Efficacy of Duloxetine on Cognition, Depression, and Pain in Elderly Patients With Major Depressive Disorder: An 8-Week, Double-Blind, Placebo-Controlled Trial
Abstract
Objective: This study compared the effects of duloxetine, 60 mg/day, versus placebo on cognition, depression, and pain in elderly patients with recurrent major depressive disorder. Method: Patients were randomly assigned (2:1) to duloxetine, 60 mg/day (N=207), or placebo (N=104) for 8 weeks in a double-blind study. The primary outcome measure was a prespecified composite cognitive score composed of four individual tests. Secondary measures included the Geriatric Depression Scale, the Hamilton Depression Rating Scale, the Visual Analogue Scale assessing pain, and standard safety and tolerability assessments. Results: Patients had a median age of 72 years (range=65–90). Duloxetine demonstrated significantly greater improvement in the composite cognitive score versus placebo (least-squares mean change from baseline to endpoint: 1.95 versus 0.76), driven by improved verbal learning and memory. Duloxetine treatment showed significantly greater baseline-to-endpoint reductions in both Hamilton depression scale (–6.49 versus –3.72) and Geriatric Depression Scale (–4.07 versus –1.34) total scores compared with placebo. Hamilton depression scale response (37.3% versus 18.6%) and remission (27.4% versus 14.7%) rates at endpoint were significantly higher for duloxetine than for placebo. Duloxetine significantly improved Visual Analogue Scale scores for back pain and time in pain while awake versus placebo. Significantly fewer patients receiving duloxetine withdrew from the study because of lack of efficacy (2.9% versus 9.6%); the incidences of discontinuation due to adverse events were similar for duloxetine and placebo (9.7% versus 8.7%). Conclusions: Duloxetine improved cognition, depression, and some pain measures and was safe and well tolerated in elderly patients with recurrent major depressive disorder.
Major depressive disorder is a common disorder in the elderly and is often associated with physical disability and cognitive deficits (1 , 2) . Studies show that cognitive function may return to normal if depression is effectively treated (3 , 4) . Cognitive impairment is an important clinical issue among elderly patients with depression and has a more complex etiology because of the variable rate of mild cerebrovascular and/or Alzheimer’s-type neurodegenerative changes associated with depression in the elderly (5) . Longitudinal studies of community-dwelling elderly have found that cognitive impairment sometimes precedes the onset of depression (6) , and follow-up studies (7) have indicated that some elderly patients with depression may have neurodegenerative changes that are precursors to dementia.
Cognitive functions, including learning, memory, and focused attention, are particularly susceptible to disruption in patients with depression. Patients with depression perform approximately one half of an SD worse than healthy comparison subjects on verbal learning and memory tests (3) . Other studies of patients with depression have found deficits most consistently in cognitive tasks involving focused attention, working memory, and decision making (3 , 4) . Cognitive impairment should be considered as important as other emotional and physical symptoms of major depressive disorder (8) . Ideally, an antidepressant for elderly patients with major depressive disorder should improve cognitive functions while improving mood and physical symptoms.
Duloxetine hydrochloride is a recently approved antidepressant medication that inhibits both serotonin (5-HT) and norepinephrine reuptake (9) . The dual action of duloxetine makes it particularly interesting in the treatment of depression with cognitive impairment because imbalance or deficiency in 5-HT and/or norepinephrine systems has been found to contribute to cognitive deficits (10 , 11) .
Because the effect of duloxetine on cognitive performance in elderly patients with depression has not been previously evaluated, the primary objective of the present study was to compare the efficacy of duloxetine, 60 mg/day, with placebo on cognition as assessed by a prespecified composite measure in a double-blind, placebo-controlled trial involving elderly patients with recurrent major depressive disorder. The conventional measures of mood were specified as secondary endpoints in the protocol.
Depression and pain are common comorbidities (12) . Pain as a physical symptom of depression affects approximately 65% of patients, leading to less favorable outcomes and greater health care utilization. Moreover, depression is a common feature in patients with chronic pain and can affect pain threshold and tolerance. Several classes of antidepressants have been used successfully in the treatment of somatic symptoms of depression and for a variety of pain syndromes (12) . A direct relationship between pain severity and depression has been identified in older patients with chronic pain (13) . Therefore, it is important to assess pain measures in elderly patients with depression.
Method
Study Design
This was a multicenter, double-blind, placebo-controlled study of 311 elderly outpatients with recurrent major depressive disorder conducted in the United States. After a 1-week screening phase and a 1-week placebo phase, patients were randomly (2:1) assigned to once-daily duloxetine, 60 mg/day (N=207), or placebo (N=104) for 8 weeks and then entered a 1-week, double-blind discontinuation phase in which the dosage of duloxetine was reduced to 30 mg/day for 4 days, followed by replacement with placebo ( Figure 1 ). If after visit 2 the patient could not tolerate the assigned drug, the dose could be reduced to 30 mg/day at the discretion of the investigator, but the full dose had to be reached by visit 5 (week 2 of treatment) or the patient was dropped from the study.

