Nursing Home Placement, Day Care Use, and Cognitive Decline in Alzheimer’s Disease
Abstract
Objective: People with Alzheimer’s disease are often placed in a nursing home, sometimes after using adult day care services. How affected persons function during this potentially difficult transition is not well understood. The aim of this study was to examine the associations of day care use and nursing home placement with the rate of cognitive decline in Alzheimer’s disease. Method: The participants were 432 older persons with Alzheimer’s disease who were recruited from health care settings in the Chicago area. At baseline, they lived in the community and were using day care services a mean 1.7 days per week. At 6-month intervals for up to 4 years, they completed nine cognitive tests from which a composite measure of global cognition was derived. Results: On average, cognition declined at a gradually increasing rate during the study period. Nursing home placement was associated with a decrease in the level of cognition and an acceleration in the rate of cognitive decline. Day care use at baseline was not related to cognitive decline in initial analyses, but it interacted with nursing home placement such that higher level of day care use substantially reduced association of placement with accelerated cognitive decline. Education interacted with placement such that more schooling was associated with a greater increase in cognitive decline upon nursing home placement, but prior day care use also attenuated this association. Conclusions: Nursing home placement is associated with accelerated short-term cognitive decline in Alzheimer’s disease. Prior experience in adult day care may lessen this association.
Progressive decline in cognitive function is the principal clinical manifestation of Alzheimer’s disease. As the dementia progresses, affected persons require increasing levels of care. A variety of adult day care programs have been developed to reduce caregiver burden and to provide therapeutic and social activities for the affected person (1 , 2) . Nonetheless, most persons with dementia are eventually placed in a nursing home (3) . Although, the transition from the community to institutional residence is a life event of enormous significance to affected persons, little is known about the relation of such placement to the course of cognitive decline in the disease or whether day care use in any way modifies this correlation.
In the current study, we examined the associations of day care use and nursing home placement with trajectories of global cognitive decline in Alzheimer’s disease. The participants were a group of about 400 community dwelling persons with clinically diagnosed Alzheimer’s disease who had cognitive function testing at 6-month intervals for up to 4 years. We used mixed-effects regression models to characterize individual paths of change in cognitive function and their relation to level of day care use at baseline and subsequent institutionalization.
Method
Participants
The study subjects were recruited from the Rush Alzheimer’s Disease Center and from adult day care centers in the Chicago metropolitan area. Eligibility was established at baseline and required a clinical diagnosis of Alzheimer’s disease, age of 65 years or older, and residence in the community. After complete description of the study to the subjects, informed consent was obtained from patients and family members. The study was approved by the institutional review board of Rush University Medical Center.
Of 1,431 persons screened, 712 were eligible for the study, and 516 (72.5%) agreed to participate. We eliminated 30 individuals who did not have a valid global cognitive score at baseline, leaving 486 persons. There were 35 deaths before initial follow-up, and of the remaining 451 people, 432 (95.8%) had at least one valid composite cognitive score upon follow-up. Analyses are based on this group. The subjects completed a mean 4.7 evaluations per person (range=2–8), 94.2% of possible evaluations in survivors. They had a mean age at baseline of 80.3 years (SD=6.4), a mean of 11.7 years of education (SD=3.6), and a mean baseline score of 14.7 (SD=7.4) on the Mini-Mental State Examination; 68.3% were women; and 69.2% were white and non-Hispanic, 27.1% were African American, and 3.7% belonged to other racial or ethnic minorities.
Clinical Evaluation
At baseline, the participants had a structured, uniform evaluation that included informant reports of medical history, a neurological examination, and cognitive testing, as described elsewhere (4 – 6) . Based on this evaluation, individuals were classified with respect to Alzheimer’s disease and other conditions known to affect cognitive function. The diagnosis of Alzheimer’s disease was made by a board-certified neurologist following the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA), which require a history of cognitive decline and evidence of impairment in memory and cognition (7) . We included 416 people who met these criteria plus another 16 persons who met criteria for NINCDS/ADRDA possible Alzheimer’s disease to help ensure a wide spectrum of cognition at baseline. Cognitive function testing in the participant’s residence or day care site and informant reports of current health history were repeated every 6 months for up to 4 years with examiners blinded to previously collected data.
