Association Between Eye Tracking Disorder in Schizophrenia and Poor Sensory Integration
Abstract
Objective:The authors tested the hypothesis that eye tracking disorder in schizophrenia is associated with neurological signs.Method:The subjects were 93 normal comparison subjects and 59 schizophrenic patients. They were evaluated with the Neurological Evaluation Scale, a standardized rating instrument that assesses sensory integration, motor coordination, sequencing of complex motor acts, and other neurological signs. Also, the schizophrenic patients’ smooth-pursuit eye movements were tested in response to a 0.3-Hz sinusoidal target by means of infrared oculography. They were divided into those with (N=18) and without (N=41) eye tracking disorder by using a previously described method, which was based on mixture analysis of the distribution of position root mean square error.Results:The patients with eye tracking disorder had significantly worse performance than the patients without eye tracking disorder with respect to sensory integration, and the effect size was moderate to large. In comparison with the normal subjects, both patient subgroups had significantly worse performance on all of the Neurological Evaluation Scale subscales. Conclusions:Although neurological signs are present generally in schizophrenia, poor sensory integration is particularly pronounced in patients with eye tracking disorder. A review of the literature shows that the two abnormalities have strikingly similar patterns of validators, including 1) familial aggregation, 2) premorbid presence, 3) syndromal specificity, 4) trait status, and 5) association with the deficit syndrome. Poor sensory integration and eye tracking disorder in schizophrenia may be various manifestations of a common, underlying pathophysiological process. Am J Psychiatry 1998; 155: 1352-1357
Two well-established and mostly independent lines of research have shown that patients with schizophrenia have abnormal smooth-pursuit eye movements (for review, see reference 1)) and neurological signs (for review, see reference 2). It has been reported that poor smooth-pursuit eye movements are associated with neurological signs in nonpsychiatric subjects (3), but it is not known whether the two abnormalities are associated in patients with schizophrenia. This possibility is suggested collectively by many studies showing that eye tracking disorder and neurological signs in schizophrenic patients share certain validators (see Discussion section).
The goal of this study was to test the hypothesis that eye tracking disorder in schizophrenia is associated with neurological signs.
METHOD
Subjects
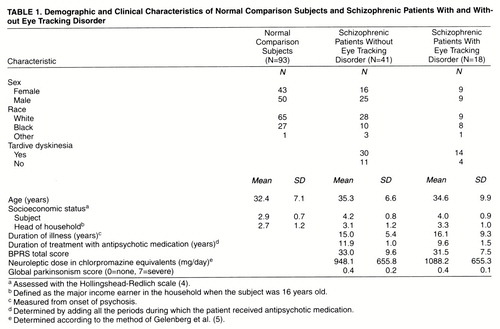
The participants in the study were 93 normal comparison subjects and 59 schizophrenic patients. The Structured Clinical Interview for DSM-III-R was administered to all subjects. Potential subjects were screened to exclude those with medical illnesses or taking medications known to adversely affect eye movements (including lithium and benzodiazepines). The socioeconomic status of each subject and the head of the household was obtained by using a previously developed scale (4). Demographic and clinical characteristics of the subjects are shown in table 1.
The normal comparison subjects were recruited from the general population through newspaper notices and flyers. They did not have any past or current DSM-III-R axis I or II disorder. They were reimbursed for their participation in the study.
Patients were recruited from outpatient and inpatient programs at the Maryland Psychiatric Research Center. All patients satisfied the DSM-III-R criteria for schizophrenia. All patients were clinically stabilized on antipsychotic medication regimens; some were taking antiparkinsonian medication. “Clinically stabilized” was defined as having taken the same antipsychotic medication for at least 4 weeks (usually longer) without a change in dose or significant change in clinical state as assessed by at least two treating clinicians, including the primary psychiatrist. The Brief Psychiatric Rating Scale (BPRS) (6) was administered to each patient within 1 week of eye movement testing. Patients were assessed with the Maryland Psychiatric Research Center’s involuntary movement scale (7) and diagnosed according to the research diagnostic criteria for tardive dyskinesia (8) (table 1). Tardive dyskinesia was diagnosed for 44 patients, and 15 were categorized as not having tardive dyskinesia.
