20).
Discussion
Three main findings emerged from this study. First, we replicated the observation of higher basal ganglia volumes in patients chronically treated with typical antipsychotic medication. Second, contrary to our hypothesis, we found no baseline differences in basal ganglia volumes between patients with first-episode schizophrenia and healthy comparison subjects. Third, we did not detect significant changes in basal ganglia volumes in first-episode patients after a mean of 1.06 years of exposure to risperidone.
In studies of nonhuman basal ganglia tissues (21, 22), haloperidol treatment increased the number of neurons immunoreactive for the Fos-like protein in the caudate nucleus and putamen. A study of rats (23) demonstrated basal ganglia enlargement after 1 to 6 months of haloperidol administration. Rat studies (24, 25) have also demonstrated increased neuronal soma volumes in the prefrontal cortex and striatum after exposure to haloperidol. These investigations suggest neuronal activation in the striatum after exposure to typical antipsychotics. In addition, functional brain studies of humans (26, 27) have demonstrated haloperidol-induced increases in deoxyglucose metabolism in basal ganglia regions. Both increased neuronal activation and increased neuronal metabolism may contribute to basal ganglia enlargement after exposure to typical antipsychotics.
The globus pallidus was approximately 25% larger in the chronically treated patients than in the healthy comparison subjects or the first-episode patients. In comparison, the caudate and putamen were larger by approximately 7% and 8%, respectively. Similar magnitudes of pallidal enlargement in patients treated with typical antipsychotics were seen in two previous studies (28, 29), and Chakos et al. (14) reported a significant correlation between basal ganglia volume and fluphenazine dose in first-episode schizophrenia. Basal ganglia enlargement may occur relatively rapidly and reach a plateau in the early phases of antipsychotic treatment, and even low-dose exposure to a typical agent may result in hypertrophy (9).
Our data did not demonstrate abnormalities of basal ganglia volumes in the early phases of illness. Corson et al. (5) reported low caudate volumes in never-medicated patients with schizophrenia, but Gur et al. (28) found no differences between drug-naive patients and healthy comparison subjects. Normal brain development involves volumetric changes during adolescence (30). Both age and gender in normal comparison subjects may require careful matching to determine true differences in subregional brain volumes.
The first-episode patients in this study did not exhibit basal ganglia enlargement after a mean of 1.06 years of continuous risperidone treatment. Studies comparing the effects of typical and atypical antipsychotics suggest that the basal ganglia enlargement induced by typical antipsychotics is reversible (8, 10, 11) and that differences in the pharmacology of these medications may be related to volume changes.
Risperidone’s dopamine D2 occupancy rates are dose dependent, and optimal doses (D2 occupancy of 65%–80%) range from 2 to 5 mg/day (31). The doses in this study (1–6 mg/day) were mostly within the optimal range. At doses above 6 mg/day, risperidone induces extrapyramidal symptoms without additional improvement in psychotic symptoms (31, 32). Similarly, optimal doses of haloperidol also have 65%–80% D2 basal ganglia occupancy rates (33), and at recommended clinical doses (i.e., at recommended D2 occupancy levels) haloperidol can increase basal ganglia volumes (8). These results suggest that the mechanism underlying basal ganglia enlargement cannot be attributed to D2 blockade alone.
Conversely, the evidence that any atypical antipsychotic causes a reduction in basal ganglia volumes below pretreatment levels remains questionable. Some groups have speculated that clozapine reduces basal ganglia volumes in animals (34) and humans (8, 10, 11). The clozapine trials with human subjects were confounded by switches from typical antipsychotics, and the animal study was based on D2 receptor autoradiography, which may not be optimal for accurate volume estimation. The current data do not support the hypothesis of basal ganglia reduction from a never-treated baseline, at least for risperidone.
We and others (20, 35–37) have documented preexisting movement disorders in some drug-naive patients. Preexisting movement disorders in schizophrenia may be masked by antipsychotic drug effects, particularly those of typical antipsychotic medications. In the present study, 10 of 11 of the chronically treated patients had extrapyramidal symptoms. Of the first-episode patients in this study, 10 of 23 had extrapyramidal symptoms at baseline. This is a higher proportion than we reported previously (20) and suggests a subtle underlying basal ganglia dysfunction in schizophrenia. Overall, the patients experienced improvement of extrapyramidal symptoms with risperidone.
The finding of no difference in basal ganglia volumes between the first-episode patients with and without extrapyramidal symptoms is in contrast to the higher basal ganglia volumes in the chronically treated patients with extrapyramidal symptoms. Enlargement of basal ganglia structures following treatment with typical antipsychotics may be closely related to the likelihood of movement disorders with traditional antipsychotic medications. The relationship of extrapyramidal symptoms and pretreatment basal ganglia volumes remains unclear.
