Toward a Better Understanding of the Mechanisms and Pathophysiology of Anhedonia: Are We Ready for Translation?
Abstract
Anhedonia—the loss of pleasure or lack of reactivity to pleasurable stimuli—remains a formidable treatment challenge across neuropsychiatric disorders. In major depressive disorder, anhedonia has been linked to poor disease course, worse response to psychological, pharmacological, and neurostimulation treatments, and increased suicide risk. Moreover, although some neural abnormalities linked to anhedonia normalize after successful treatment, several persist—for example, blunted activation of the ventral striatum to reward-related cues and reduced functional connectivity involving the ventral striatum. Critically, some of these abnormalities have also been identified in unaffected, never-depressed children of parents with major depressive disorder and have been found to prospectively predict the first onset of major depression. Thus, neural abnormalities linked to anhedonia may be promising targets for prevention. Despite increased appreciation of the clinical importance of anhedonia and its underlying neural mechanisms, important gaps remain. In this overview, the author first summarizes the extant knowledge about the pathophysiology of anhedonia, which may provide a road map toward novel treatment and prevention strategies, and then highlights several priorities to facilitate clinically meaningful breakthroughs. These include a need for 1) appropriately controlled clinical trials, especially those embracing an experimental therapeutics approach to probe target engagement; 2) novel preclinical models relevant to anhedonia, with stronger translational value; and 3) clinical scales that incorporate neuroscientific advances in our understanding of anhedonia. The author concludes by highlighting important future directions, emphasizing the need for an integrated, collaborative, cross-species, and multilevel approach to tackling anhedonic phenotypes.
Victoria, a middle-aged professional and a dedicated long-distance runner since young adulthood, has totally lost interest in running. When asked by her partner about this change, she explains that what used to be her favorite hobby does not provide any joy and, in fact, has become a burden. Concerned by her mounting sleep difficulties (mostly early awakening) and weight loss, Victoria reconnects with the psychiatrist who successfully treated her first major depressive episode a decade ago. At the intake session, Victoria discloses multiple ongoing stressors, including her mother’s progressing dementia, difficulties at work, and growing financial debt. Unfortunately, the selective serotonin reuptake inhibitor (SSRI) that had previously led to remission does not work this time, and augmentation strategies bring little benefit. After 9 months of unabating symptoms, Victoria’s mental state worsens, and she is hospitalized after attempting suicide.
With the lifting of COVID-related restrictions, Antonne’s parents had hoped that he would reconnect with his friends and be eager to return to in-person classes. Throughout the pandemic, Antonne, a timid 14-year-old boy, has been isolating himself from his friends and falling behind in school. A reserved child from early age who often needed encouragement to socialize, Antonne has spent an increasing amount of time playing video games in his room. Alarmed by his apathic demeanor and failing grades, his parents reach out to their pediatrician, who is unsure how best to help. When prompted about these developments, Antonne describes no motivation in initiating social activities. He acknowledges that he still enjoys spending time with one soccer teammate who is also a gamer, but most of the time he does not feel like doing anything.
Victoria and Antonne are fictional, but they illustrate a key point. Specifically, although they both exhibit anhedonic behaviors, those behaviors are different and are likely associated with distinct pathophysiologies (1–3), which may respond to different treatment strategies. Victoria exhibits anhedonia as it is classically understood: she shows loss of pleasure, which may have been triggered by chronic, uncontrollable stressors. Antonne, on the other hand, can experience pleasure but has difficulty initiating behavior in pursuit of some pleasurable experiences. Such difficulty emerged early in development, apparently without any objective external trigger.
As exemplified by these fictional cases, anhedonia is complex and its treatment remains a critical, unmet need. One of the two cardinal symptoms of major depressive disorder (MDD), anhedonia is reported by 37%–72% of individuals with MDD (4–6). In MDD, anhedonia has been linked to chronic disease course (7), worse outcome (8), poor response to pharmacological (9), psychological (10), and neurostimulation treatments (11), and increased risk of completed suicide (12). Critically, and highlighting the clinical relevance of anhedonia, individuals with MDD conceptualize remission as the restoration of positive affect, rather than the alleviation of depressive symptoms (13, 14). Why has the treatment of anhedonia remained such an unmet need, despite decades of preclinical and clinical research?
In this overview, I attempt to answer this question by identifying three areas that require attention. These areas pertain to the need for 1) appropriately controlled clinical trials, especially those embracing an experimental therapeutics approach to probe target engagement; 2) novel preclinical models relevant to anhedonia with stronger translational value; and 3) clinical scales that incorporate neuroscientific advances in our understanding of anhedonia. Before addressing these points, I summarize the extant knowledge about the pathophysiology of anhedonia, which may provide a road map toward filling these knowledge gaps.
