Are There Sex Differences in Neuropsychological Functions Among Patients With Schizophrenia?
Abstract
Objective:Studies of sex differences in neuropsychological performance in schizophrenia report inconsistent results, due in part to methodological artifacts. The study presented here was specifically designed to examine sex differences in neuropsychological performance. It was hypothesized that schizophrenic women would exhibit fewer neuropsychological deficits than schizophrenic men and that their performance would be more similar to that of normal women than schizophrenic men’s performance would be to that of normal men. Method:Thirty-one outpatients with DSM-III-R-defined schizophrenia were systematically sampled from an extensive service network serving a large urban catchment area for seriously mentally ill persons. Twenty-seven normal comparison subjects were matched within sex on the basis of age, parental socioeconomic status, ethnicity, and handedness. An extensive neuropsychological test battery was administered, and multivariate analysis of variance was used to test for the effects of sex and group and sex-by-group interactions.Results:Male patients were significantly impaired across all functions in comparison with normal male subjects and on tests of attention, verbal memory, and executive functions in comparison with female patients. Female patients performed significantly worse than female normal comparison subjects only on tests of attention, executive functions, visual memory, and motor functions.Conclusions:The findings suggest that women with schizophrenia may be less vulnerable to particular cognitive deficits, especially those involving verbal processing, than schizophrenic men. Am J Psychiatry 1998; 155: 1358-1364
There are substantial neuropsychological data indicating that schizophrenic patients are pervasively impaired in contrast to normal comparison subjects. However, there are relatively few studies reporting on sex differences in neuropsychological performance in subjects with schizophrenia. Sex differences in biobehavioral characteristics among schizophrenic patients are plausible, because studies have shown poorer premorbid histories, earlier age at onset, and worse outcomes among schizophrenic men than among schizophrenic women (1, 2). These clinical characteristics tend to be associated with poorer neuropsychological functioning.
Sex differences in neuropsychological performance may reflect differential illness effects or premorbid capacities or both. Studies of subjects at high risk for schizophrenia have identified significantly more premorbid abnormalities among boys than among girls in neuromotor function (3, 4), aggression and social withdrawal (4), impulse control and IQ (5, 6), electrodermal response (5), and attention (3, 5). These studies and our work (7) suggest that schizophrenic males may be at higher risk than schizophrenic females for more severe neurocognitive consequences because of early developmental deficits.
Some studies of adults indicated that schizophrenic males have more impairment than females in sustained attention, language, executive functions, intelligence, and olfactory identification (7-10). However, three studies (11-13) did not find significant sex differences in cognition in schizophrenia, and still others (14, 15) found that females exhibited greater cognitive deficits. We and others have argued that the inconsistency in study results is due to different sampling strategies and inadequate sample sizes to test for sex effects (16-18) and/or failure to match normal comparison subjects within sex (18). The issue of sampling bias stems from the likelihood that study groups composed of persons with chronic schizophrenia may include higher proportions of severely ill women than schizophrenic women in general, given that schizophrenic women are less likely to become chronic patients. Thus, study groups of chronic patients may result in attenuated sex effects. The chronicity of illness in study groups may be reflected in 1) the source of sampling (i.e., tertiary-care facilities), 2) the high male-to-female ratio of cases (i.e., men are more likely to be chronic patients), and 3) the early age at onset (i.e., the early 20s) among the women in the studies, which is typically associated with schizophrenia in males (1, 2).
An additional sampling-related issue is the reliance on acutely ill patients for analyses of neuropsychological functioning in schizophrenia. Acutely ill patients tend to be more cognitively impaired than patients in remission (19). Using acutely ill patients to test for sex effects may produce results reflecting clinical state differences rather than those due to traits associated with the sex of the subjects. For example, in two studies (11, 14), the inpatient women had more symptoms than the inpatient men.
Finally, none of these studies included matched normal comparison subjects within sex to test for sex differences in schizophrenia. The inclusion of within-sex-matched normal comparison subjects is important because even though there is substantial evidence of sex differences in cognition among normal subjects (20), the sex effect size is small. Matching normal comparison subjects within sex provides a better control of extraneous variation, thus increasing the statistical power to find sex effects, particularly in small groups.