There are several limitations to the present study. First, the results may have been influenced by the MRI acquisition protocol, slice thickness, the presence of interslice gaps, and the clarity of the white-to-gray tissue contrast. Typically, volumes acquired from two-dimensional and three-dimensional MRIs produce lower basal ganglia volumes than are found in postmortem studies (3, 4, 38). The volumes and error variances obtained in the present study are similar to values determined by imaging protocols with thinner coronal slices (5, 8, 14, 39). Interstudy comparisons do not indicate that slice thickness affects the accuracy or reliability of basal ganglia volume acquisition. Both accuracy and reliability of basal ganglia volumes are most affected by measurement methods. Our measurement protocols were clearly defined and highly reliable (ICC>0.90 for all regions). Second, subtle differences in basal ganglia morphology are difficult to identify in small study groups. The present study had a relatively small number of subjects because of the inherent difficulty in obtaining appropriate subjects and keeping subjects involved during follow-up. Third, if progressive changes in the basal ganglia are occurring over the early years of illness, we may not have detected differences between the healthy volunteers and patients with schizophrenia in the relatively short follow-up period of 1 year. However, as already noted, studies of nonhuman subjects suggest that changes related to treatment with typical antipsychotics occur relatively rapidly.
There are several implications of the present results. Typical antipsychotics are associated with extrapyramidal symptoms, which appear to be related to enlargement of the basal ganglia. While motor disturbances may also be present in never-medicated patients with schizophrenia, the structural correlates are less clear. Last, the effects of risperidone on basal ganglia structures appear to be different from those of typical antipsychotics. These observations suggest there may be lasting advantages to the use of risperidone (and possibly other atypical antipsychotics) in first-episode schizophrenia.
 |
Presented in part at the 10th Biennial Winter Workshop on Schizophrenia, Davos, Switzerland, Feb. 5–11, 2000. Received Aug. 1, 2000; revision received Nov. 3, 2000; accepted Nov. 15, 2000. From the Molecular Psychiatry & Therapeutics Laboratory, Department of Psychiatry, University of British Columbia; and Psychiatry Research, Department of Psychiatry, Dalhousie University, Queen Elizabeth-II Health Sciences Centre, Halifax, Nova Scotia, Canada. Address reprint requests to Dr. Honer, Molecular Psychiatry & Therapeutics Laboratory, Department of Psychiatry, University of British Columbia, Room 203, 828 West 10th Ave., Vancouver, British Columbia, Canada V5Z 1L8; [email protected] (e-mail). Supported by a Canadian Institutes of Health Research Scientist Award to Dr. Honer and by a grant from the Norma Calder Foundation for Schizophrenia Research to Ms. Lang. Partial funding for MRI scanning was provided by an investigator-initiated grant from Janssen-Ortho of Canada; additional funding for MRI scanning was provided by the Queen Elizabeth-II Hospital Health Science Research Foundation and the Department of Psychiatry, Dalhousie University. The authors thank Melissa M. Butler, R.T.N.M., C.C.R.C., Charlene A. Day, R.N., M.N., Carol P. Fredrickson, B.A., Janet L. Gallant, R.N., B.S., C.N., Heather M. Milliken, M.D, F.R.C.P.C., and David Whitehorn, Ph.D., M.Sc.N., for their help with this study.
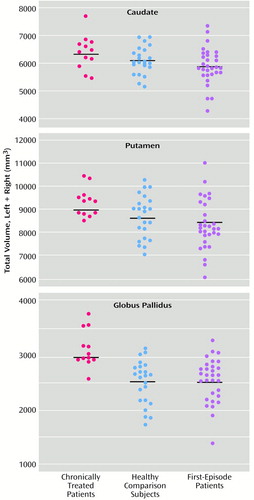
Figure 1. Total Baseline Caudate, Putamen, and Globus Pallidus Volumes in 12 Patients With Chronic Schizophrenia Previously Treated With Typical Antipsychotics, 23 Healthy Subjects, and 30 Patients With First-Episode Schizophreniaa
aHorizontal lines represent mean values.