The Neurobiology of Anhedonia
Decades of neuroscientific research in experimental animals and humans has emphasized the role of the mesocorticolimbic circuit in different subdomains of reward processing, including reward responsiveness (e.g., reward anticipation, reward consumption), reward learning (e.g., positive reward prediction errors, which encode that an outcome is better than expected and is critically implicated in reward learning), and reward valuation (e.g., deciding to exert effort to pursue a possible reward) (15–17). These findings have contributed to an understanding of anhedonia as being composed of discrete subcomponents (2, 3, 18, 19) (Figure 1), which has also been captured by the Research Domain Criteria (RDoC) initiative from the National Institute of Mental Health (NIMH) (https://www.nimh.nih.gov/research/research-funded-by-nimh/rdoc/constructs/positive-valence-systems).
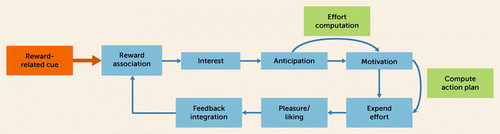
FIGURE 1. Subprocesses and subdomains implicated in reward processinga
a Specific anhedonic behaviors may be chiefly associated with disruption in one or several of these subdomains. (Modified after references 18, 19.)
Originating from the ventral tegmental area (VTA), the dopaminergic (DA) mesocorticolimbic pathway projects to the ventral (nucleus accumbens [NAc]) and dorsal (caudate, putamen) striatum, and then runs to the orbitofrontal cortex (OFC), more dorsal aspects of the prefrontal cortex (PFC), and various subregions of the anterior cingulate cortex (ACC) (3, 20, 21). The main regions of the reward system (e.g., VTA → nucleus accumbens) are anatomically connected by the medial forebrain bundle (20, 22)—a white matter tract that has been strongly implicated in the experience of pleasure and motivated behavior (23, 24). Although a detailed summary of neurobiological mechanisms implicated in anhedonic behaviors is beyond the scope of this overview, abundant preclinical data highlight that anhedonic phenotypes in experimental animals, which are often induced by exposure to chronic, uncontrollable, and inescapable stressors, are linked to blunted DA transmission in the ventral striatum, with potentiated DA transmission in the VTA and medial PFC (or functionally homologous regions in rodents) (3). In particular, rodent models relevant to depression have linked anhedonia and reduced goal-directed behaviors to increased phasic bursting and excitability of VTA DA neurons, which characterized only vulnerable animals and could be reversed by chronic antidepressant treatment (21, 25–27). Similarly, optogenetic activation of VTA DA neurons during chronic social defeat stress exacerbated depressive phenotypes (28, 29), whereas optogenetic inhibition of VTA-NAc DA neurons reversed anhedonia elicited by chronic social defeat (30). Collectively, these data demonstrate that normative hedonic behaviors are supported by an adaptive and flexible DA-mediated interplay among the VTA, the striatum (especially the ventral striatum), and the PFC.
The Neural Correlates of Anhedonia
The past decade has seen substantial progress with respect to neural mechanisms that underlie anhedonia and reward processing dysfunction in MDD. Several recent reviews focusing on functional neuroimaging have highlighted frontostriatal abnormalities in MDD during different reward processes, including incentive motivation (reward anticipation), valuation (reward consumption), and reward learning (31–34). Specifically, in depression, reduced dorsal (e.g., caudate, putamen) and ventral (NAc) activation and reduced perigenual anterior cingulate cortex (pgACC) activation have emerged in tasks probing reward consumption (35, 36), reward anticipation (3, 35, 37, 38), and reward learning (39–42). Reduced ventral striatal activation to reward receipt was associated with anhedonic symptoms (38), whereas larger reward prediction error signals (which captured the difference between expected and actual reward outcome) in the ventral striatum predicted reduced anhedonia 6 months later (43). In addition, during reward consumption or anticipation, MDD has been linked to reduced activation in the dorsal anterior cingulate cortex (dACC) (36) as well as the central and medial OFC (44), with blunted reward-related central OFC (areas 11 and 13) activation correlating with more anhedonic symptoms in adolescents with MDD (44). In tasks harnessing computational modeling to estimate expected value and reward prediction errors during Pavlovian, instrumental, or reversal learning tasks, MDD has been linked to reduced reward prediction error in the ventral and dorsal striatum (39–41; but see also 45, 46), pgACC (47), dACC (42), and medial OFC (46). Similarly, in the decision phase of an instrumental reinforcement learning task, MDD was characterized by reduced reward value encoding in the pgACC but higher subgenual anterior cingulate cortex (sgACC) activation (48). Notably, reward-related blunting in these regions has been accompanied by hyperactivation in the medial prefrontal cortex (mPFC), ventromedial prefrontal cortex (vmPFC; including the sgACC), and dorsolateral prefrontal cortex (dlPFC) (33, 38, 49). Findings highlighting disruption in key hubs of the brain reward system have been complemented by reports that functional connectivity between the caudal vmPFC and various reward regions (NAc, VTA, OFC) while listening to pleasant music was negatively correlated with anhedonia (50).
