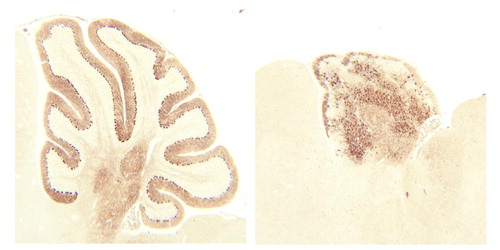
Brain Development, VIII: The Reeler Mouse
Mutant mouse strains have been useful to scientists and clinicians in understanding aspects of normal CNS development. One such spontaneous mutation produces a mouse with abnormal movements and, on microscopic analysis, abnormal cortical neuronal organization. The gene responsible for this mutant mouse strain is called reeler and was recently cloned. The reeler mouse mutation causes an abnormal pattern in the layering of neurons in the neocortex and the cerebellum. Instead of the cells arranging themselves in the typical “inside-out” fashion as described by P. Rakic (Images in Neuroscience, Am J Psychiatry 1998; 155:1150–1151), they order themselves in an “outside-in” pattern (figure). As a consequence, the earliest-born neurons abnormally migrate to the surface in the reeler mouse, the next generation of neurons settle immediately below, and the final neurons form the deepest cortical layer—the exact opposite of what is normally found. These mutant mice lack a functional gene product, the protein called reelin. Reelin is highly expressed during the period of development when brain cells are migrating into the cortex. It is produced by neurons near the cortical surface, is secreted into the surrounding extracellular matrix, and is thought to guide newly formed neurons to their proper final destination. It is only then that normal synaptic connections may be established between neurons. The lack of this protein guide leaves the neuronal patterning in disarray. It is intriguing to speculate that subtle mutations in this, or related genes, may contribute to the etiology of some developmental disorders of childhood, as well as other psychiatric illnesses.
Address reprint requests to Dr. Tamminga, Maryland Psychiatric Research Center, University of Maryland, P.O. Box 21247, Baltimore, MD 21228. Image is courtesy of Dr. Goldowitz.