Patients
All patients in this study were age ≥65 years with recurrent major depressive disorder. All patients met diagnostic criteria for major depressive disorder as defined in the DSM-IV. The diagnosis was confirmed by the Mini International Neuropsychiatric Interview (14) , a standardized diagnostic interview based on DSM-IV criteria. Baseline disease severity was defined by patients’ scores on the Hamilton Depression Rating Scale with 17 items (HAM-D) (15) . Patients were required to have a HAM-D total score ≥18 at visits 1 and 2; a Mini-Mental State Examination (MMSE) (16) score ≥20 with or without mild dementia; and at least one previous episode of major depression. Patients with an MMSE score of 20 to 23 were categorized as having mild dementia, while those with a score of ≥24 were categorized as having no dementia.
Patients were excluded for the following reasons: current primary axis I diagnosis other than major depressive disorder or mild dementia (including dysthymia or psychotic depression); previous diagnosis of psychotic disorder; organic mental disorder, moderate to severe dementia, or mental retardation; serious or unstable medical illness, psychological condition, or clinically significant laboratory abnormality that, in the opinion of the investigator, would compromise participation in this study or be likely to lead to hospitalization during the course of the study; or an alanine transaminase, aspartate transaminase, or gamma glutamyl transferase level >1.5 times the upper limit of normal, based on Eli Lilly and Company’s reference ranges (17) . Each patient provided written informed consent before any study procedures or administration of study drug, in accordance with the Declaration of Helsinki.
Efficacy Measures
The primary outcome measure was a protocol-specified composite cognitive score based on four cognitive tests: 1) the Verbal Learning and Recall Test , 2) the Symbol Digit Substitution Test, 3) the Two-Digit Cancellation Test, and 4) the Letter-Number Sequencing Test. These tests were selected because they assess aspects of cognition shown previously to be most impaired in patients with depression, specifically verbal learning and memory, attention, executive function, and working memory (3 , 4) . Additionally, each of these tests has been used extensively in clinical psychopharmacology and can be completed in a short period of time. Secondary measures included the Geriatric Depression Scale, the HAM-D, the Visual Analogue Scale for pain, and the Clinical Global Impression (CGI) severity scale.
For the Verbal Learning and Recall Test, which was adapted from the Rey Auditory Verbal Learning Test (18) , the patient was given three trials to learn a list of 15 common nouns presented on cards one at a time. The learning score was the average number of words recalled for the three trials. The delayed recall score was the number of words correctly recalled after the other cognitive tests had been administered. The Symbol Digit Substitution Test is an attention-demanding component of the Wechsler Adult Intelligence Scale, 3rd ed. (WAIS-III) (19) . The Symbol Digit Substitution Test score was the number of digits coded correctly in a 90-second test period.
The Two-Digit Cancellation Test (20) was designed to assess drug effects on visual attention and executive function in persons with mild cognitive impairment. The patients had 45 seconds to search for two target digits among rows of numbers on a page. The Two-Digit Cancellation Test score is the number of targets hit minus the number incorrectly marked (errors) minus the number of times the patient had to be reminded of the task.
The Letter-Number Sequencing Test is another component of the WAIS-III (19) and assesses working memory and executive functioning. The test administrator read aloud a group of numbers and letters (e.g., 9-C-3) then asked the patient to repeat them after rearranging them with the numbers first, in order from smallest to largest, followed by the letters in alphabetical order. The sequence length (2–8) was increased until the patient failed three trials of a given length. The score was the sum of the item scores (0–21).
The composite cognitive score, ranging from 0 to 51, was constructed so that the contribution of each test was roughly proportional to the time spent in administering the test. Tests stressing learning and memory were weighted only slightly more than the combination of those stressing attention or executive function. The composite cognitive score was defined as the sum of the following:
The average number of words recalled on the three learning trials of the Verbal Learning and Recall Test (score 0–15) and the number of words recalled on the delayed recall test of the Verbal Learning and Recall Test (score 0–15)
The fraction of all possible targets correct on the Symbol Digit Substitution Test (number correct divided by 133) multiplied by 7 (score 0–7)
The number of targets hit minus the number incorrect minus the number of times the patient had to be reminded of the task divided by the possible number correct (40) on the Two-Digit Cancellation Test multiplied by 7 (score 0–7, set to 0 if negative)
The total score on the Letter-Number Sequencing Test (0–21) divided by 3 (score 0–7)
The Geriatric Depression Scale (21) was developed as a basic screening measure for depression in older adults. The Visual Analogue Scale (22) for pain consists of six questions regarding the experience of overall pain, headache, back pain, shoulder pain, pain interference with daily activities, and time in pain while awake. The Visual Analogue Scale is used widely in the assessment of pain.