Several recent findings deserve special emphasis. First, frontostriatal abnormalities in MDD emerge early in the disease course, as demonstrated by reduced striatal and pgACC activation—but potentiated PFC (specifically mPFC, dlPFC) activation—during reward consumption in children and adolescents with MDD (33, 51, 52), with blunted pgACC activation during reward consumption correlating with higher anhedonia (51). Across studies, mPFC and dlPFC overrecruitment during reward processing was interpreted as pointing to possible overcompensation for reduced striatal responses to rewards (33, 52, 53). Critically, in a large sample of youths (N=1,576; mean age, ∼14 years), reduced ventral striatal activation during reward anticipation was associated with anhedonia and predicted transition to depression 2 years later among previously healthy youths (54; see also 55). Along similar lines, blunted striatal activation during reward anticipation predicted greater increases in adolescent depressive symptoms over 2 years (55).
Second, while some abnormalities, such as blunted reward-related striatal activation (56) and reduced reward prediction error signals in the ventral striatum (57), normalize after remission, others persist and point to possible trait-like abnormalities. Abnormalities that do not normalize include blunted OFC activation to rewards (58), reduced ability to sustain ventral striatal activation to positive cues (56), and greater reward prediction error in the VTA (42). Along similar lines, never-depressed children of parents with MDD showed blunted striatal activation during reward anticipation (59) and consumption (60) as well as reduced NAc activation in response to happy faces (61). Altogether, these studies suggest that reward-related dysfunction can precede the initial onset of MDD and thus represents a vulnerability risk.
Third, some of these abnormalities show acute treatment-related changes. For example, a single ketamine infusion was associated with normalization of sgACC hyperactivation to positive incentives (which was associated with more anhedonia) as well as dACC hypometabolism (62, 63). Similarly, administration of a single low dose of amisulpride—hypothesized to increase DA transmission via autoreceptor blockade—normalized frontostriatal abnormalities in unmedicated individuals with MDD. Specifically, relative to placebo, 50 mg of amisulpride increased ventral and dorsal striatal hypoactivation to reward-related cues and decreased lateral OFC and vmPFC hyperactivation in MDD (64, 65).
Fourth, evidence of frontostriatal abnormalities has emerged not only in reward tasks but also during resting (i.e., task-free) states. In the Adolescent Brain Cognitive Development (ABCD) study, among children ages 9–10 years from unreferred community samples (N=2,455), decreased resting-state functional connectivity (rsFC) between the ventral striatum and the cingulo-opercular network was observed in children with anhedonia but not those with low mood (66), which highlights specificity in relation to anhedonia. In a notable study involving a large community-based sample of 9-year-old children (N=637), rsFC between the ventral striatum and other key reward hubs (vmPFC, dACC, VTA) predicted new onset of depressive disorders 3 years later, but not attention deficit hyperactivity disorder, anxiety disorders, or substance use disorders (53). Thus, disrupted frontostriatal coupling not only characterizes current MDD but also represents a vulnerability marker for MDD. The same research group recently extended these findings (67, in this issue) by analyzing data from the IMAGEN Consortium (N=305, ages 13–15 years at baseline) and examining intrinsic FC between the ventral striatum and the rest of the brain reward network. Several interesting findings emerged. First, in logistic regression models, right ventral striatal intrinsic FC at baseline (age 14) was positively associated with depressive disorders (but not anxiety disorders) at age 14. Similarly, left ventral striatal intrinsic FC at baseline correlated positively with anhedonia (but not low mood) at age 14. Second, structural equation modeling showed that left ventral striatum FC predicted anhedonia 2 years later, whereas right ventral striatum FC predicted anhedonia 4 years later. Based on these findings, the authors speculate that excessive FC between the ventral striatum and the rest of the reward network may reflect a lack of flexibility to respond to reward-related cues in the environment. Future studies incorporating ecological momentary assessments probing flexible responding to potential rewards in daily activities would be well positioned to evaluate this interesting interpretation.
Finally, evidence of disrupted mesocorticolimbic pathways in anhedonia has also emerged from structural studies. For example, studies probing the integrity of the medial forebrain bundle have found that anhedonia in MDD is associated with decreases in tract volume and the number of tracts in the left superolateral branch of the medial forebrain bundle (68, 69), which projects through the anterior limb of the internal capsule and connects the VTA to the PFC. Notably, severity of anhedonia was also associated with increased structural connectivity between the VTA and the medial PFC (69), a finding interpreted as reflecting a possible compensatory mechanism in severe anhedonia. These structural connectivity findings have been complemented by reports of reduced striatal (in particular, dorsal) and OFC volume correlating with anhedonia (35, 70) or polygenic risk for anhedonia (71).