The schizophrenic patients were classified into those with (N=18) and without (N=41) eye tracking disorder according to a previously described method (9). In that study, mixture analysis showed that the distribution of position root mean square (RMS) error was best fit by a mixture of two normal distributions. That finding provided a rationale for determining a cutoff point that could be used to divide the patients into those with and without eye tracking disorder.
The patient subgroups and normal comparison subjects were compared to assess the presence of potential confounds. Chi-square tests were used for categorical variables, and independent t tests were used for continuous variables.
The three subject groups (normal comparison subjects, patients with eye tracking disorder, and patients without eye tracking disorder) did not differ significantly with respect to age, sex, race, or socioeconomic status of head of household (p>0.05 in all cases). The patients with and without eye tracking disorder did not differ significantly with respect to the subject’s socioeconomic status, duration of psychotic illness, duration of treatment with neuroleptic medication, neuroleptic dose in chlorpromazine equivalents, global parkinsonism score (from the involuntary movement scale) or total BPRS score within 1 week of eye movement testing (p>0.05 in all cases).
The patients with and without tardive dyskinesia did not differ significantly with respect to age, sex, or subject’s socioeconomic status (p>0.05 in all cases). They also did not differ significantly with respect to the presence or absence of eye tracking disorder (Fisher’s exact test, p=1.00; odds ratio=1.28). (The subjects were classified into the four cells as follows: both eye tracking disorder and tardive dyskinesia, 14 of 59 subjects [24%]; tardive dyskinesia without eye tracking disorder, 30 of 59 subjects [51%]; eye tracking disorder without tardive dyskinesia, four of 59 subjects [7%]; neither disorder, 11 of 59 subjects [19%].) The patients with and without tardive dyskinesia did not differ significantly with respect to scores on any of the subscales from the Neurological Evaluation Scale (to be discussed); for all cases, p>0.15 and d<0.4 (effect size [10]).
After complete description of the study to the subjects, written informed consent was obtained.
Oculographic Methods
Data collection
Eye movements were tested and analyzed by using our previously described methods (9) , which will be repeated here only briefly. Eye movements were measured with infrared oculography. The head was stabilized with a chin rest, forehead rest, and headband. The smooth-pursuit target consisted of eight cycles of a 0.3-Hz sine wave traversing ±10 degrees of visual angle. The data were converted from analog to digital format and stored for later interactive analysis.
Data analysis
From the eight cycles of the sinusoidal target, three contiguous cycles (10 sec) were chosen for analysis according to our previously described method (9, 11, 12). Position RMS error was calculated by using the following definition:
where ri is the position of the eye (response) at each ith point in time, si is the position of the stimulus at the same time, and n is the number of data points measured. Position RMS error was measured after exclusion of nontracking epochs and anticipatory saccades, which were thought to reflect general inattention (1). Position RMS error was used to classify each patient as having or not having eye tracking disorder, by using our previouly described method, which was based on mixture analysis of position RMS error (9).
Neurological Evaluation
All subjects were neurologically examined with the Neurological Evaluation Scale (13) , and this examination will be described only briefly here. The Neurological Evaluation Scale is designed to standardize the assessment of neurological impairment in schizophrenia. It consists of 26 items, which are divided into the following four subscales: 1) sensory integration, 2) motor coordination, 3) sequencing of complex motor acts, and 4) other neurological signs.
The sensory integration subscale measures the ability to integrate major sensory modalities, such as vision, hearing, and touch. It includes the following items: audiovisual integration (the ability to match a pattern of sounds to a visual diagram); stereognosis (the ability to identify common objects by touch); graphesthesia (the ability to identify numbers written on the skin); extinction (in response to bilateral and simultaneous somatosensory stimulation); and right/left confusion.
The motor coordination subscale measures the ability to coordinate movements. It includes the following items: tandem walk (walking toe to heel); diadochokinesis (rapidly alternating movements of the hands); finger-thumb opposition; and finger-nose test (touching one’s nose after closing one’s eyes).
The complex motor sequencing subscale measures the ability to rhythmically and regularly alternate hand positions. It includes the following items: fist-ring test; fist-edge-palm test; Ozeretski test; and rhythm tapping test, version B (generate a series of tapping sounds).