Cognitive measures and the MMSE were recorded once before random assignment and at the last visit of the acute treatment phase. The Geriatric Depression Scale, the HAM-D, the CGI severity scale, and the Visual Analogue Scale were recorded once before random assignment and at every visit of the acute treatment phase.
Response and remission rates were also secondary efficacy outcome measures, with response defined as a ≥50% decrease in the HAM-D total score from baseline to endpoint and remission as a HAM-D total score ≤7 at endpoint.
Safety and Tolerability Measures
Safety and tolerability measures recorded at every visit included spontaneously reported adverse events, concomitant medications, weight, and supine and standing blood pressure and heart rate. An ECG, a urine sample, and blood for chemistry and hematology tests were collected at screening and at the last visit of the acute treatment phase.
Sustained elevation in systolic (diastolic) blood pressure was defined as supine systolic (diastolic) blood pressure ≥140 (90) mm Hg and an increase from baseline ≥10 mm Hg for three or more consecutive visits in the acute treatment phase. Sustained elevation in blood pressure was defined as sustained elevation in either systolic or diastolic blood pressure.
Treatment-emergent orthostatic hypotension was defined as 1) having the standing diastolic blood pressure at least 10 mm Hg less than the supine diastolic blood pressure or the standing systolic blood pressure at least 20 mm Hg less than the supine systolic blood pressure at any time in the acute treatment phase and 2) not meeting these criteria at any visit in the baseline interval. Values for abnormal laboratory tests were based on Covance (Covance Central Laboratory Services, Indianapolis) reference limits.
Statistical Analysis
All analyses were conducted on an intent-to-treat basis. For treatment comparisons, the term “significant” indicates statistical significance (two-sided, p≤0.05). No adjustments for multiple comparisons were made. Unless otherwise specified, “baseline” refers to the last nonmissing observation before random assignment. “Endpoint” refers to the last nonmissing observation during the acute treatment phase. The baseline used for determination of elevated blood pressure was the maximum observation before random assignment.
Changes in continuous efficacy variables from baseline to endpoint were analyzed with a fixed-effects analysis of covariance (ANCOVA) model that included terms for treatment, investigator, and baseline score. Continuous baseline variables; changes from baseline to endpoint in vital signs, weight, and ECG intervals; and changes in laboratory results were evaluated by using a fixed-effects analysis of variance (ANOVA) model that included terms for treatment and investigator. Laboratory variables were rank-transformed because they are often skewed to the right and not normally distributed. These analyses are referred to as mean change analyses. Some efficacy variables measured over time were analyzed by using a mixed-effects model, likelihood-based, repeated-measures approach. The model included the fixed categorical effects of treatment, investigator, visit, and treatment-by-visit interaction, as well as the continuous fixed covariates of baseline score and baseline score-by-visit interaction. The unstructured covariance structure was used to model the within-patient errors. Categorical measures, such as rates of response and remission, adverse events, and discontinuations, were analyzed with Fisher’s exact test. Throughout this article, “mean” refers to the raw mean unless the least-squares mean is specified.
Subgroup analyses of the composite cognitive score and the Geriatric Depression Scale and HAM-D total scores were performed by adding the relevant subgroup and treatment-by-subgroup interaction terms to the ANCOVA model. Interaction effects were tested at a two-sided, 0.10 significance level. The subgroups were defined a priori in the protocol, namely: investigator, age (<75, ≥75), gender, origin (Caucasian, other), comorbidity (yes, no), mild dementia at baseline (yes, no), baseline HAM-D score (<24, ≥24), HAM-D anxiety (yes, no), HAM-D insomnia (yes, no), number of previous episodes of depression (<median, ≥median), Geriatric Depression Scale total score, CGI severity scale score, and number of previous drugs received for depression (0, ≥1). Treatment-emergent adverse events were reported using preferred terms for version 7.0 of the Medical Dictionary for Regulatory Activities.