Interim Summary
Across tasks probing different subdomains of reward processing but also during task-free (resting) states, MDD has been linked to abnormal activation within and functional connectivity across nodes of the brain reward pathways (Figure 2). Although some inconsistencies exist, MDD is generally characterized by reduced activation to reward-related cues within ventral and dorsal striatal regions, perigenual and dorsal ACC regions, and central and medial OFC. These hypoactivations contrast with overrecruitment of medial frontal pole (BA10), vmPFC (including sgACC), and dlPFC regions in response to reward-related cues, which has been interpreted as reflecting a compensatory mechanism owing to reduced reward-related striatal activation. Highlighting the clinical importance of these findings, some of these markers were cross-sectionally and prospectively related to anhedonia and predicted first onset of MDD. Complementing these findings, certain abnormalities—specifically, reduced reward-related striatal activation and frontostriatal resting-state functional connectivity—were also observed in unaffected, never-depressed children of parents with MDD, indicating that they may represent vulnerability markers. A key question for future research is whether such vulnerability markers might be targeted for prevention.
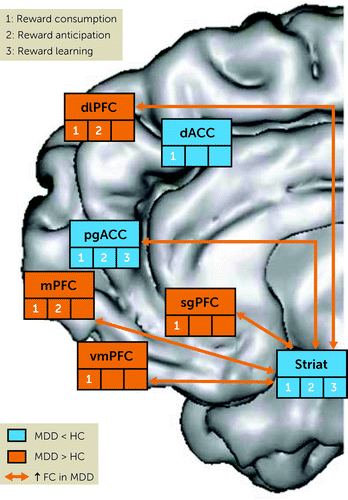
FIGURE 2. Summary of abnormalities emerging from functional MRI in individuals with major depressive disorder (MDD) or at risk for MDD using tasks probing reward-related processes or evaluating resting-state functional connectivity within the brain reward systema
aRegions highlighted in orange and blue show higher activation and lower activation, respectively, in MDD samples than in healthy control samples (HC). Orange arrows denote higher functional connectivity (FC) in MDD samples than healthy control samples. dACC=dorsal anterior cingulate cortex; dlPFC=dorsolateral prefrontal cortex; mPFC=medial prefrontal cortex; pgACC=perigenual anterior cingulate cortex; sgACC=subgenual anterior cingulate cortex; Striat=striatum; vmPFC=ventromedial prefrontal cortex. (Modified after reference 34.)
The Need for Placebo-Controlled Clinical Trials With an Experimental Therapeutics Approach
As mentioned above, the treatment of anhedonia in MDD remains a formidable challenge. These challenges are present in both first-line psychological (e.g., cognitive-behavior therapy) and pharmacological treatments. In light of these unmet needs, several targeted psychological interventions have been developed in recent years, including behavioral activation treatment (e.g., 72) and positive affect treatment (73). Positive affect treatment, in particular, was specifically designed to target deficits in reward sensitivity, and it includes modules involving planning for pleasurable activities (reward approach-motivation), reinforcing connections between behaviors and mood effects (reward learning), and “in-the-moment” savoring (reward consumption). Initial results are promising (73), and future studies should evaluate whether this intervention normalizes neural abnormalities associated with anhedonia. (For promising evidence that modulation of reward-related neural circuitry may underlie reduction in anhedonia with behavioral activation, see reference 74.)
With respect to pharmacological treatments, anhedonia has received surprisingly modest attention vis-à-vis rigorous placebo-controlled trials. For example, in a recent qualitative review, Cao and colleagues (75) summarized results from 17 studies that evaluated the efficacy of different pharmacotherapies for anhedonia. These strategies included melatonergic antidepressants (agomelatine; eight studies), SSRIs (escitalopram, sertraline, fluoxetine; four studies), serotonin-noradrenaline reuptake inhibitors (extended-release venlafaxine, extended-release levomilnacipran; two studies), norepinephrine-dopamine reuptake inhibitors (bupropion; one study), serotonin-norepinephrine-dopamine reuptake inhibitors (amitifadine; one study), reversible inhibitors of monoamine oxidase A (moclobemide; one study), tricyclic antidepressants (clomipramine; one study), glutamatergic agents (ketamine and riluzole; one study), stimulants (methylphenidate; one study), and psychedelics (psilocybin; one study). Of note, nine of these 17 studies were open-label, and 10 included 30 or fewer patients in each treatment arm. Agomelatine showed some promise in alleviating anhedonia, but none of the eight studies published included a placebo-control arm. Although the low number of studies and high level of heterogeneity prevented a quantitative meta-analysis, Cao and colleagues concluded that melatonergic antidepressants (agomelatine), monoaminergic antidepressants, glutamatergic agents, psychedelics, and stimulants have shown initial promise in addressing anhedonia.
Recently, three relatively novel mechanisms have attracted substantial interest as promising antianhedonic treatments: kappa opioid receptor (KOR) antagonism, potassium channel (KCNQ) modulation, and NMDA receptor antagonism.