The study presented here was specifically designed to test for sex effects in neuropsychological performance. We hypothesized that women with schizophrenia would exhibit fewer neuropsychological deficits than men with schizophrenia, and that their performance would be more similar to that of normal comparison women than the performance of schizophrenic men would be to that of their comparison subjects. We hypothesized specific sex differences in language, attention, and executive functions on the basis of previously identified sex differences in these domains among normal subjects (20), among high-risk offspring of schizophrenic subjects (21), and in our previous study of adult schizophrenic patients (7).
METHOD
Subjects
Patients were drawn from an extensive public network of outpatient services for seriously mentally ill persons that serves a large urban catchment area. Out of the universe of current outpatients at the initiation of the study (N=345), the first 136 charts (of 75 men and 61 women) were reviewed to find those meeting our inclusion criteria, since funding was available for only 60 subjects (i.e., approximately 30 patients). Thirty-six (16 men and 20 women) of the 136 patients were excluded on the basis of a history of severe substance abuse or dependence, 25 or more ECTs in their lifetime, or their medical history, and one was deceased. On the basis of the record review, 79% of the men (N=59) and 67% of the women (N=41) met our criteria, including a clinical diagnosis of DSM-III-R schizophrenia. These 100 patients were stratified by sex and then randomly sampled to yield 31 patients.
We chose outpatients to ensure that patients would be stable when tested. The 31 recruited patients (17 male and 14 female) were representative of all schizophrenic patients in the service network who met our inclusion criteria. One woman dropped out of the study, and another was randomly chosen from the list of eligible cases.
Normal comparison subjects were recruited through advertisements and notices posted on bulletin boards at two hospitals serving the same catchment area. The majority were already participating in an ongoing family study of the neuropsychology of schizophrenia (22). Twenty-seven normal comparison subjects (13 male and 14 female) were proportionately matched within sex on age, handedness, parental socioeconomic status, and ethnicity. Written informed consent was obtained from all subjects after the procedures had been fully explained.
The subjects ranged from 20 to 60 years of age. They had no history of organic mental disorder, documented head trauma associated with loss of consciousness, ECT within the past year, DSM-III-R substance dependence, or substance abuse within the past 6 months. Potential subjects were excluded if they had sensory impairments interfering with performance or a systemic medical condition affecting CNS functioning. Other inclusion criteria were fluency in English, at least a 10th-grade education, and an IQ of 70 or greater.
The mean age of the schizophrenic patients was 39.1 years (SD=7.0). This group was primarily (85%) non-Hispanic white, most had some college education (mean=13.2 years, SD=1.9), and they came from middle class socioeconomic backgrounds (the mean parental socioeconomic status [Hollingshead four-factor score] was 2.3, SD=1.4). They also performed at an average level on the Wide Range Achievement Test—Revised (WRAT-R) (23) reading test (mean standardized score=101.2, SD=15) and spelling test (mean standardized score=102.9, SD=16.7). The comparison group did not significantly differ on any sociodemographic variable or on the standardized, age-scaled WRAT-R reading and spelling scores.
The patients’ mean age at onset was 22.0 years (SD=5.2, range=14–35), the mean number of hospitalizations was 7.6 (SD=5.5), and the mean duration of illness was 17.8 years (SD=9.1). All of the patients were in a stable clinical state when tested, as measured by our ratings of cooperation and degree of overt psychotic agitation (22); possible scores ranged from 0, reflecting normal effort on the task or no current overt psychosis, to 6, reflecting very poor effort or a high degree of psychosis. The mean cooperation and psychosis ratings were 0.65 (SD=1.0) and 0.84 (SD=1.3), respectively, reflecting mild symptoms. The patients were receiving a mean daily neuroleptic dose of 520 mg (SD=428) in chlorpromazine equivalents (for the men, mean=624.7 mg, SD=511.9; for the women, mean=402.9 mg, SD=190.2; t=1.65, df=21.1, p<0.11).