Path analysis (23) was performed post hoc to test the direct treatment effect on the change in the composite cognitive score. In the analysis, prespecified pathways described the causal relationships for the testing: the treatment has effect on change in the composite cognitive score (direct effect), and the treatment effect on depressive symptoms (as measured by the Geriatric Depression Scale and HAM-D total scores) has effect on change in the composite cognitive score (indirect). Indirect effects through the two depression measures were evaluated separately. The significance of the direct treatment effect was tested by Student’s t test in the regression model, in which change in the composite cognitive score was the dependent variable and treatment, baseline composite cognitive score, baseline Geriatric Depression Scale (or HAM-D) total score, and change in Geriatric Depression Scale (or HAM-D) total score were regressors. In addition, with another regression model for change in Geriatric Depression Scale (or HAM-D) total score, the indirect treatment effect by means of change in Geriatric Depression Scale (or HAM-D) total score was a result of multiplying the treatment coefficient by the coefficient of the corresponding change in the first described regression model. The percentages of direct and indirect effects on the total treatment effect were computed.
Two hundred duloxetine- and 100 placebo-treated patients provide 80% power to detect an effect size (difference between mean changes in the composite cognitive score divided by the common SD) of 0.35 by using a 5% two-sided significance level and assuming that 95% of patients have data for the analysis. All analyses were performed using SAS software (SAS Institute, Cary, N.C.).
Results
Patient Characteristics
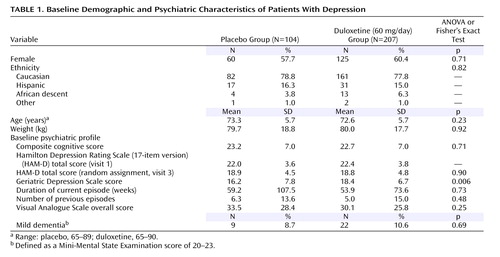
Patient characteristics at baseline are summarized in Table 1 . The duloxetine and placebo groups did not significantly differ in any variable except for the Geriatric Depression Scale total score, which was higher for the duloxetine group than for the placebo group (p<0.01).
Efficacy in Cognitive Measures
Figure 2 presents the mean change from baseline to endpoint in the composite cognitive score for all randomly assigned patients and by subgroups based on baseline HAM-D scores (<24 versus ≥24). Duloxetine significantly improved cognitive performance compared with placebo in all randomly assigned patients (p<0.02). The advantage of duloxetine over placebo was slightly greater in patients with more severe depression compared with patients with less depression, but the treatment-by-baseline HAM-D interaction was not significant (p=0.82). On average, the composite cognitive score worsened in patients with more severe depression taking placebo. For the composite score, there was no significant interaction of treatment with investigator; gender; age; origin; number of previous drugs for depression; number of previous episodes of depression; Geriatric Depression Scale total score; CGI severity scale score; HAM-D anxiety; HAM-D insomnia; comorbid arthritis, diabetes, vascular disease, or any of these; or mild dementia.

a Interaction (left): p=0.82; mean baseline scores (right): learning trials, ∼7; delayed recall, ∼7; symbol digit substitution, ∼36; two-digit cancellation, ∼22; letter-number sequencing, ∼9.
b p<0.02 versus placebo.
c p=0.13 versus placebo.
d p=0.03 versus placebo.
e p=0.02 versus placebo.
As Figure 2 also indicates, the mean baseline scores for the individual tests were all near the middle of the range of possible scores, suggesting that all tests had sufficient dynamic range to measure both improvement and deterioration due to drug treatment. Compared with placebo, patients taking duloxetine had significant improvement in both Verbal Learning and Recall Tests (learning trials, p=0.003; delayed recall, p=0.02), but not other cognitive tests ( Figure 2 ). Composite cognitive score improvement in patients treated with duloxetine was driven mainly by verbal learning and memory. Least-squares mean changes from baseline for MMSE scores were similar for duloxetine- and placebo-treated patients (0.29 versus 0.24, p=0.87).
Path analysis showed that for improvement of the composite cognitive score, there was a 90.9% direct effect (p=0.03) and a 9.1% indirect effect through improvement in the Geriatric Depression Scale total score. There was an 81.3% direct effect (p=0.07) and an 18.7% indirect effect through improvement in the HAM-D total score.
Efficacy in Depression Measures
Results from the repeated measures and mean change analyses of the depression efficacy measures are shown in Figure 3 . Patients treated with duloxetine had significantly greater improvement in Geriatric Depression Scale total score than did placebo-treated patients beginning 1 week after random assignment and continuing through the end of treatment (p≤0.01 at weeks 1 and 2 and p≤0.001 at weeks 4 and 8) and at endpoint (p≤0.001) ( Figure 3 , top). Duloxetine also showed significantly greater improvement in HAM-D total scores and CGI severity scale scores starting at week 4 and week 2, respectively, through the end of treatment and at endpoint ( Figure 3 , middle and bottom).

a Mean baseline Geriatric Depression Scale score: ∼18 (top); mean baseline Hamilton Depression Rating Scale score: ∼19 (middle).
b p≤0.01 versus placebo.
c p<0.001 versus placebo.