Kappa Opioid Receptor Antagonists
KOR antagonism has been proposed as a possible treatment for anhedonia based on robust preclinical data implicating these receptors in modulating reward processing and stress regulation (76, 77). Specifically, preclinical studies had previously shown that stressors trigger release of dynorphin, which binds to KOR receptors and inhibits DA release in the NAc via ventral tegmental area neurons (77–81). KOR antagonists have been hypothesized to exert antianhedonic effects by blocking CREB-mediated upregulation of dynorphin function, which in turn normalizes the mesolimbic DA system (81). Consistent with this hypothesis, in rodents KOR antagonists have shown antidepressant effects (e.g., 82–84); moreover, when delivered in the NAc, KOR antagonists led to a 175% increase in DA release in this region (85). In a transdiagnostic sample of 89 individuals with MDD, bipolar disorder, or anxiety disorders with some level of anhedonia, a KOR antagonist (aticaprant, formerly JNJ-67953964) was associated with greater pre- to posttreatment changes in self-reported, behavioral (performance on the probabilistic reward task [PRT]), and neural (i.e., ventral striatal activation during reward anticipation) measures of anhedonia (86, 87) relative to placebo. Moreover, on the PRT, which uses an asymmetric reinforcement schedule to objectively assess participants’ ability to learn from rewards, trial-level computational modeling indicated that the KOR antagonist affected learning rate (the ability to learn from rewards) while leaving reward sensitivity (the hedonic response) unaffected.
KCNQ Channel Modulators
A different strategy to restore DA signaling consists of affecting membrane excitability by means of modulation of membrane-bound ion channels (88). Interestingly, in mice exposed to chronic social defeat, resilient animals were characterized by upregulation of KCNQ2/3 channels in the VTA, which was associated with normative phasic firing of the VTA and protection against anhedonic behaviors. Of note, administration of ezogabine (a selective KCNQ2/3 channel opener) restored VTA homeostasis and reversed anhedonic and pro-depressive behaviors among defeated animals (89). Inspired by these preclinical findings, Tan and colleagues evaluated the effects of 10 weeks of treatment with ezogabine (900 mg/day) on self-reported, behavioral (performance on the PRT), and neural measures of anhedonia, using an open-label single-arm design (90). Participants included 18 individuals with MDD and clinically significant symptoms of anhedonia. From baseline to the 10-week endpoint, ezogabine increased reward learning on the PRT and reduced depression severity and anhedonia. Two additional, notable findings emerged. First, the decrease in anhedonic symptoms remained after controlling for depression severity. Second, improvements in self-reported anhedonia correlated with reduced rsFC between the ventral caudate and the midcingulate cortex, a region that has been implicated in responding to salient stimuli (91). These findings were recently confirmed and extended by the same group (92), who randomized 45 individuals with MDD and elevated anhedonia to 5-week treatment with ezogabine (900 mg/day; N=21) or placebo (N=24). Relative to placebo, ezogabine was associated with a larger reduction in depressive and anhedonic symptoms and an increase, short of significance, in ventral striatal activation during reward anticipation.
NMDA Antagonists (e.g., Ketamine)
Ketamine, an NMDA receptor antagonist, is a glutamatergic modulator that has attracted substantial interest due to its rapid antidepressant effects (e.g., 93). NMDA-receptor-mediated inhibition of inhibitory GABAergic interneurons in the PFC has been implicated in ketamine’s antidepressant mechanism of action (94). Preclinical studies have shown that increased synaptic glutamate release leads to potentiated AMPA receptor activation and, ultimately, synaptic plasticity via the mTOR pathway (95). This, in turn, has been hypothesized to increase DA tone in mesocorticolimbic pathways (96) and thereby exert antianhedonic effects. In line with these hypotheses, retrospective analyses combining data from multiple studies (N=203 individuals with MDD) showed that treatment with four intravenous infusions of racemic ketamine (0.50–0.75 mg/kg) over the course of 1–2 weeks was associated with significant reductions in self-reported anhedonia (97). Interestingly, reductions in anhedonia partially mediated reductions in symptoms of depression, anxiety, and suicidal ideation (for a recent review of ketamine’s antianhedonic effects, see reference 9). These findings have been complemented by reports that reduced anhedonia after ketamine infusion correlated with 1) increased glucose metabolism in the dACC (63, 98), putamen (98), and OFC (63); 2) increased rsFC within a frontostriatal network involving the PFC, OFC, and pgACC (99); and 3) normalization (i.e., reduction) of sgACC hyperactivation to positive feedback. This latter finding was particularly interesting in light of recent findings in marmosets implicating sgACC hyperactivation in anhedonic behaviors (100).
Interim Summary
Findings from these studies are exciting not only because they point to potentially novel mechanisms for tackling anhedonia but also because they show that reduced anhedonia correlates with changes in the brain reward system (9, 86, 90). Thus, by taking an experimental therapeutics approach, these studies provided important corroboration that the “target” (in this case, ventral striatal activation to reward cues) was engaged. Although an in-depth discussion of this point is beyond the scope of this overview (for elaboration, see references 101–103), the importance of an experimental therapeutics approach in order to avoid false positive findings and meaningfully interpret failed clinical trials cannot be overemphasized. Harnessing this approach early in drug discovery will be critically important to accelerate the development of better treatment for anhedonia and to avoid investing resources and time in therapeutics that do not show target engagement. I believe that this approach, coupled with the use of preclinical models with more direct translational value (see the next section), offers the strongest path forward.