Table 1 shows the demographic and clinical characteristics of the patients and the comparison subjects stratified by sex. The two diagnostic groups were proportionately matched within sex on age, parental socioeconomic status, ethnicity, and handedness. There were no significant differences between the male and female schizophrenic patients in age at onset, duration of illness, number of hospitalizations, or ratings on psychosis and cooperation. Finally, there were no significant differences in age, ethnicity, parental socioeconomic status, and handedness between the male and female patients and their same-sex normal comparison subjects (table 1).
Diagnostic Assessments
The patients with schizophrenia were diagnosed according to the DSM-III-R criteria on the basis of structured research interviews and a systematic record review of the history and course of their symptoms and their medical history. Patients were clinically interviewed with the Schedule for Affective Disorders and Schizophrenia (25). Consensus diagnoses were made on the basis of information from all sources by two experienced diagnosticians (J.M.G. and L.J.S.), who were unaware of the patients’ neuropsychological data. The normal comparison subjects received a brief clinical interview and the MMPI-I to screen for current psychopathology. They were included if their T scale scores on the MMPI-I were less than or equal to 70 (22).
Neuropsychological Assessment
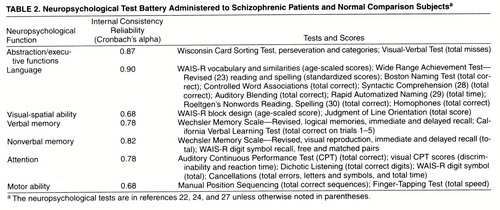
As in our previous work and others’ (11, 13, 22, 26, 27), we used an extensive battery of tests grouped into domains of neuropsychological functions on the basis of theory and practice as applied to schizophrenia (27). The neuropsychological battery consisted of tasks relating to intelligence, verbal and nonverbal memory, abstraction/executive functions, motor skills, visual-spatial processing, smell identification, and visual and auditory attention, as well as an extensive language battery, including tests of phonology, semantics, and grammar. Smell identification was the topic of another article (10) and thus is not discussed here. The examiners had extensive experience in clinical and neuropsychological testing with schizophrenic patients. It was not possible for them to be blind to patient status, although they did not conduct the diagnostic interviews. The neuropsychological tests and the associated internal consistency reliabilities (Cronbach’s alpha for standardized variables) are presented in table 2.
In general, the internal consistency reliabilities of the functions were excellent. The somewhat lower reliabilities (alphas) for visual-spatial ability and motor skills were most likely due to the use of only two tests to operationalize these domains.
Functions were created by standardizing all of the variables to a mean of 0 and standard deviation of 1, as is typically done to create z scores. The z scores were created by using the mean and standard deviation of the whole study group. Standardized scores on each test were averaged within a function. In previous work on neuropsychological functions in schizophrenia (22, 31), the control mean and standard deviation have been used to standardize tests within a function after adjustment for age and parental socioeconomic status. However, in this study, the variance of test scores within the group of patients was significantly larger than that within the normal comparison group. Thus, if the patients’ deviation scores were divided by the comparison group’s standard deviation, they would be divided by an artifactually small number, that is, the significantly smaller comparison group standard deviation. This would result in an overestimation of the patients’ standardized deficit scores. Analyses of the data with the use of both approaches demonstrated that the differences between male and female patients and their comparison subjects were in the same direction but even larger when the latter method was used. However, we used the former z score approach, to avoid overestimating the differences between patients and normal comparison subjects.
Data Analyses
We used multivariate analysis of covariance to test for significant sex differences across neuropsychological functions, with independent predictors being sex, group, and sex-by-group interaction. The interaction term tested whether there was a "different difference" between schizophrenic women and men than between normal comparison women and men. Effect sizes (32) were estimated for sex differences within group and group differences within sex. Effect sizes represent the mean difference, in standard deviation units, between groups of interest. Wilks's lambda was used as the multivariate test for three effects: sex, group, and their interaction. We did not expect a significant overall interaction effect, since not all cognitive functions were hypothesized to differ by sex. Given the previous literature, we hypothesized that the interaction would be significant for attention, language, and executive functions in particular.