Assessment of Cognitive Function
Nine cognitive tests were administered as part of each evaluation. As shown in Table 1 , the tests assessed multiple domains of cognition. One test, the Mini-Mental State Examination (8) , assessed global cognition. There were two measures of episodic memory: immediate and delayed recall of 25 ideas in story A from the Wechsler Memory Scale—Revised (9) . Semantic memory was assessed with a 15-item version (10) of the Boston Naming Test (11) , which requires naming pictured objects; verbal fluency, which involves generating exemplars from two semantic categories (animals, fruits and vegetables) in separate 1-minute trials (10 , 12) ; and body part identification in which the participant points to body parts named by the examiner (13) . Working memory was assessed with the digit span backward test from the Wechsler Memory Scale—Revised (9) in which participants are read sequences of digits and asked to say them in reverse order. Visuospatial ability was assessed with a 12-item form of the Standard Progressive Matrices (14) , which involves selecting the missing element from an abstract visual design among a set of alternatives, and the eight-item Figural Recognition Test (15) , in which perception of abstract line drawings is assessed in a match-to-sample format.
To minimize floor and ceiling effects and other sources of measurement error, a composite measure of global cognition based on all nine tests was used in analyses. The composite measure was formed by converting the raw scores on each test to z scores, using the baseline mean and SD in the full cohort, and then averaging the z scores, as previously described (4 – 6) .
Day Care Use and Nursing Home Placement
At the baseline evaluation, the family member with the most contact with the participant was asked about use of adult day care services in the past 3 months and the average number of days per week of attendance. To ascertain nursing home placement, the informant was interviewed in person or by telephone at 3-month intervals for the duration of the study. The interviewer ascertained the date of nursing home admission for permanent care; a temporary stay for rehabilitation was not treated as nursing home placement, as previously described (16) .
Assessment of Other Covariates
We used the Rosow-Breslau Scale (17) to assess physical disability at each evaluation. The scale addresses three tasks requiring mobility and strength: walking up and down a flight of stairs, walking a half mile, and doing heavy housework such as washing windows, walls, or floors. A knowledgeable informant was asked if the participant could perform each task without help. The score was the number of tasks for which assistance was required, as previously described (18) .
Hallucinations were assessed at each evaluation with four structured questions posed to the informant about visual or auditory hallucinations inferred from something the participant said or did, as previously reported (5 , 19) . Hallucinations were treated as present or absent (at the time of nursing home entry) in analyses.
Informant responses to structured questions, adapted from items on the Diagnostic Interview Schedule (20) , were used to assess mood at each evaluation. Depressed mood required sadness persisting for at least 2 weeks accompanied by feelings of worthlessness, hopelessness, or excessive guilt. We focused exclusively on mood because other more physical components of depression (e.g., weight loss, fatigue, psychomotor agitation or retardation) can also be manifestations of Alzheimer’s disease.
Data Analysis
We used linear mixed-effects regression models (21) to characterize individual paths of change in cognitive function and to test the relation of covariates to level of cognition and rate of change. All models included terms for time in years since baseline and time squared to account for the accelerating nature of cognitive decline in Alzheimer’s disease and terms to control for the potentially confounding effects of age, sex, race, and education on initial level of function and rate of change. The initial model included a term for frequency of day care use at baseline and its interaction with time.