The Need for Stronger Cross-Species Translational Models of Anhedonic Behaviors
As highlighted by several recent reviews (e.g., 15, 18, 104), one important limitation and potential translational “leak” is the use of vastly different approaches to assess anhedonia across species. Whereas human studies overwhelmingly rely on clinical scales, rodent (and sometimes nonhuman primate) studies rely on the sucrose preference test or other tasks involving palatable food, and in the case of rodents, intracranial self-stimulation (e.g., within the medial forebrain bundle). Despite the evolutionary conservation of brain reward pathways, and acknowledging that these approaches have contributed to important discoveries, the translational value of this work remains unclear. As a result, in recent years there has been growing interest in developing and optimizing experimental procedures that are functionally analogous—and, in some cases, identical—across species, with the hope that these platforms might accelerate translation. A comprehensive review is beyond the scope of this overview, but the interested reader is referred to several recent reviews on this topic (105–107). Here, I briefly highlight our experience using the PRT, which assesses reward learning or the ability to modulate behavior as a function of rewards. Originally developed for humans (108), the PRT has been back-translated to nonhuman primates (109), rats (110), and mice (unpublished), most recently using touchscreen technology (Figure 3A). Using tasks with identical reinforcement contingencies (e.g., 3-to-1 reward ratio for correct identification of one stimulus vs. another), identical sensory modalities (e.g., visual stimuli), and similar psychometric properties (e.g., overall accuracy between 70% and 90%), and using identical signal-detection equations to derive measures of response bias (i.e., the preference for the more frequently rewarded stimulus), our lab and others have described similar findings in humans and rodents given interventions hypothesized to increase or decrease DA signaling (e.g., pramipexole, stimulants, nicotine withdrawal) or exposed to stressors (111–116). Critically, anhedonic symptoms among depressed individuals (Figure 3B), as well as anhedonic behavior induced by early-life stress in rats (Figure 3C), were associated with similarly blunted reward learning on the PRT (117, 118). Finally, in humans, individual differences in the ability to acquire a response bias have been linked to functional, electrophysiological, and molecular markers of the mesocorticolimbic system (119, 120) (Figure 3D). Interestingly, a recent study in rats (116) showed that blunted reward learning after exposure to chronic mild stress could be reversed by systemic injection of a low dose of the D2/D3 antagonist amisulpride (hypothesized to increase DA signaling in the striatum via autoreceptor blockade) (116). Thus, transient increase of DA signaling in the striatum rescued stress-induced anhedonic behavior in rats, which parallels prior findings in MDD showing that a single low dose of amisulpride (50 mg) normalized blunted reward-related activation in the dorsal and ventral striatum (64, 65). Finally, and highlighting potential clinical utility, two recent studies in independent samples showed that more normative PRT reward learning before treatment predicted better antidepressant and antianhedonic response to bupropion (121) and pramipexole (122). Critically, in the study by Ang and colleagues (121), better reward learning predicted better response to bupropion after failing to respond to 8 weeks of treatment with the SSRI sertraline. If replicated, these findings suggest that objectively assessed anhedonic phenotypes may be more homogeneous than the syndrome of “MDD,” which may facilitate treatment selection for at least a subgroup of depressed individuals. Future studies should also evaluate whether subgrouping patients based on objective measures of anhedonia might outperform self-reported assessments of anhedonia (for initial evidence, see references 123, 124), particularly when using “first-generation” clinical anhedonia scales, which, as discussed next, do not differentiate among different subdomains of reward processing.