RESULTS
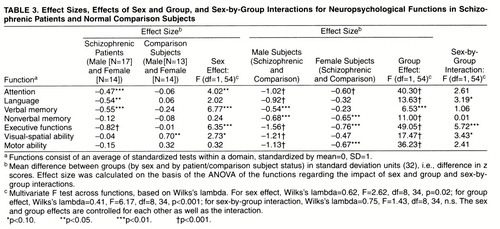
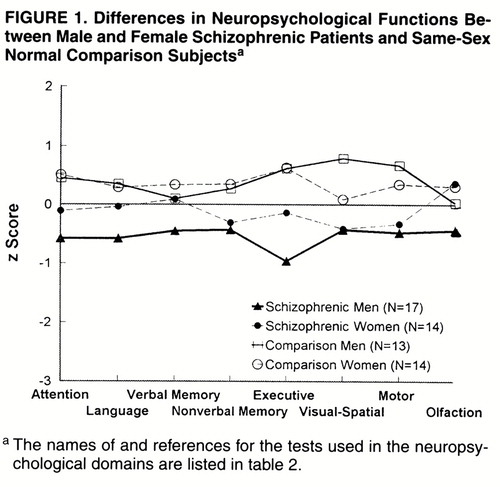
Table 3 presents the multivariate test results for the overall effects of group and sex and their interaction. In addition, effect sizes were calculated, on the basis of univariate analyses of variance of the functions, for the effects of sex within group (table 3, columns 2 and 3) and group within sex (columns 5 and 6). Finally, univariate F tests are shown for the effects of sex (column 4), group (column 7), and their interaction (column 8) across functions. Figure 1 shows the standardized scores for each neuropsychological domain for male and female patients with schizophrenia and male and female comparison subjects.
Table 3 shows that the multivariate test (Wilks’s lambda) for an overall effect of group across all neuropsychological functions and the overall effect of sex were significant. The overall test of the interaction of group by sex was not significant.
When neuropsychological functions were compared by group within sex (table 3, columns 2–4, and figure 1), the results showed that the male schizophrenic patients performed significantly more poorly across all neuropsychological domains than the male comparison subjects. The female schizophrenic patients performed significantly more poorly than the female comparison subjects on attention, nonverbal memory, executive functions, and motor function. However, they were not significantly different from the female comparison subjects on language, verbal memory, and visual-spatial ability. In general, the effect sizes for the men, patients versus comparison subjects, were two to three times larger than the effect sizes among the women, except for nonverbal memory (table 3).
The sex effects within group showed that female and male normal comparison subjects did not significantly differ in performance on most of the neuropsychological domains, except visual-spatial ability (table 3, column 3); the male comparison subjects performed significantly better on visual-spatial ability and on motor speed than the females. In contrast, the male patients performed significantly more poorly than the female patients on attention, language, verbal memory, and executive functions (table 3, column 2). The male patients did not significantly differ from the female patients on nonverbal memory, visual-spatial ability, and motor speed because of poorer functioning among the men. The difference in effect sizes for men and women within groups was substantial (two- to eightfold), except for nonverbal memory (table 3, columns 2 and 3), even though the interaction of sex and group was not significant.
DISCUSSION
As is typical in schizophrenia, the patients showed significant decrements in neuropsychological performance across many functional domains. Although the overall interaction of sex by group was not significant, the effect sizes suggest that there are differences in deficits for male and female patients compared with normal subjects. Male patients were significantly impaired across all domains of functioning in comparison with male normal subjects. Female patients were significantly worse than female normal subjects on attention, executive functions, visual memory, and motor functions, with relatively preserved function in language, verbal memory, and visual-spatial ability. Further, male patients performed significantly worse than female patients on tasks of attention, verbal memory, and executive functions, three domains thought to be central in the neuropsychology of schizophrenia (21, 26, 27, 31, 33-35).
The results consistently showed medium to large differences in effect size between patients and comparison subjects within sex (table 3, columns 5 and 6) and between the sexes within groups (table 3, columns 2 and 3), suggesting that there is widespread dysfunction among male patients and greater preservation of functioning among female patients. Even for functions in which female patients showed deficits (i.e., executive functions or attention), the effect size for the deficits among male patients was twofold greater than that for female patients (table 3, columns 5 and 6).