To model the effects of nursing home placement, we repeated the initial analyses but with a change point added for the time of entry into a nursing home. In this analysis, the effect of nursing home placement on cognitive decline is measured by two components. One component describes the acute effect on level of cognitive function at the change point: time of nursing home placement. It is modeled by an indicator variable that equals one for all follow-up observations after a participant was placed into nursing home and zero otherwise. The other component describes the effect of nursing home placement on change in cognitive function. It is modeled by terms for time since placement and time since placement squared for each follow-up observation after a participant was placed into a nursing home and zero otherwise. We subsequently added terms for the interactions of day care use with placement time and placement time squared.
We repeated this analysis controlling for level of disability and the presence of hallucinations and depressed mood at the time of nursing home placement. In separate subsequent analyses, we tested whether education, age, sex, or race modified the association of nursing home placement with cognitive decline. We also tested for three-way interactions of day care use by education by nursing home times. Programming was done in SAS (22) . Models were graphically and analytically validated.
Results
Day Care Use and Nursing Home Placement
At the time of the baseline evaluation, 196 participants (45.4%) were using day care services from 2 to 6 days a week for an overall mean of 1.7 days per week (SD=2.1) in the group as a whole. Use of day care was associated with older age (t=4.0, df=430, p<0.001), less education (t=4.8, df=377, p<0.001), and belonging to a racial or ethnic minority group (χ 2 =6.0, df=2, p=0.05) but not with sex (χ 2 =0.4, df=1, p=0.51).
During up to 4 years of follow-up (mean=2.2, SD=0.9), 155 persons (35.9%) were placed in a nursing home for a mean period of 0.54 years (SD=0.81, range=0.00–3.25). Nursing home placement was not associated with age (t=1.7, df=430, p=0.08), education (t=0.5, df=430, p=0.59), or sex (χ 2 =0.0, df=1, p=0.86). White non-Hispanic persons were slightly more likely to be institutionalized than African Americans and other racial or ethnic minorities (40.5% versus 28.2% and 6.3%; χ 2 =11.8, df=2, p=0.003).
Day Care Use, Nursing Home Placement, and Cognitive Decline
At baseline the composite measure of global cognition ranged from –1.662 to 2.361 (mean=0.063, SD=0.726), with higher scores indicating better function. We examined the relation of day care and nursing home use with individual patterns of change in cognitive function in a series of mixed-effects models adjusted for age, sex, race, and education.
The initial analysis ( Table 2 , model A) included terms for study time (in years since baseline) and study time squared to account for linear and nonlinear change in cognitive function and for day care use (in days per week at baseline) and its interaction with study time to test the association of day care use with initial level of cognition and rate of change. The effects for study time and study time squared indicate a gradually accelerating rate of global cognitive decline in the group as a whole. Higher level of day care use at baseline was associated with a lower level of cognitive function, as shown by the term for day care use but not with the rate of cognitive decline, as shown by the interaction term.
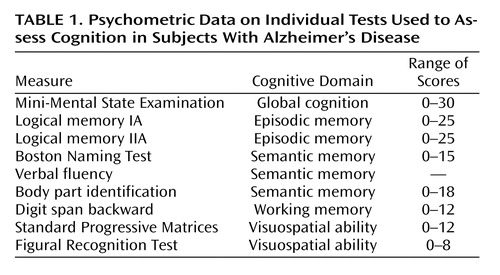
To examine the effects of nursing home placement, we repeated the analysis with a term to indicate whether or not the participant was in a nursing home at the time of testing and with terms for time since placement and time since placement squared ( Table 2 , model B). Placement in a nursing home was associated with a lower level of cognition and an accelerated rate of cognitive decline. Figure 1 shows the predicted 3-year paths of change for two typical participants: with nursing home placement, both level of cognitive impairment and rate of cognitive decline increased.
a The dashed segment at 2 years represents the 6-month time interval for data collection.