When changes from baseline to endpoint in Geriatric Depression Scale and HAM-D total scores were analyzed in subgroups by baseline HAM-D scores, there was a significant treatment-by-baseline HAM-D interaction (p=0.09 and p=0.03, respectively), with a greater advantage of duloxetine over placebo in the patients with more severe depression compared with those with less severe depression ( Figure 4 ).

a Interaction (left): p<0.09; interaction (right): p<0.03.
b p<0.001 versus placebo.
c p<0.02 versus placebo.
d p<0.08 versus placebo.
e p<0.01 versus placebo.
When changes from baseline to endpoint in HAM-D total score were analyzed by baseline Geriatric Depression Scale total score (≤18 and >18) and CGI severity scale score (≤4 and >4), no significant interactions were found. However, for change in the Geriatric Depression Scale total score, a significant interaction was found between treatment and baseline Geriatric Depression Scale total score (p=0.05), with a greater advantage of duloxetine over placebo in patients with higher baseline Geriatric Depression Scale total scores compared with those with lower baseline Geriatric Depression Scale total scores (data not shown). There was no significant interaction between treatment and baseline CGI severity scale scores. For changes in both Geriatric Depression Scale and HAM-D scores, there were no significant treatment-by-mild-dementia subgroup interactions. Patients treated with duloxetine also had significantly greater HAM-D response (p<0.001) and remission (p<0.02) rates than did placebo-treated patients ( Figure 5 ).
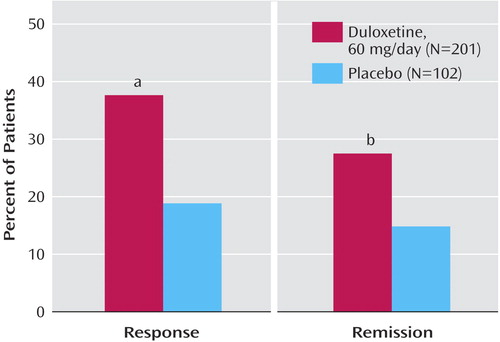
a p<0.001 versus placebo.
b p<0.02 versus placebo.
Efficacy in Pain Measures
Patients treated with duloxetine showed a significantly greater improvement in Visual Analogue Scale for back pain scores ( Figure 6 , top) and amount of time in pain while awake compared with placebo-treated patients ( Figure 6 , middle). The improvements for other pain measures, including overall pain ( Figure 6 , bottom), headache, shoulder pain, and pain interference with daily activities, were not significantly different between patients treated with duloxetine or given placebo.
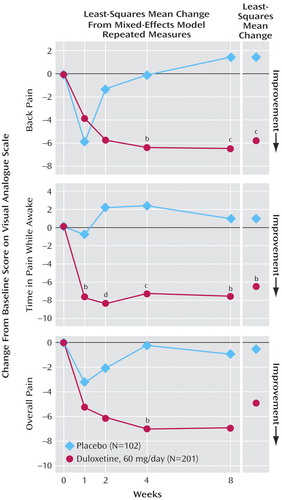
a Mean baseline pain severity: ∼26 (top); ∼35 (middle), ∼32 (bottom).
b p<0.05 versus placebo.
c p<0.01 versus placebo.
d p<0.001 versus placebo.
Safety and Tolerability Measures
Seventeen (8.3%) of 205 patients treated with duloxetine and one (1.0%) of 104 patients treated with placebo had a subsequent dose reduction. Overall discontinuation rates for any reason did not significantly differ between the duloxetine and placebo groups (21.7% versus 23.1%). However, discontinuation because of lack of efficacy was significantly less likely in the duloxetine group (2.9%, p<0.03) than in the placebo group (9.6%). Discontinuations due to adverse events were similar for duloxetine-treated patients compared with placebo-treated patients (9.7% versus 8.7%, p=0.84) ( Table 2 ). Treatment-emergent adverse events for which the incidence among duloxetine-treated patients was at least 5.0% and twice the placebo rate are presented in Table 3 . Of these events, those occurring significantly more frequently for duloxetine than for placebo were dry mouth (14.5% versus 1.9%, p<0.001), nausea (12.6% versus 3.8%, p<0.02), and diarrhea (8.2% versus 1.9%, p<0.05). Five patients (one duloxetine-treated patient and four placebo-treated patients) experienced serious adverse events. No patients died during the study.
Blood pressure and pulse changes were modest and did not differ significantly between duloxetine- and placebo-treated patients. The rates of sustained hypertension were 0.5% for duloxetine and 1.0% for placebo. The rates of treatment-emergent orthostatic hypotension were 15.6% for duloxetine and 20.5% for placebo. There was a significant decrease in weight for duloxetine- compared with placebo-treated patients (–0.73 versus –0.13 kg, p=0.009).