Other studies examining neuropsychological functioning in schizophrenia have shown diffuse deficits among first-episode and chronic schizophrenic patients (13, 26, 33) and selective impairment in verbal memory (31). One possibility suggested by our work is that these findings apply primarily to male schizophrenic patients. In fact, the groups in these other studies (13, 26, 31, 33) included a majority of men (73%, 62%–87%, and 74%, respectively). Thus, it is possible that the effects were mainly accounted for by the men.
However, two recent studies, one of which (15) was specifically designed to study sex differences in neuropsychological functioning, reported no significant sex differences (11) or greater impairment in women (15). What could account for the inconsistency between our results and theirs? One possible methodological artifact may be that neither of the other studies matched schizophrenic and normal subjects within sex. This is important, since the variability of cognitive functions within sex in normal subjects is greater than the differences between the sexes (20). Thus, since sex effect sizes are small, it is important to control for as much extraneous variation as possible within sex-specific groups, such as using carefully matched normal comparison subjects within sex. Otherwise, the significance of the sex effects may not be detected, even with large study groups (11, 15).
Second, although one study (11) involved a large study group from three sources, it did not include normal comparison subjects. The absence of sex effects could potentially provide some insights into sex differences in particular cognitive functions in which normal subjects have been found to differ (20).
A third possibility is that there were sex differences in premorbid capacities among our subjects, which resulted in sex differences in cognitive performance after illness. In our schizophrenic group, the men had significantly lower reading scores and IQs than the normal comparison men, perhaps indicating that they were drawn from a population with lower intellectual capacity and thus exhibited lower cognitive performance as adults than the comparison men or schizophrenic women. This would suggest that our findings were due to sampling bias rather than to the effects of illness. We tested this by conducting additional analyses of covariance of the functions with group, sex, and their interaction, and statistically controlling for reading ability. We controlled for reading rather than IQ, since we and others have argued that IQ is more susceptible to illness effects than single-word oral reading (36, 37). Controlling for IQ would therefore “overadjust” by controlling for intellectual capacity and illness effects (36); thus, reading can be considered a better matching variable (36, 37). The results remained the same even when we controlled for reading. That is, the sex effects were still significant for language (when reading was dropped from the definition of the domain, since reading was used as a covariate), attention, verbal memory, and executive functions. Controlling for reading is a conservative test of our results, since it is possible that premorbid reading ability may also be subtly susceptible to illness effects early in the developmental course of the illness and more likely to be expressed by males (6, 7).
Our sample of women was not atypical of a large outpatient service network serving an extensive urban catchment area, since we systematically ascertained subjects by using a strict stratified-by-sex, random ascertainment procedure. The women were long-term, clinically stable outpatients, carefully matched to comparison subjects within sex. We did not initially design a study of sex differences that would include chronic patients, since we and others (16, 17) have argued that among chronic subjects, sex effects would be attenuated. Finding significant sex effects even among this chronic group of patients is meaningful, since the female patients had somewhat longer, although not significantly different, illnesses and more hospitalizations than the men and still functioned better in selective domains.
Given that the size of sex-specific groups was relatively small, the findings in this study must be replicated in larger groups with the use of similar methodological designs. However, we do not believe that group size accounted for the lack of significant differences in some functions among the women, since we found a differential effect among the men, and the male group sizes were similar to the females’. Further, we had excellent reliabilities on the functions, thus increasing the power to find significant effects even in small groups. Finally, although we had a small study group and thus low statistical power to detect significant interactions, differences in the effect sizes of the functions for sex within group and group within sex were substantial (two- to eightfold) and consistent. If we were to be conservative in our interpretation of the results because of the limitations of group size, then the results would still show that the differences in functions between female patients and female comparison subjects were much smaller than the differences between male patients and their comparison subjects, indicating that male patients are more impaired on specific functions than female patients. The lower, twofold difference in effect size for verbal memory may reflect sex differences in deficits in encoding (i.e., an executive function) or in verbal ability in general rather than memory deficits per se. Thus, it is important to investigate further the aspects of each overall domain in which men and women with schizophrenia differ.