The “other neurological signs” subscale includes items that have been traditionally used or are of theoretical interest and that could not be clearly classified in any of the preceding categories. It includes the following items: adventitious overflow test; Romberg test; resting tremor; memory (remembering four words at 5 and 10 minutes); rhythm tapping test, version A (reproduce a series of tapping sounds); mirror movements; synkinesis (the ability to follow slowly moving object with the eyes only, not the head); convergence (ocular motor); gaze impersistence (in response to fixation of a peripheral, stationary target); and frontal release signs (glabellar, snout, suck, and grasp).
The neurological evaluations were completed by master’s- and doctoral-level clinicians who were trained in the use of the instruments and were blind to the results of the ocular motor analyses. Each sign was judged to be present or absent. The data were reduced by taking the subtotal of the number of signs present for each subscale. The interrater reliabilities for the subscales have been found to be at least moderately strong, with intraclass correlation coefficients ranging from 0.71 to 0.99 (13).
Statistical Analyses
The distributions of the data were examined for normality and homogeneity of variance. The variance of each of the four subscales of the Neurological Evaluation Scale differed significantly between groups (Brown-Forsythe test, df=2, 149, p<0.05 in all cases); the variances of the normal comparison group were larger than those of the patient subgroups. Therefore, a square-root transformation was applied to the data and was successful in removing the significant differences between groups (Brown-Forsythe test, df=2, 149, p>0.05 in all cases). Consequently, the transformed data were used in subsequent analyses, including between-groups comparisons and calculations of effect sizes.
A multivariate analysis of variance (MANOVA) was used to test the hypothesis that the three groups (normal comparison subjects, patients with eye tracking disorder, and patients without eye tracking disorder) differed significantly with respect to the four Neurological Evaluation Scale subscales. Significant omnibus tests were followed up by post hoc Tukey’s honestly significant difference tests, in order to make the following comparisons: 1) patients with eye tracking disorder versus patients without eye tracking disorder, 2) patients with eye tracking disorder versus normal comparison subjects, and 3) patients without eye tracking disorder versus normal comparison subjects.
RESULTS
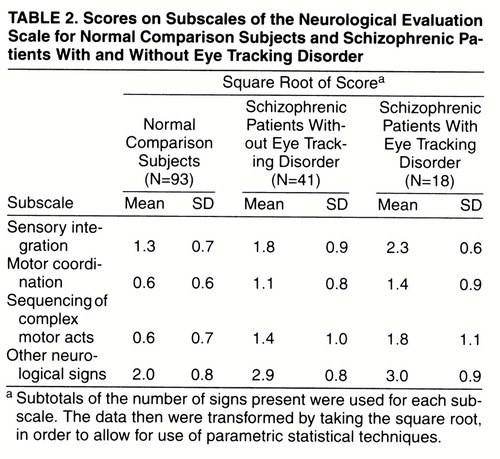
Scores on the subscales of the Neurological Evaluation Scale are shown in table 2. A MANOVA, using group as a between-subjects factor and the four subscale scores as the dependent variables, revealed a significant effect of group (Wilks’s lambda=0.63, F=42.90, df=2, 149, p<0.0001).
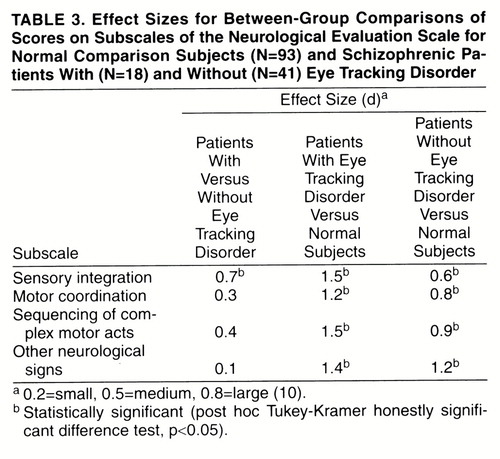
The results of the post hoc comparisons of the individual groups, by means of Tukey-Kramer honestly significant difference tests (table 3), revealed the following: 1) sensory integration was significantly worse in the patients with eye tracking disorder than in the patients without eye tracking disorder (p<0.05); 2) the patients with and without eye tracking disorder did not differ significantly on any of the other Neurological Evaluation Scale subscales (p>0.05 in all cases); and 3) the scores of the patients with and without eye tracking disorder were significantly worse than those of the normal comparison subjects on all subscales (p<0.05 in all cases).