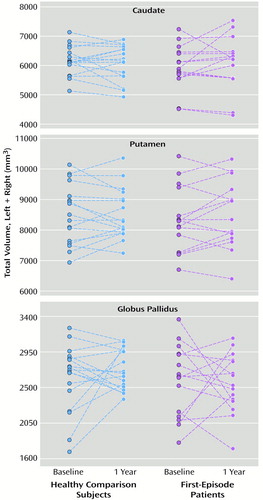
Figure 2. Total Caudate, Putamen, and Globus Pallidus Volumes at Baseline and 1-Year Follow-Up in 17 Healthy Subjects and 15 Patients With First-Episode Schizophrenia Treated With Risperidone
1. Aylward EH, Li Q, Stine OC, Ranen N, Sherr M, Barta PE, Bylsma FW, Pearlson GD, Ross CA: Longitudinal change in basal ganglia volume in patients with Huntington’s disease. Neurology 1997; 48:394–399Crossref, Medline, Google Scholar
2. Hardman CD, Halliday GM: The internal globus pallidus is affected in progressive supranuclear palsy and Parkinson’s disease. Exp Neurol 1999; 158:135–142Crossref, Medline, Google Scholar
3. Bogerts B, Meertz E, Schönfeldt-Bausch R: Basal ganglia and limbic system pathology in schizophrenia: a morphometric study of brain volume and shrinkage. Arch Gen Psychiatry 1985; 42:784–791Crossref, Medline, Google Scholar
4. Heckers S, Heinsen H, Heinsen Y, Beckmann H: Cortex, white matter, and basal ganglia in schizophrenia: a volumetric postmortem study. Biol Psychiatry 1991; 29:556–566Crossref, Medline, Google Scholar
5. Corson PW, Nopoulos P, Andreasen NC, Heckel D, Arndt S: Caudate size in first-episode neuroleptic-naive schizophrenic patients measured using an artificial neural network. Biol Psychiatry 1999; 46:712–720Crossref, Medline, Google Scholar
6. Keshavan MS, Rosenberg D, Sweeney JA, Pettegrew JW: Decreased caudate volume in neuroleptic-naive psychotic patients. Am J Psychiatry 1998; 155:774–778Abstract, Google Scholar
7. Shihabuddin L, Buchsbaum MS, Hazlett EA, Haznedar MM, Harvey PD, Newman A, Schnur DB, Spiegel-Cohen J, Wei T, Machac J, Knesaurek K, Vallabhajosula S, Biren MA, Ciaravolo TM, Luu-Hsia C: Dorsal striatal size, shape, and metabolic rate in never-medicated and previously medicated schizophrenics performing a verbal learning task. Arch Gen Psychiatry 1998; 55:235–243Crossref, Medline, Google Scholar
8. Chakos MH, Lieberman JA, Alvir J, Bilder R, Ashtari M: Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. Lancet 1995; 345:456–457Crossref, Medline, Google Scholar
9. Keshavan MS, Bagwell WW, Haas GL, Sweeney JA, Schooler NR, Pettegrew JW: Changes in caudate volume with neuroleptic treatment (letter). Lancet 1994; 344:1434Crossref, Medline, Google Scholar
10. Frazier JA, Giedd JN, Kaysen D, Albus K, Hamburger S, Alaghband-Rad J, Lenane MC, McKenna K, Breier A, Rapoport JL: Childhood-onset schizophrenia: brain MRI rescan after 2 years of clozapine maintenance treatment. Am J Psychiatry 1996; 153:564–566Link, Google Scholar
11. Corson PW, Nopoulos P, Miller DD, Arndt S, Andreasen NC: Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am J Psychiatry 1999; 156:1200–1204Google Scholar
12. Human Resources Development Canada: National Occupational Classification. Scarborough, Ont, Canada, ITP Nelson, 1993Google Scholar
13. Bezchlibnyk-Butler KZ, Jeffries JJ (eds): Clinical Handbook of Psychotropic Drugs, 9th revised ed. Toronto, Hogrefe & Huber, 1999, pp 71–73Google Scholar
14. Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M: Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry 1994; 151:1430–1436Google Scholar
15. Chouinard G, Ross-Chouinard A, Annable L, Jones BD: The Extrapyramidal Symptom Rating Scale (abstract). Can J Neurol Sci 1980; 7:233Google Scholar
16. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
17. Paty DW, McFarland H: Magnetic resonance techniques to monitor the long term evolution of multiple sclerosis pathology and to monitor definitive clinical trials. J Neurol Neurosurg Psychiatry 1998; 64(suppl 1):S47–S51Google Scholar
18. Rasband W: NIH Image. Bethesda, Md, National Institutes of Health, 1997Google Scholar
19. Duvernoy HM: The Human Brain: Surface, Three-Dimensional Sectional Anatomy and MRI. New York, Springer-Verlag, 1991, pp 88–116Google Scholar
20. Kopala LC, Good KP, Fredrikson DC, Whitehorn D, Lazier L, Honer WG: Risperidone in first-episode schizophrenia: improvement in symptoms and pre-existing extrapyramidal signs. Int J Psychiatry in Clin Practice 1998; 2:519–525Google Scholar