Of the five hepatic enzymes, alkaline phosphatase was significantly increased in duloxetine-treated patients compared with placebo-treated patients, although mean changes were not considered clinically relevant (0.41 versus –2.41 U/liter, p<0.02). No significant differences existed between the duloxetine and the placebo treatment groups in the incidence of treatment-emergent abnormal laboratory values at any time.
Discussion
Duloxetine treatment for 8 weeks resulted in a significant improvement compared with placebo on a composite measure of cognitive functions previously demonstrated to be impaired in patients with recurrent major depressive disorder (2 , 3) . Most of the improvement was driven by verbal learning and memory, whereas measures of focused attention and executive functioning showed no duloxetine-placebo difference. Duloxetine also significantly improved both self- and clinician-rated depression measures and lessened the severity of some self-reported pain measures.
Although cognitive deficits in patients with major depressive disorder have been demonstrated in many studies (3) , antidepressant medications, such as paroxetine and nortriptyline, do not routinely improve cognition in these patients (24) . Follow-up studies of elderly patients treated for major depressive disorder coexisting with dementia have shown that some patients demonstrate poor performance on cognitive tests after imipramine treatment (25) . The effect of duloxetine to augment 5-HT and norepinephrine activity may make it particularly beneficial in treating the cognitive deficiency of depression. The precursor to dopamine, l -dopa, was found to improve cognitive function, mainly verbal learning and longer-term memory, but had little antidepressant effect in patients with major depressive disorder (26) . The effect of duloxetine on cognition may have been more clearly shown in the current study because it included some patients with mild dementia. Path analysis suggested that the effect of duloxetine on improvement of the composite cognitive score was mainly a direct treatment effect rather than an indirect effect through improvement of depression measures.
The results from this trial demonstrate that duloxetine, 60 mg/day, may be an effective treatment for elderly patients with major depressive disorder. The Geriatric Depression Scale total score was a more sensitive scale than the HAM-D and CGI severity scale for measuring the onset of treatment effect of duloxetine, as indicated by rapid separation from placebo as early as week 1. The remission rate was also significantly greater for duloxetine than for placebo (27.4% versus 14.7%, p<0.02), which is in agreement with a recent report showing that duloxetine, 60 mg/day, was effective in the treatment of major depressive disorder in patients age 55 years and older (27) . The absolute response and remission rates were relatively low, perhaps attributable to a relatively short 8-week study duration, a fixed dosing schedule, and low placebo response rates. However, the relative advantages of duloxetine over placebo in response and remission rates were substantial, as evidenced by the fact that they were twice the placebo rates.
Several placebo-controlled antidepressant trials in elderly patients can be compared with the present study (28 – 32) . In general, antidepressant trials in the elderly are more difficult to show positive efficacy results for active treatments over placebo than trials in general populations (28) . In a recent placebo-controlled study of fluoxetine and venlafaxine, there were no significant differences among the three treatment groups in the change in HAM-D (21-item version), Montgomery-Åsberg Depression Rating Scale, or CGI severity scale scores, and there was no statistically significant difference in the proportion of remitters at the last on-therapy visit (29) . Similarly, in community-dwelling patients over 75 years of age, citalopram was no more effective than placebo for the treatment of depression (30) . However, in two larger studies in patients with depression over 60 years of age, sertraline or fluoxetine were more effective than placebo (31 , 32) . The remission rates in these studies were low in general, and the differences between treatment groups were small (20%–35% for active treatments, 18%–33% for placebo).
The number needed to treat is believed by many clinical epidemiologists to be the most clinically meaningful and useful measure of a treatment effect (33) . In this study, the duloxetine versus placebo number needed to treat for response was 5.3, and the number needed to treat for remission was 7.9. For duloxetine registration studies (all adults), the duloxetine versus placebo number needed to treat for response ranged from 3.7 to 15.2, and the number needed to treat for remission ranged from 4.7 to 52.6. The fact that the number needed to treat for response and remission in elderly patients lies within these corresponding ranges suggests that duloxetine efficacy in the elderly is comparable to that in the general adult population. The number needed to treat for response was very similar to the mean number needed to treat of five for tricyclic/placebo comparisons in the elderly reported in the meta-analysis by Taylor and Doraiswamy (34) . It is better than the mean number needed to treat of eight reported for selective serotonin reuptake inhibitor (SSRI) antidepressant studies (and for antidepressants overall) in that review. Similar results were found in a recent report by Cookson et al. (35) .
Duloxetine demonstrated statistically significant improvement on two pain measures, as evidenced by separation from placebo on the Visual Analogue Scale for time in pain while awake at week 1. However, patients enrolled in this study were not selected specifically for pain, and the pain reported was generally not severe. It is possible that more or less pain efficacy for duloxetine might be observed in patients with depression with higher initial pain severity.