DISCUSSION
Main Findings
The schizophrenic patients with eye tracking disorder had significantly worse performance on tests of sensory integration than did schizophrenic patients without eye tracking disorder, and the effect size was moderate to large. This finding is consistent with a previous finding of an association between poor smooth pursuit and neurological signs (including sensory integration) in a nonpatient college group (3). More generally, the finding that patients with eye tracking disorder have worse performance than patients without eye tracking disorder on tests of sensory integration supports the validity of classifying patients into subgroups on the basis of eye tracking performance.
Both patient subgroups differed significantly from the normal comparison subjects on all of the Neurological Evaluation Scale subscales (sensory integration, motor coordination, sequencing of complex motor acts, and other neurological signs). The patients with and without eye tracking disorder did not differ significantly on any subscale other than sensory integration. Therefore, although neurological signs are present generally in schizophrenia, poor sensory integration is particularly pronounced in patients with eye tracking disorder.
Eye Tracking Disorder and Poor Sensory Integration: Similar Patterns of Validators
The association between eye tracking disorder and neurological signs brings together two important areas of research that previously have been conducted almost completely in parallel. Both areas of research have been active for several decades. In more recent years, a significant amount of effort has been made toward refining the measures of eye tracking and neurological signs (13, 14). Despite limitations in measurement techniques, the abnormalities have proven to be robust, and various tests of their validity have been replicated many times.
In reviewing the literature, it was reasonable to consider a variety of smooth-pursuit measures (e.g., qualitative ratings, global quantitative ratings, and pursuit gain) as adequate measures of eye tracking disorder, because of the high correlations between these measures and validators shared by them (for reviews, see references 9 and 15). In reviewing studies of neurological signs, batteries of tests that included sensory integration generally were assumed to be adequate measures. Thus, this approach to literature review was based on the robustness of these measures and was intended to serve a heuristic function. Certainly, continued refinement of these measures will be important for advancement of these areas of research, and conclusions based on this review will be limited accordingly.
Review of the literature suggests that eye tracking disorder and neurological signs share many validators, as follows.
1. Each abnormality aggregates in families
Family and twin studies have shown that eye tracking disorder aggregates in families, that family members with schizophrenia-spectrum disorders are especially likely to have eye tracking disorder, and that the familial aggregation is at least partly due to genetic factors (for review, see reference 1; for more recent work, see reference 16). Similarly, neurological signs (including poor sensory integration) have been reported to aggregate in families of patients with schizophrenia (for review, see reference 2; for more recent work, see reference 17).
2. Each abnormality is present before the onset of psychosis
Presence of the abnormality before onset of psychosis has been reported for both smooth-pursuit dysfunction (18, 19) and neurological signs, including poor sensory integration (for review, see reference 2; for more recent works, see references 20 and (21)).
3. The prevalence of each abnormality is higher in schizophrenic patients than in normal comparison subjects
This basic finding has been extremely robust for eye tracking disorder (for review, see reference 1; for more recent works, see references 9, 12, 22, and 23). The finding has also been very robust for neurological signs, including poor sensory integration (for review, see reference 2; for more recent works, see references 13, 17, 24, and 25).
4. The prevalence of each abnormality is higher in schizophrenic patients than in other psychiatric patients
This conclusion is generally true for eye tracking disorder, particularly when patients with acute illness are excluded (for review, see reference 1; for more recent work, see reference 23). It is also generally true for neurological signs, including poor sensory integration (for review, see reference 2).
5. Each abnormality is trait-related, not state-related
In addition to being present before the onset of psychosis, both disorders are present at the onset of psychotic illness and persist during the longitudinal course of the illness, despite fluctuations in clinical state. Generally, the abnormalities do not seem to be due simply to secondary factors, such as medication. For eye tracking disorder, the literature relevant to this point has been reviewed previously (1) (for more recent work, see reference 26). For neurological signs, including poor sensory integration, the literature relevant to this point has been reviewed previously (2) (for more recent works, see references (24, 25, and 27).
6. Each abnormality is associated with primary and enduring negative features of psychopathology
The evidence for this conclusion is moderately strong. For eye tracking disorder, the literature relevant to this point has been reviewed previously (9). For neurological signs, including poor sensory integration, the literature relevant to this point has been reviewed previously (2) (for more recent work, see reference 28).