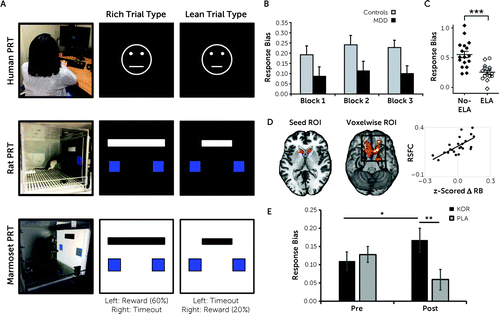
FIGURE 3. Cross-species reward learning assaya
aPanel A shows task schematics for the human (top) (108), rat (middle) (110), and marmoset (bottom) (109) probabilistic reward task (PRT) (for a review, see reference 137). Using a signal-detection approach involving an asymmetric reinforcement schedule and two difficult-to-discriminate stimuli, the PRT objectively assesses subjects’ ability to develop a response bias toward a more frequently rewarded stimulus, which is taken as a measure of reward learning. (Reprinted by permission from Springer Nature GmBH, Toward a quantification of anhedonia: unified matching law and signal detection for clinical assessment and drug development, Luc OT et al, Perspect Behav Sci, Copyright 2021.) In panel B, relative to healthy control subjects, unmedicated individuals with major depressive disorder (MDD) have significantly lower response bias (117). (Reprinted from J Psychiatr Res, vol 43, no 1, Pizzagalli et al, Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task, pp 76–87, Copyright 2008, with permission from Elsevier.) In panel C, relative to a no-stress (control) group, rats exposed to early-life adversity (ELA) (limited bedding and nesting paradigm) between postnatal days 2 and 9 were characterized by significantly blunted response bias (118). (Reprinted with permission.) In panel D, among healthy control subjects, individual differences in response bias (RB) are positively associated with resting-state functional connectivity (RSFC) between the ventral striatum and the ventromedial prefrontal cortex (as assessed with functional MRI) and negatively associated with dopamine transporter binding potential (not shown; as assessed using positron emission tomography). The latter finding suggests that low dopamine transporter availability, and thus higher dopamine availability in the synaptic cleft, is associated with better reward learning abilities (119). (Reprinted from Kaiser RH et al, Frontostriatal and dopamine markers of individual differences in reinforcement learning: a multi-modal investigation, Cereb Cortex, 2018, vol 28, no 12, pp 4281–4290, by permission of Oxford University Press.) In panel E, in a placebo-controlled clinical trial, a kappa-opioid receptor antagonist (KOR) was associated with better response bias than placebo (PLA) in a transdiagnostic sample with elevated anhedonia (87). (Reprinted with permission.)
The Need for Neuroscientifically Informed, Psychometrically Sound Clinical Scales of Anhedonia
Informed by historical conceptualizations of anhedonia, early scales focused exclusively on the assessment of pleasure (consummatory anhedonia) (for a recent review, see reference 125). For example, the Snaith-Hamilton Pleasure Scale (SHAPS) (126), which is arguably the most widely used self-report scale of anhedonia, assesses the ability to experience pleasure in the context of five type of rewards (food/drinks, sensory experiences, social interaction, pastimes, and achievements). Similarly, the Chapman Anhedonia Scale focuses exclusively on consummatory pleasure (125). Feasibly, individuals such as Antonne—who, as described at the start of this article, can experience pleasure but has difficulty mounting motivated behavior in pursuit of potentially pleasurable experiences—may not generate high scores on such measures, despite clearly displaying anhedonic phenotypes. Consistent with this speculation, a recent meta-analysis comparing SHAPS scores across neuropsychiatric disorders concluded that use of the SHAPS may underestimate the number of depressed individuals with anhedonia (127). To address these limitations, and informed by more modern (neuroscientific) conceptualizations of anhedonia, second-generation scales, such as the Dimensional Anhedonia Rating Scale (128) and the Temporal Experience of Pleasure Scale (129), have been developed to probe different subdomains, including anticipatory versus consummatory anhedonia. Finally, a recently published scale, the Positive Valence Systems Scale (130), explicitly uses domains and subdomains from the NIMH RDoC initiative (131) to provide a more fine-grained assessment of anhedonic behaviors, by parsing seven subdomains (reward valuation, reward expectancy, effort valuation, reward anticipation, action selection, initial responsiveness, and reward satiation). Future studies should use these more modern anhedonia scales to identify what are expected to be more biologically homogeneous subgroups of patients. In addition, studies are needed to confirm the hypothesis that patients featuring dysfunction in specific reward processing subdomains are indeed characterized by different neural alterations. Collectively, these studies might suggest how best to conceptualize treatment approaches for the individuals in such subgroups.
Conclusions and Future Directions
Treating anhedonia associated with MDD as well as with other neuropsychiatric disorders (for a full overview, see reference 132) remains a daunting clinical challenge. Loss of pleasure, as well as blunted motivation to pursue pleasurable activities and learn from them, negatively impacts our ability to see purpose in life, to function across domains (e.g., family, work, society), and to be resilient when challenged by life stress. Restoring motivation and the ability to feel pleasure is seen by individuals as pivotal to remission. Thanks to substantial progress in our understanding of reward processing across species, but also to novel conceptualizations of psychopathology (e.g., the RDoC initiative), the past 10 years have seen remarkable innovation and promise in addressing anhedonia. Such progress has been fueled by several developments, including 1) an appreciation that different reward processing subdomains are governed by partially nonoverlapping brain networks; 2) the development and optimization of objective tasks to probe reward processing subdomains that are functionally identical across species; and 3) the identification of novel (nonmonoaminergic) targets for anhedonia treatment. Moreover, this knowledge has spurred significant innovation, including the development of psychological treatments that specifically target anhedonia (such as positive affect treatment [73]) and the harnessing of virtual reality to address anhedonic symptoms (133); the evaluation of the medial forebrain bundle as a novel target for deep brain stimulation (23, 134); and the development of second-generation, circuity-targeted neurostimulation strategies targeting anhedonia (11).