The tolerability of duloxetine in the elderly patients is comparable with the profile observed in a general population of adult patients with depression (36) . The dropout rate of this study can be compared with the aforementioned other published studies of elderly patients with antidepressants (30 , 31) , although no direct comparisons can be made. In this study, overall discontinuation rates for any reason did not significantly differ between the duloxetine and placebo groups, similar to what was found in an 8-week study of sertraline in elderly patients (31) . The dropout rates were significantly higher in elderly patients treated with citalopram (21.4%) compared with patients treated with placebo (12.2%) (30) . In the present study, the rates of adverse events reported as a reason for discontinuation were similar for patients treated with duloxetine and placebo, except for the discontinuation rate due to nausea, which was actually significantly higher in patients treated with placebo than in patients treated with duloxetine (3.8% versus 0.5%, p<0.05). The most frequently reported treatment-emergent adverse events for patients treated with duloxetine in the present study were dry mouth (14.5%) and nausea (12.6%); they were reported at rates comparable to those observed in several studies conducted with elderly patients treated with SSRIs (37 , 38) .
This study had several limitations, including 1) duration of treatment for only 8 weeks; 2) exclusion of patients with serious or unstable medical illnesses, including psychosis; 3) exclusion of first-episode patients and those with significant psychiatric comorbidities; and 4) limited dose flexibility during the study.
In conclusion, the present results suggest that duloxetine, 60 mg/day, is effective in improving cognition and depression measures in patients with recurrent major depressive disorder age ≥65 years. Duloxetine also may be an effective treatment for some pain symptoms in these patients. The dual action of duloxetine on 5-HT and norepinephrine reuptake inhibition may explain its efficacy in treating the traditional symptoms of depression as well as improving some cognitive and pain symptoms. The study results also suggest that duloxetine, 60 mg/day, is well tolerated in elderly patients. Collectively, the present results indicate that duloxetine, 60 mg/day, may represent a new treatment option for older patients with recurrent major depressive disorder.
1. Steffens DC, Skoog I, Norton MC, Hart AD, Tschanz JT, Plassman BL, Wyse BW, Welsh-Bohmer KA, Breitner JC: Prevalence of depression and its treatment in an elderly population: the Cache County Study. Arch Gen Psychiatry 2000; 57:601–607Google Scholar
2. Mecocci P, Cherubini A, Mariani E, Ruggiero C, Senin U: Depression in the elderly: new concepts and therapeutic approaches. Aging Clin Exp Res 2004; 16:176–189Google Scholar
3. Burt DB, Zembar MJ, Niederehe G: Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull 1995; 117:285–305Google Scholar
4. Cohen RM, Weingartner H, Smallberg SA, Pickar D, Murphy DL: Effort and cognition in depression. Arch Gen Psychiatry 1982; 39:593–597Google Scholar
5. Serby M, Yu M: Overview: depression in the elderly. Mt Sinai J Med 2003; 70:38–44Google Scholar
6. Vinkers DJ, Gussekloo J, Stek ML, Westendorp RG, Van Der Mast RC: Temporal relation between depression and cognitive impairment in old age: prospective population based study. BMJ 2004; 329:881Google Scholar
7. Reding M, Haycox J, Blass J: Depression in patients referred to a dementia clinic: a three-year prospective study. Arch Neurol 1985; 42:894–896Google Scholar
8. Austin MP, Mitchell P, Goodwin GM: Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry 2001; 178:200–206Google Scholar
9. Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG, Shaw JL, Thompson L, Nelson DL, Hemrick-Luecke SK, Wong DT: Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology 2001; 25:871–880Google Scholar
10. Ressler KJ, Nemeroff CB: Role of norepinephrine in the pathophysiology of neuropsychiatric disorders. CNS Spectr 2001; 6:663–666, 670Google Scholar
11. Reneman L, Lavalaye J, Schmand B, De Wolff FA, Van Den BW, Den Heeten GJ, Booij J: Cortical serotonin transporter density and verbal memory in individuals who stopped using 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”): preliminary findings. Arch Gen Psychiatry 2001; 58:901–906Google Scholar
12. Williams LJ, Jacka FN, Pasco JA, Dodd S, Berk M: Depression and pain: an overview. Acta Neuropsychiatr 2006; 18:79–87Google Scholar