Significant neuropsychological sex differences have been reported by other investigators as well. In fact, Hoff and colleagues (13) found a significant overall sex effect, although there was no significant result for any particular domain upon inspection of the univariate analysis. This may have been due to the small number of female patients compared with males (approximately 1:3). Further, these researchers controlled for education, which may have attenuated illness effects. Females, in general, in their study performed relatively better on verbal memory than males, and males, in general, performed better on visual-spatial ability, consistent with our results for female patients and male comparison subjects, respectively. Our findings are also consistent with previous studies of schizophrenic patients (7-9) and studies of high-risk offspring of schizophrenic parents (3–5), 21). These studies reported significant sex differences in neuropsychological performance, with males showing more deficits than females in particular areas, such as attention, language, and executive functions.
Our findings suggest that female schizophrenic patients may be less vulnerable to deficits involving verbal processing (e.g., language, verbal abstraction, and verbal memory) and visual-spatial ability. We believe this to be associated, in part, with normal sexual brain dimorphism (18, 20, 38) and normal sex differences in cognition (20) and brain physiology (39). Studies of cognition in normal subjects have reported that, on average, women perform better on tasks of verbal fluency and verbal memory, and men perform better on particular kinds of visual-spatial and mathematical reasoning tasks (20). Such normal cognitive differences may be associated with sex differences in brain structures. Preliminary magnetic resonance imaging (MRI) studies of small groups of normal subjects have reported that with adjustment for total brain volume, women had a larger hippocampus (40), dorsolateral prefrontal cortex, and superior temporal gyrus (40), 41) than men. Some MRI studies of schizophrenic patients have also indicated sex differences in brain volumes in areas such as the thalamus and ventricular system (42). The brain areas just mentioned contribute to long-term and working memory, sustained attention, abstract verbal reasoning, and language production. If these recent MRI findings are replicated, then women with schizophrenia may be less vulnerable to consequences from particular prenatal/perinatal or genetically determined insults that may differentially affect these areas of the brain in schizophrenia. Replication of our findings in this study is important, since the area of sex differences in neuropsychological functioning remains controversial.
Received June 2, 1997; revisions received Dec. 10, 1997, and March 23, 1998; accepted April 9, 1998. From the Harvard Institute of Psychiatric Epidemiology and Genetics, Laboratory of Neuropsychology, Department of Psychiatry, Harvard Medical School at Massachusetts Mental Health Center; the Department of Psychiatry, Harvard Medical School at Brockton VA Medical Center; the Department of Epidemiology, Harvard School of Public Health, Boston; and the Cognitive Neurology and Alzheimer’s Disease Center, Departments of Neurology and Psychiatry, Northwestern University Medical School, Chicago.. Address reprint requests to Dr. Goldstein, Massachusetts Mental Health Center, 74 Fenwood Rd., Boston, MA 02115; [email protected] (e-mail). Supported initially by a Young Investigator Award to Dr. Goldstein from the National Alliance for Research on Schizophrenia and Depression and later by NIMH Scientist Development Award MH-00976 to Dr. Goldstein and NIMH Merit Award MH-43518 to Dr. Tsuang.
 |
 |
 |
1. Lewine RR: Gender and schizophrenia, in Handbook of Schizophrenia: Nosology, Epidemiology, and Genetics of Schizophrenia. Edited by Tsuang MT, Simpson JC. Amsterdam, Elsevier, 1988, pp 379–397Google Scholar