7. Each abnormality may be associated with dysfunction of the parietal heteromodal cortex
Poor smooth-pursuit eye movements in schizophrenia have been found to be associated with low glucose utilization in the angular gyri (29). There is no direct evidence implicating the parietal cortex in sensory integration, but theory suggests that this would be the case. For example, it is likely that audiovisual integration is subserved by a circuit that includes the posterior parietal cortex. Furthermore, indirect evidence comes from the finding that the deficit syndrome, which is associated with eye tracking disorder and poor sensory integration, is characterized by low glucose utilization in the parietal lobes (30).
Conclusions
Smooth pursuit eye tracking disorder and poor sensory integration in schizophrenia may be various signs of a common underlying pathophysiology of neural circuits. In the future, it will be important to continue refining the measurement of these abnormalities in order to make advances in these areas of research.
Received July 14,1997; revision received May 14, 1998; accepted May 28, 1998. From the Department of Psychiatry, Maryland Psychiatric Research Center, University of Maryland at Baltimore; and the Department of Psychiatry, Medical College of Virginia, Virginia Commonwealth University, Richmond.. Address reprint requests to Dr. Ross, Central State Hospital, P.O. Box 4030, Petersburg, VA 23803; [email protected] (e-mail). Supported in part by the Scottish Rite Schizophrenia Research Program and NIMH grants MH-40279, MH-43031, and MH-49826.The authors thank Kirsten Hahn and Ashton Morgan for assistance in collection and analysis of the data and Brian Kirkpatrick, M.D., for comments regarding this manuscript.
 |
 |
 |
1. Levy D, Holzman P, Matthysse S, Mendell N: Eye tracking dysfunction and schizophrenia: a critical perspective. Schizophr Bull 1993; 19:461–505Crossref, Medline, Google Scholar
2. Heinrichs DW, Buchanan RW: Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry 1988; 145:11–18Link, Google Scholar
3. Siever LJ, Coursey RD, Alterman IS, Zahn T, Brody L, Bernad P, Buchsbaum M, Lake CR, Murphy DL: Clinical, psychophysiological, and neurological characteristics of volunteers with impaired smooth pursuit eye movements. Biol Psychiatry 1989; 26:35–51Crossref, Medline, Google Scholar
4. Hollingshead AB, Redlich FC: Social Class and Mental Illness: A Community Study. New York, John Wiley & Sons, 1958Google Scholar
5. Gelenberg A, Bassuk E, Schoonover S: The Practitioner’s Guide to Psychoactive Drugs. New York, Plenum, 1991Google Scholar
6. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
7. Cassady SL, Thaker GK, Summerfelt A, Tamminga CA: The Maryland Psychiatric Research Center Scale and the characterization of involuntary movements. Psychiatry Res 1997; 70:21–37Crossref, Medline, Google Scholar
8. Schooler NR, Kane JM: Research diagnoses for tardive dyskinesia (letter). Arch Gen Psychiatry 1982; 39:486–487Medline, Google Scholar
9. Ross DE, Thaker GK, Buchanan RW, Kirkpatrick B, Lahti AC, Medoff D, Bartko JJ, Goodman J, Tien AY: Eye tracking disorder in schizophrenia is characterized by specific ocular motor defects and is associated with the deficit syndrome. Biol Psychiatry 1997; 42:781–796Crossref, Medline, Google Scholar
10. Cohen J: Statistical Power Analysis. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988Google Scholar
11. Ross DE, Ochs AL, Hill MP, Goldberg SC, Pandurangi AK, Winfrey J: The erratic nature of eye movements in schizophrenic patients as revealed by high resolution techniques. Biol Psychiatry 1988; 24:675–688Crossref, Medline, Google Scholar
12. Ross DE, Ochs AL, Pandurangi AK, Thacker LR, Kendler KS: Mixture analysis of smooth pursuit eye movements in schizophrenia. Psychophysiology 1996; 33:390–397Crossref, Medline, Google Scholar
13. Buchanan RW, Heinrichs DW: The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res 1989; 27:335–350Crossref, Medline, Google Scholar
14. Abel LA, Ziegler AS: Smooth pursuit eye movements in schizophrenics—what constitutes assessment? Biol Psychiatry 1988; 24:746–761Google Scholar