13. Andersson HI, Ejlertsson G, Leden I, Rosenberg C: Chronic pain in a geographically defined general population: studies of differences in age, gender, social class, and pain localization. Clin J Pain 1993; 9:174–182Google Scholar
14. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC: The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59:22–33Google Scholar
15. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Google Scholar
16. Folstein MF, Folstein SE, McHugh PR: “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Google Scholar
17. Thompson WL, Brunelle RL, Enas GG, Simpson PJ: Routine laboratory tests in clinical trials: interpretation of results. J Clin Res Drug Development 1987; 1:95–119Google Scholar
18. Rey A: L’examen psychologique dans les cas d’encephalopathie traumatique. Archives De Psychologie 1941; 28:286–340Google Scholar
19. Wechsler D: Wechsler Adult Intelligence Scale, 3rd ed. San Antonio, Tex, Psychological Corporation, 1997Google Scholar
20. Neisser U: Visual search. Sci Am 1964; 210:94–102Google Scholar
21. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO: Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982; 17:37–49Google Scholar
22. Deloach LJ, Higgins MS, Caplan AB, Stiff JL: The Visual Analog Scale in the immediate postoperative period: intrasubject variability and correlation with a numeric scale. Anesth Analg 1998; 86:102–106Google Scholar
23. Retherford RD, Choe MK: Statistical Methods for Causal Analysis. John Wiley & Sons, 1993Google Scholar
24. Butters MA, Becker JT, Nebes RD, Zmuda MD, Mulsant BH, Pollock BG, Reynolds CF III: Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry 2000; 157:1949–1954Google Scholar
25. Teri L, Reifler BV, Veith RC, Barnes R, White E, McLean P, Raskind M: Imipramine in the treatment of depressed Alzheimer’s patients: impact on cognition. J Gerontol 1991; 46:372–377Google Scholar
26. Murphy DL, Henry GM: Catecholamines and memory: enhanced verbal learning during L-dopa administration. Psychopharmacologia 1972; 27:319–326Google Scholar
27. Nelson JC, Wohlreich MM, Mallinckrodt CH, Detke MJ, Watkin JG, Kennedy JS: Duloxetine for the treatment of major depressive disorder in older patients. Am J Ger Psychiatry 2005; 13:227–235Google Scholar
28. Walsh BT, Sysko R: Placebo control groups in trials of major depressive disorder among older patients. J Clin Psychopharmacol 2005; 25:S29–S33Google Scholar
29. Schatzberg A, Roose S: A double-blind, placebo-controlled study of venlafaxine and fluoxetine in geriatric outpatients with major depression. Am J Geriatr Psychiatry 2006; 14:361–370Google Scholar
30. Roose SP, Sackeim HA, Krishnan KR, Pollock BG, Alexopoulos G, Lavretsky H, Katz IR, Hakkarainen H, Old-Old Depression Study Group: Antidepressant pharmacotherapy in the treatment of depression in the very old: a randomized, placebo-controlled trial. Am J Psychiatry 2004; 161:2050–2059Google Scholar
31. Sheikh JI, Cassidy EL, Doraiswamy PM, Salomon RM, Hornig M, Holland PJ, Mandel FS, Clary CM, Burt T: Efficacy, safety, and tolerability of sertraline in patients with late-life depression and comorbid medical illness. J Am Geriatr Soc 2004; 52:86–92Google Scholar
32. Tollefson GD, Bosomworth JC, Heiligenstein JH, Potvin JH, Holman S: A double-blind, placebo-controlled clinical trial of fluoxetine in geriatric patients with major depression: the Fluoxetine Collaborative Study Group. Int Psychogeriatr 1995; 7:89–104Google Scholar
33. Cook RJ, Sackett DL: The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995; 310:452–454Google Scholar
34. Taylor WD, Doraiswamy PM: A systematic review of antidepressant placebo-controlled trials for geriatric depression: limitations of current data and directions for the future. Neuropsychopharmacology 2004; 29:2285–2299Google Scholar
35. Cookson J, Gilaberte I, Desaiah D, Kajdasz DK: Treatment benefits of duloxetine in major depressive disorder as assessed by number needed to treat. Int Clin Psychopharmacol 2006; 21:267–273Google Scholar
36. Detke MJ, Lu Y, Goldstein DJ, McNamara RK, Demitrack MA: Duloxetine 60 mg once daily dosing versus placebo in the acute treatment of major depression. J Psychiatr Res 2002; 36:383–390Google Scholar
37. Dunner DL, Cohn JB, Walshe T III, Cohn CK, Feighner JP, Fieve RR, Halikas JP, Hartford JT, Hearst ED, Settle EC Jr: Two combined, multicenter double-blind studies of paroxetine and doxepin in geriatric patients with major depression. J Clin Psychiatry 1992; 53:57–60Google Scholar
38. Bondareff W, Alpert M, Friedhoff AJ, Richter EM, Clary CM, Batzar E: Comparison of sertraline and nortriptyline in the treatment of major depressive disorder in late life. Am J Psychiatry 2000; 157:729–736Google Scholar