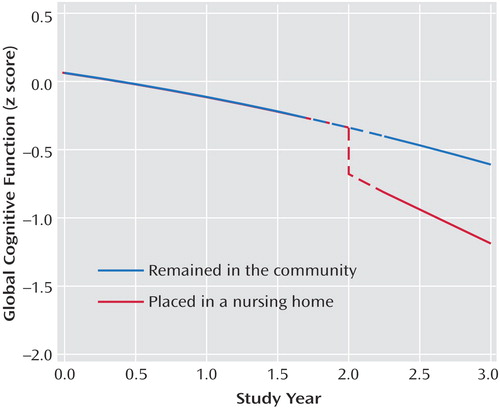
With nursing home placement accounted for in the model, higher level of day care use was associated with a slower rate of cognitive decline ( Table 2 , model B). To determine whether day care use modified the association of nursing home placement with cognition, we repeated the analysis with terms for the interaction of day care use with nursing home placement. Day care use interacted with both nursing home time (estimate=0.158, SE=0.037, p<0.001) and nursing home time squared (estimate= –0.046, SE=0.016, p=0.005). To illustrate these results, we plotted the 3-year paths of decline predicted by the model for two typical participants who each entered a nursing home after 2 years in the study: one with no day care use at study onset and the other with high use (3.5 days per week); prior day care use markedly attenuated the association of nursing home placement with accelerated cognitive decline ( Figure 2 ).
a The dashed segments at 2 years represent the 6-month time interval for data collection.
To determine whether the associations among day care use, nursing home placement, and cognitive decline depended on noncognitive features of Alzheimer’s disease, we repeated the analysis, controlling for three variables at the time of nursing home placement: depressed mood (present in 5.8%) and two characteristics previously associated with nursing home placement, cognitive decline, and death in this cohort (5 , 16) : hallucinations (present in 39.1%) and physical disability (45.4% with substantial mobility limitation). In this analysis, nursing home entry continued to be related to more rapid cognitive decline, and the interactions of day care with placement time (estimate=0.156, SE=0.037, p<0.001) and placement time squared (estimate=–0.046, SE=0.016, p=0.006) were unchanged.
We next assessed whether the association of nursing home placement with cognitive decline varied along demographic lines. Education had a robust interaction with both nursing home time (estimate=–0.088, SE=0.021, p<0.001) and nursing home time squared (estimate=0.032, SE=0.008, p<0.001). The association of nursing home placement with accelerated cognitive decline was stronger at higher levels of education compared to lower levels. In another analysis, there were three-way interactions of education and day care with nursing home time (estimate=0.046, SE=0.011, p<0.001) and nursing home time squared (estimate=–0.010, SE=0.005, p=0.04). As shown in Figure 3 , among those with no day care at baseline, education strongly modified the association of nursing home placement with cognitive decline (upper panel), whereas among those with high day care use (3.5 days per week), education had little modifying effect (lower panel). In subsequent analyses, the association of nursing home placement did not strongly vary by gender or race, but there was an interaction of age with nursing home time (estimate=0.026, SE=0.007, p<0.001), indicating less of a nursing-home-related acceleration in decline for older compared to younger patients.

a The upper panel shows paths for people with eight (red line) or 16 (blue line) years of education with no baseline day care, and the lower panel shows the predicted paths for eight (red line) or 16 (blue line) years of education and 3.5 days of care per week at baseline. The dashed segments at 2 years represent the 6-month time interval for data collection.
Discussion
We examined a group of about 400 persons with Alzheimer’s disease at 6-month intervals for up to 4 years. Placement in a nursing home during the study was associated with both a decrease in level of cognitive function and a more rapid rate of cognitive decline. Higher level of day care use at study onset attenuated the association of nursing home placement with accelerated cognitive decline in a dose-dependent fashion. The findings suggest that experience in adult day care may help persons with Alzheimer’s disease make the transition from the community to institutional residence.