21. Deutch AY, Lewis DA, Whitehead RE, Elsworth JD, Iadarola MJ, Redmond DE Jr, Roth RH: Effects of D2 dopamine receptor antagonists on Fos protein expression in the striatal complex and entorhinal cortex of the nonhuman primate. Synapse 1996; 23:182–191Crossref, Medline, Google Scholar
22. MacGibbon GA, Lawlor PA, Bravo R, Dragunow M: Clozapine and haloperidol produce a differential pattern of immediate early gene expression in rat caudate-putamen, nucleus accumbens, lateral septum and islands of Calleja. Brain Res Mol Brain Res 1994; 23:21–32Crossref, Medline, Google Scholar
23. Chakos MH, Shirakawa O, Lieberman J, Lee H, Bilder R, Tamminga CA: Striatal enlargement in rats chronically treated with neuroleptic. Biol Psychiatry 1998; 44:675–684Crossref, Medline, Google Scholar
24. Benes FM, Paskevich PA, Davidson J, Domesick VB: The effects of haloperidol on synaptic patterns in the rat striatum. Brain Res 1985; 329:265–273Crossref, Medline, Google Scholar
25. Benes FM, Paskevich PA, Davidson J, Domesick VB: Synaptic rearrangements in medial prefrontal cortex of haloperidol-treated rats. Brain Res 1985; 348:15–20Crossref, Medline, Google Scholar
26. Holcomb HH, Cascella NG, Thaker GK, Medoff DR, Dannals RF, Tamminga CA: Functional sites of neuroleptic drug action in the human brain: PET/FDG studies with and without haloperidol. Am J Psychiatry 1996; 153:41–49Link, Google Scholar
27. Bartlett EJ, Brodie JD, Simkowitz P, Dewey SL, Rusinek H, Wolf AP, Fowler JS, Volkow ND, Smith G, Wolkin A: Effects of haloperidol challenge on regional cerebral glucose utilization in normal human subjects. Am J Psychiatry 1994; 151:681–686Link, Google Scholar
28. Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC: Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry 1998; 155:1711–1717Google Scholar
29. Hokama H, Shenton ME, Nestor PG, Kikinis R, Levitt JJ, Metcalf D, Wible CG, O’Donnell F, Jolesz FA, McCarley RW: Caudate, putamen, and globus pallidus volume in schizophrenia: a quantitative MRI study. Psychiatry Res 1995; 61:209–229Crossref, Medline, Google Scholar
30. Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL: Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21:1185–1201Google Scholar
31. Kapur S, Zipursky RB, Remington G: Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry 1999; 156:286–293Abstract, Google Scholar
32. Nyberg S, Eriksson B, Oxenstierna G, Halldin C, Farde L: Suggested minimal effective dose of risperidone based on PET-measured D2 and 5-HT2A receptor occupancy in schizophrenic patients. Am J Psychiatry 1999; 156:869–875Link, Google Scholar
33. Kapur S, Zipursky R, Jones C, Remington G, Houle S: Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry 2000; 157:514–520Link, Google Scholar
34. Lee H, Tarazi FI, Chakos M, Wu H, Redmond M, Alvir JM, Kinon BJ, Bilder R, Creese I, Lieberman JA: Effects of chronic treatment with typical and atypical antipsychotic drugs on the rat striatum. Life Sci 1999; 64:1595–1602Google Scholar
35. Caligiuri MP, Lohr JB, Jeste DV: Parkinsonism in neuroleptic-naive schizophrenic patients. Am J Psychiatry 1993; 150:1343–1348Google Scholar
36. Chatterjee A, Chakos M, Koreen A, Geisler S, Sheitman B, Woerner M, Kane JM, Alvir J, Lieberman JA: Prevalence and clinical correlates of extrapyramidal signs and spontaneous dyskinesia in never-medicated schizophrenic patients. Am J Psychiatry 1995; 152:1724–1729Google Scholar
37. Kopala LC, Good KP, Honer WG: Extrapyramidal signs and clinical symptoms in first-episode schizophrenia: response to low-dose risperidone. J Clin Psychopharmacol 1997; 17:308–313Crossref, Medline, Google Scholar
38. Bogerts B, Falkai P, Haupts M, Greve B, Ernst S, Tapernon-Franz U, Heinzmann U: Post-mortem volume measurements of limbic system and basal ganglia structures in chronic schizophrenics: initial results from a new brain collection. Schizophr Res 1990; 3:295–301Crossref, Medline, Google Scholar
39. Aylward EH, Li Q, Habbak QR, Warren A, Pulsifer MB, Barta PE, Jerram M, Pearlson G: Basal ganglia volume in adults with Down syndrome. Psychiatry Res 1997; 74:73–82Crossref, Medline, Google Scholar