2. Goldstein JM: The impact of gender on understanding the epidemiology of schizophrenia, in Gender and Psychopathology. Edited by Seeman MV. Washington, DC, American Psychiatric Press, 1995, pp 159–199Google Scholar
3. Erlenmeyer-Kimling L, Kestenbaum C, Bird H, Hilldoff U: Assessment of the New York high-risk project subjects in sample A who are now clinical deviants, in Children at Risk for Schizophrenia: A Longitudinal Perspective. Edited by Watt N, Anthony EJ, Wynne LC, Rolf JE. Cambridge, England, Cambridge University Press, 1984, pp 227–239Google Scholar
4. Marcus J, Hans SL, Nagler S, Auerbach JG, Mirsky AF, Aubrey A: Review of the NIMH Israeli kibbutz-city study and the Jerusalem infant development study. Schizophr Bull 1987; 13:425–438Crossref, Medline, Google Scholar
5. Mednick SA, Schulsinger F, Teasdale TW, Schulsinger H, Venables PH, Rock DR: Schizophrenia in high-risk children: sex differences in predisposing factors, in Cognitive Defects in the Development of Mental Illness. Edited by Serban G. New York, Brunner/Mazel, 1978, pp 169–197Google Scholar
6. Aylward E, Walker E, Bettes B: Intelligence in schizophrenia. Schizophr Bull 1984; 10:430–459Crossref, Medline, Google Scholar
7. Goldstein JM, Seidman LJ, Santangelo S, Knapp P, Tsuang MT: Are schizophrenic men at higher risk for developmental deficits than schizophrenic women? implications for adult neuropsychological functions. J Psychiatr Res 1994; 28:483–498Crossref, Medline, Google Scholar
8. Haas GL, Sweeney JA, Hien DA, Goldman D, Deck M: Gender differences in schizophrenia (abstract). Schizophr Res 1991; 4:277Crossref, Google Scholar
9. Kopala L, Clark C: Implications of olfactory agnosia for understanding sex differences in schizophrenia. Schizophr Bull 1990; 16:255–261Crossref, Medline, Google Scholar
10. Seidman LJ, Goldstein JM, Goodman JM, Koren D, Turner WM, Faraone SV, Tsuang MT: Sex differences in olfactory identification and Wisconsin Card Sorting performance in schizophrenia: relationship to attention and verbal ability. Biol Psychiatry 1997; 42:104–115Crossref, Medline, Google Scholar
11. Goldberg TE, Gold JM, Torrey EF, Weinberger DR: Lack of sex differences in the neuropsychological performance of patients with schizophrenia. Am J Psychiatry 1995; 152:883–888Link, Google Scholar
12. Andia AM, Zisook S, Heaton RK, Hesselink J, Jernigan T, Kuck J, Moranville J, Braff DL: Gender differences in schizophrenia. J Nerv Ment Dis 1995; 183:522–528Crossref, Medline, Google Scholar
13. Hoff AL, Riordan H, O’Donnell D, Stritzke P, Neale C, Boccio A, Anand AK, DeLisi LE: Anomalous lateral sulcus asymmetry and cognitive function in first-episode schizophrenia. Schizophr Bull 1992; 18:257–272Crossref, Medline, Google Scholar
14. Perlick D, Mattis S, Stastny P, Teresi J: Gender differences in cognition in schizophrenia. Schizophr Res 1992; 8:69–73Crossref, Medline, Google Scholar
15. Lewine RRJ, Walker EF, Shurett R, Caudle J, Haden C: Sex differences in neuropsychological functioning among schizophrenic patients. Am J Psychiatry 1996; 153:1178–1184Link, Google Scholar
16. Walker EF, Lewine RRJ: Sampling biases in studies of gender and schizophrenia. Schizophr Bull 1993; 19:1–7Crossref, Medline, Google Scholar
17. Goldstein JM: Sampling biases in studies of gender and schizophrenia: a reply. Schizophr Bull 1993; 19:9–14Crossref, Google Scholar
18. Goldstein JM: Sex and brain abnormalities in schizophrenia: fact or fiction? Harvard Rev Psychiatry 1996; 4:110–115Google Scholar
19. Sweeney JA, Haas GL, Keilp JG, Long M: Evaluation of the stability of neuropsychological functioning after acute episodes of schizophrenia: one-year follow up study. Psychiatry Res 1991; 38:63–76Crossref, Medline, Google Scholar
20. Collaer ML, Hines M: Human behavioral sex differences: a role for gonadal hormones during early development? Psychol Bull 1995; 118:55–107Google Scholar
21. Cornblatt B, Erlenmeyer-Kimling N: Global attentional deviance as a marker of risk for schizophrenia: specificity and predictive validity. J Abnorm Psychol 1985; 94:470–486Crossref, Medline, Google Scholar