15. Ross DE, Thaker GK, Buchanan RW, Lahti AC, Conley R, Medoff D: Specific measures account for most of the variance in qualitative ratings of smooth pursuit eye movements in schizophrenia. Arch Gen Psychiatry 1998; 55:184–185Crossref, Medline, Google Scholar
16. Arolt V, Lencer R, Nolte A, Muller-Myhsok B, Purmann S, Schurmann M, Leutelt J, Pinnow M, Schwinger E: Eye tracking dysfunction is a putative phenotypic susceptibility marker of schizophrenia and maps to a locus on chromosome 6p in families with multiple occurrence of the disease. Am J Med Genet 1996; 67:564–579Crossref, Medline, Google Scholar
17. Rossi A, De Cataldo S, Di Michele V, Manna V, Ceccoli S, Stratta P, Casacchia M: Neurological soft signs in schizophrenia. Br J Psychiatry 1990; 157:735–739Crossref, Medline, Google Scholar
18. Mather JA: Eye movements of teenage children of schizophrenics: a possible inherited marker of susceptibility to the disease. J Psychiatr Res 1985; 19:523–532Crossref, Medline, Google Scholar
19. Ross RG, Hommer D, Radant A, Roath M, Freedman R: Early expression of smooth-pursuit eye movement abnormalities in children of schizophrenic parents. J Am Acad Child Adolesc Psychiatry 1996; 35:941–949Crossref, Medline, Google Scholar
20. Fish B, Marcus J, Hans SL, Auerbach JG, Perdue S: Infants at risk for schizophrenia: sequelae of a genetic neurointegrative defect: a review and replication analysis of pandysmaturation in the Jerusalem Infant Development Study. Arch Gen Psychiatry 1992; 49:221–235Crossref, Medline, Google Scholar
21. Marcus J, Hans SL, Auerbach JG, Auerbach AG: Children at risk for schizophrenia: the Jerusalem Infant Development Study, II: neurobehavioral deficits at school age. Arch Gen Psychiatry 1993; 50:797–809Crossref, Medline, Google Scholar
22. Ciuffreda KJ, Alpert M, Blackstone T, Fudge R, Thaler J: Pursuit eye movements in chronic schizophrenics: relationship between increased saccades and negative symptoms. Ophthalmic Physiol Opt 1994; 14:79–81Crossref, Medline, Google Scholar
23. Friedman L, Jesberger JA, Siever LJ, Thompson P, Mohs R, Meltzer HY: Smooth pursuit performance in patients with affective disorders or schizophrenia and normal controls: analysis with specific oculomotor measures, RMS error and qualitative ratings. Psychol Med 1995; 25:387–403Crossref, Medline, Google Scholar
24. Rubin P, Vorstrup S, Hemmingsen R, Andersen HS, Bendsen BB, Stromso N, Larsen JK, Bolwig TG: Neurological abnormalities in patients with schizophrenia or schizophreniform disorder at first admission to hospital: correlations with computerized tomography and regional cerebral blood flow findings. Acta Psychiatr Scand 1994; 90:385–390Crossref, Medline, Google Scholar
25. Sanders RD, Keshavan MS, Schooler NR: Neurological examination abnormalities in neuroleptic-naive patients with first-break schizophrenia: preliminary results. Am J Psychiatry 1994; 151:1231–1233Link, Google Scholar
26. Lieberman J, Jody D, Alvir J, Ashtari M, Levy D, Bogerts B, Degreef G, Mayerhoff D, Cooper T: Brain morphology, dopamine and eye tracking in first episode schizophrenia: prevalence and clinical correlates. Arch Gen Psychiatry 1993; 50:357–368Crossref, Medline, Google Scholar
27. King DJ, Wilson A, Cooper SJ, Waddington JL: The clinical correlates of neurological soft signs in chronic schizophrenia. Br J Psychiatry 1991; 158:770–775Crossref, Medline, Google Scholar
28. Buchanan R, Kirkpatrick B, Heinrichs DW, Carpenter WT Jr: Clinical correlates of the deficit syndrome of schizophrenia. Am J Psychiatry 1990; 147:290–294Link, Google Scholar
29. Ross DE, Thaker GK, Holcomb HH, Cascella NG, Medoff DR, Tamminga CA: Abnormal smooth pursuit eye movements in schizophrenic patients are associated with cerebral glucose metabolism in oculomotor regions. Psychiatry Res 1995; 58:53–67Crossref, Medline, Google Scholar
30. Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, Carpenter WT: Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry 1992; 49:522–530Crossref, Medline, Google Scholar