Progressive cognitive decline is the principal clinical manifestation of Alzheimer’s disease, but rates of decline differ substantially among affected persons for reasons that are not well understood. As dementia progresses, many families turn to adult day care services and eventually to nursing home placement. Little is known about how either of these important life experiences is related to cognitive decline in Alzheimer’s disease. One study documented cognitive decline in nursing home residents during a 6-month period, consistent with the present results, but there was no cognitive testing prior to nursing home entry, no control group, and no clinical diagnoses of the participants (23) . Another study found less cognitive decline during a 9-month period in day care patients compared to untreated control subjects, partially in line with the current findings, but the sample included people without dementia and the association was seen for only one of four tests (24) . Each of these studies had fewer than 100 participants.
Higher level of education was associated with accelerated cognitive decline upon nursing home placement. Because education appears to diminish the deleterious effect of Alzheimer’s disease pathology on cognition (25) , a high level of education or related factors in a person with dementia suggests substantial accumulation of pathology and more rapid decline late in the disease course, which has been observed in this (4) and other (26) cohorts. Of interest, day care use markedly reduced the association of education with accelerated cognitive decline in the nursing home, further evidence of the robustness of the association between day care experience and cognition during the transition to institutional residence.
The bases of these findings are uncertain. One possibility is that nursing home placement is simply a sign of increased severity of Alzheimer’s disease. Yet, the nursing-home-related increase in cognitive decline was observed even after simultaneous control for cognitive and noncognitive (i.e., depressed mood, hallucinations, physical disability) indicators of dementia severity at the time of nursing home entry. Alternatively, the increased cognitive decline upon placement may reflect difficulty adapting to an unfamiliar environment, consistent with clinical reports of increased confusion and behavior problems in those with dementia during acute hospitalizations or trips away from home. Regardless of its basis, the association of nursing home placement with cognitive decline decreased as prior use of adult day care services increased. Adult day care programs are thought to help care providers become accustomed to the idea of nursing home placement (1 , 2) . The present results suggest that this experience in an institutional setting may affect the patient’s ability to adapt to full-time institutional living. Alternatively, Alzheimer’s disease patients who use day care services regularly may differ from those using the services sporadically or not at all in terms of tractability or some related characteristic that makes them better able to conform to the demands of institutionalized living. Further research will be needed to evaluate these and other possibilities.
This study has several strengths. The clinical classification of Alzheimer’s disease was based on a uniform clinical evaluation and well established criteria applied by a board-certified neurologist. Cognition was assessed with a previously established composite measure that could accommodate a wide range of performance. The psychometric properties of this measure and a mean of about five evenly spaced testings per participant enhanced our ability to characterize nonlinear patterns of change in cognitive function and their associations with critical life experiences.
These results have important limitations. Because this is an observational study, we cannot conclude that nursing home placement or day care use actually caused the observed changes in the rate of cognitive decline; research on these questions is needed. In addition, participants were selected from health care settings, so results may not generalize beyond such settings, and findings are based on a mean of less than 3 years of observation.
In summary, in this observational study of persons with Alzheimer’s disease, nursing home placement was associated with a decrease in level of cognitive function and an increased rate of cognitive decline. As level of day care use at study onset increased, the association of nursing home placement with accelerated cognitive decline decreased. The findings suggest that the transition from the community to a nursing home is particularly difficult for people with Alzheimer’s disease and that those planning for their care should consider the possibility that experience in adult day care programs may help prepare affected persons for institutional living.