22. Faraone SV, Seidman LJ, Kremen WS, Pepple JR, Lyons MJ, Tsuang MT: Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: a diagnostic efficiency analysis. J Abnorm Psychol 1995; 104:286–304Crossref, Medline, Google Scholar
23. Jastak S, Wilkinson GS: Wide Range Achievement Test—Revised. Wilmington, Del, Jastak Associates, 1984Google Scholar
24. Lezak M: Neuropsychological Assessment, 3rd ed. New York, Oxford University Press, 1995Google Scholar
25. Spitzer RL, Endicott J: Schedule for Affective Disorders and Schizophrenia (SADS), 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1978Google Scholar
26. Heaton R, Paulsen J, McAdams LA, Kuck J, Zisook S, Braff D, Harris J, Jeste D: Neuropsychological deficits in schizophrenics: relationship to age, chronicity, and dementia. Arch Gen Psychiatry 1994; 51:469–476Crossref, Medline, Google Scholar
27. Seidman LJ, Cassens GP, Kremen WS, Pepple JR: Neuropsychology of schizophrenia, in Clinical Syndromes in Adult Neuropsychology: The Practitioner’s Handbook. Edited by White RF. Amsterdam, Elsevier, 1992, pp 381–449Google Scholar
28. Caplan D, Hildebrant N: Modified Battery for the Assessment of Syntactic Comprehension. Boston, Massachusetts General Hospital, Department of Neurology, Neuropsychology Laboratory, 1992Google Scholar
29. Katz WF, Curtiss S, Tallal P: Rapid automatized naming and gesture by normal and language-impaired children. Brain Lang 1992; 43:623–641Crossref, Medline, Google Scholar
30. Roeltgen DP: Phonological error analysis, development and empirical evaluation. Brain Lang 1992; 43:190–229Crossref, Medline, Google Scholar
31. Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester B, Strafiniak P: Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry 1991; 48:618–624Crossref, Medline, Google Scholar
32. Cohen J: Statistical Power Analysis for the Behavioral Sciences, revised ed. New York, Academic Press, 1977Google Scholar
33. Braff D, Heaton R, Kuck J, Cullum M, Moranville J, Grant I, Zissok S: The generalized pattern of neuropsychological deficits in outpatients with chronic schizophrenia with heterogeneous Wisconsin Card Sorting Test results. Arch Gen Psychiatry 1991; 48:891–898Crossref, Medline, Google Scholar
34. Bilder RM, Mukherhjee S, Rieder RO, Pandurangi AK: Symptomatic and neuropsychological components of defect states. Schizophr Bull 1985; 11:409–419Crossref, Medline, Google Scholar
35. Weinberger DR, Berman KF, Suddath R, Torrey EF: Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry 1992; 149:890–897Link, Google Scholar
36. Kremen WS, Seidman LJ, Faraone SV, Pepple JR, Lyons MJ, Tsuang MT: The “3R’s” and neuropsychological function in schizophrenia: an empirical test of the matching fallacy. Neuropsychology 1996; 10:22–31Crossref, Google Scholar
37. Dalby JT, Williams R: Preserved reading and spelling ability in psychotic disorder. Psychol Med 1986; 16:171–175Crossref, Medline, Google Scholar
38. Lewine RRJ, Seeman MV: Gender, brain and schizophrenia: anatomy of differences/differences of anatomy, in Gender and Psychopathology. Edited by Seeman MV. Washington, DC, American Psychiatric Press, 1995, pp 131–158Google Scholar
39. Gur RE, Gur RC: Gender differences in regional cerebral blood flow. Schizophr Bull 1990; 16:247–254Crossref, Medline, Google Scholar
40. Filipek PA, Richelme C, Kennedy DN, Caviness VS: The young adult human brain: an MRI-based morphometric study. Cereb Cortex 1994; 267:344–360Crossref, Google Scholar
41. Schlaepfer TE, Harris GJ, Tien AY, Peng L, Lee S, Pearlson GD: Structural differences in the cerebral cortex of healthy female and male subjects: a magnetic resonance imaging study. Psychiatr Res: Neuroimaging 1995; 61:129–135Crossref, Medline, Google Scholar
42. Andreasen NC, Ehrhardt JC, Swayze VW II, Alliger RJ, Yuh WT, Cohen G, Ziebell S: Magnetic resonance imaging of the brain in schizophrenia: the pathophysiologic significance of structural abnormalities. Arch Gen Psychiatry 1990; 47:35–44Crossref, Medline, Google Scholar