1. Gaugler JE, Jarrott SE, Zarit SH, Stephens MA, Townsend A, Greene R: Respite for dementia caregivers: the effects of adult day service use on caregiving hours and care demands. Int Psychogeriatr 2003; 15:37–58Google Scholar
2. Gaugler JE, Zarit SH: The effectiveness of adult day care services for disabled older people. J Aging Soc Pol 2001; 12:23–47Google Scholar
3. Smith GE, Kokmen E, O’Brien PC: Risk factors for nursing home placement in a population-based dementia cohort. J Am Geriatr Soc 2000; 48:519–525Google Scholar
4. Wilson RS, Li Y, Aggarwal NT, Barnes LL, McCann JJ, Gilley DW, Evans DA: Education and the course of cognitive decline in Alzheimer disease. Neurology 2004; 63:1198–1202Google Scholar
5. Wilson RS, Tang Y, Aggarwal NT, Gilley DW, McCann JJ, Bienias JL, Evans DA: Hallucinations, cognitive decline, and death in Alzheimer’s disease. Neuroepidemiology 2006; 26:68–75Google Scholar
6. Wilson RS, Li Y, Aggarwal NT, McCann JJ, Gilley DW, Bienias JL, Barnes LL, Evans DA: Cognitive decline and survival in Alzheimer’s disease. Int J Geriatr Psychiatry 2006; 21:356–362Google Scholar
7. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS/ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939–944Google Scholar
8. Folstein M, Folstein S, McHugh P: “Mini-Mental State”: a practical method of grading the mental state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Google Scholar
9. Weschler D: Wechsler Memory Scale—Revised Manual. San Antonio, Tex, Psychological Corp, 1987Google Scholar
10. Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A: The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part V: a normative study of the neuropsychological battery. Neurology 1994; 44:609–614Google Scholar
11. Kaplan E, Goodglass H, Weintraub S: The Boston Naming Test. Philadelphia, Lea & Febiger, 1983Google Scholar
12. Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA: Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 2002; 17:179–193Google Scholar
13. Goodglass H, Kaplan E: The Assessment of Aphasia and Related Disorders, 2nd Ed. Philadelphia, Lea & Febiger, 1983Google Scholar
14. Raven JC, Court JH, Raven J: Manual for Raven’s Progressive Matrices and Vocabulary: Standard Progressive Matrices. Oxford, England, Oxford Psychologists Press, 1992Google Scholar
15. Wilson RS, Gilley DW, Bennett DA, Beckett LA, Evans DA: Person-specific paths of cognitive decline in Alzheimer’s disease and their relation to age. Psychol Aging 2000; 15:18–28Google Scholar
16. McCann JJ, Hebert LE, Li Y, Wolinsky FD, Gilley DW, Aggarwal NT, Miller JM, Evans DA: The effect of adult day care services on time to nursing home placement in older adults with Alzheimer’s disease. Gerontologist 2005; 45:754–763Google Scholar
17. Rosow I, Breslau N: A Guttman health scale for the aged. J Gerontol 1966; 21:556–559Google Scholar
18. Wilson RS, Buchman AS, Arnold SE, Shah RC, Tang Y, Bennett DA: Harm avoidance and disability in old age. Exp Aging Res 2006; 32:243–261Google Scholar
19. Wilson RS, Krueger KR, Kamenetsky JM, Tang Y, Gilley DW, Bennett DA, Evans DA: Hallucinations and mortality in Alzheimer’s disease. Neuroepidemiology 2005; 13:984–990Google Scholar
20. Robins LN, Helzer JE, Ratcliff KS, Seyfried W: Validity of the Diagnostic Interview Schedule, II: DSM-III diagnoses. Psychol Med 1982; 12:855–870Google Scholar
21. Laird N, Ware J: Random-effects models for longitudinal data. Biometrics 1982; 38:963–974Google Scholar
22. SAS Institute Inc: SAS/STAT User’s Guide, Version 8. Cary, NC, SAS Institute, 2000Google Scholar
23. Scocco P, Rapattoni M, Fantoni G: Nursing home institutionalization: a source of eustress or distress for the elderly. Int J Geriatr Psychiatry 2006; 21:281–287Google Scholar
24. Zank S, Schacke C: Evaluation of geriatric day care units: effects on patients and caregivers. J Gerontol Psychol Sci 2002; 57B:P348–P357Google Scholar
25. Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, Barnes LL, Bienias JL: Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 2003; 60:1909–1915Google Scholar
26. Wilson RS, Bennett DA, Gilley DW, Beckett LA, Barnes LL, Evans DA: Premorbid reading activity and patterns of cognitive decline in Alzheimer’s disease. Arch Neurol 2000; 57:1718–1723Google Scholar