Despite this progress and promise, there are important remaining gaps and future directions the field will need to pursue. First, there is insufficient understanding of how specific anhedonic phenotypes should be treated: should Victoria and Antonne, as described at the beginning of this review, be treated similarly or differently? Treatment studies with large samples that afford the use of machine learning or clustering approaches are needed to identify reward-related biotypes and their response to treatments (135). For instance, it is unclear why individuals with a more normative brain reward system at baseline (as evidenced by a better ability to learn from rewards and by stronger functional coupling between the NAc and the pgACC) fail to benefit from an 8-week treatment course with an SSRI (sertraline) but go on to respond to an atypical antidepressant (bupropion) (121; see also 122). Do these findings challenge the traditional pharmacology deficiency model (e.g., SSRI ↔ monoaminergic hypothesis of MDD), and do they suggest that anhedonia might follow a capitalization model, which assumes that a treatment should be provided to match relative strengths rather than deficits? (For a discussion of the capitalization vs. compensation approaches in the context of psychotherapy for depression, see reference 136.) If specific neural markers (e.g., blunted striatal recruitment while anticipating or receiving rewards, and/or disrupted rsFC between the ventral striatum and other key reward hubs) are present in young, unaffected, never-depressed children of depressed parents, should we implement preventive strategies to thwart the emergence of MDD and anhedonia? If so, which strategies should we use? Ultimately, for people like Victoria and Antonne, the best chances for remission will stem from a rigorous, integrated, cross-species, multilevel investigation of anhedonia, which promises to lead to much-needed therapeutic and preventive breakthroughs.
1 : Endogenomorphic depression: a conceptual and terminological revision. Arch Gen Psychiatry 1974; 31:447–454 Crossref, Medline, Google Scholar
2 : Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev 2011; 35:537–555 Crossref, Medline, Google Scholar
3 : Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol 2014; 10:393–423 Crossref, Medline, Google Scholar
4 : Anhedonia in schizophrenia and major depression: state or trait? Ann Gen Psychiatry 2009; 8:22 Crossref, Medline, Google Scholar
5 : The efficacy of vortioxetine on anhedonia in patients with major depressive disorder. Front Psychiatry 2019; 10:17 Crossref, Medline, Google Scholar
6 : Inflammatory cytokines, complement factor H, and anhedonia in drug-naïve major depressive disorder. Brain Behav Immun 2021; 95:238–244 Crossref, Medline, Google Scholar
7 : Symptom-based predictors of a 10-year chronic course of treated depression. J Nerv Ment Dis 1999; 187:360–368 Crossref, Medline, Google Scholar
8 : Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Acta Psychiatr Scand 2001; 103:122–130 Crossref, Medline, Google Scholar
9 : Pharmacological treatments for anhedonia. Curr Top Behav Neurosci (Online ahead of print, May 1, 2022) Google Scholar
10 : Psychological treatments for anhedonia. Curr Top Behav Neurosci (Online ahead of print, December 22, 2021) Google Scholar
11 : Circuit-targeted neuromodulation for anhedonia. Curr Top Behav Neurosci (Online ahead of print, May 24, 2022) Google Scholar
12 : Anhedonia and Suicide. Curr Top Behav Neurosci (Online ahead of print, April 19, 2022) Google Scholar
13 : What is important in being cured from depression? Discordance between physicians and patients (1). J Affect Disord 2015; 174:390–396 Crossref, Medline, Google Scholar
14 : How should remission from depression be defined? The depressed patient’s perspective. Am J Psychiatry 2006; 163:148–150 Link, Google Scholar
15 : Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat Rev Neurosci 2018; 19:470–484 Crossref, Medline, Google Scholar
16 : Parsing anhedonia: translational models of reward-processing deficits in psychopathology. Curr Dir Psychol Sci 2013; 22:244–249 Crossref, Medline, Google Scholar
17 : The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci 2012; 35:68–77 Crossref, Medline, Google Scholar
18 : Assessing anhedonia in depression: potentials and pitfalls. Neurosci Biobehav Rev 2016; 65:21–35 Crossref, Medline, Google Scholar
19 : The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. Eur Neuropsychopharmacol 2014; 24:725–736 Crossref, Medline, Google Scholar
20 : The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010; 35:4–26 Crossref, Medline, Google Scholar
21 : Circuit-based frameworks of depressive behaviors: the role of reward circuitry and beyond. Pharmacol Biochem Behav 2018; 174:42–52 Crossref, Medline, Google Scholar
22 : The medial forebrain bundle of the rat, I: general introduction. J Comp Neurol 1982; 206:49–81 Crossref, Medline, Google Scholar
23 : Cross-species affective functions of the medial forebrain bundle: implications for the treatment of affective pain and depression in humans. Neurosci Biobehav Rev 2011; 35:1971–1981 Crossref, Medline, Google Scholar
24 : Hedonic tone is associated with left supero-lateral medial forebrain bundle microstructure. Psychol Med 2015; 45:865–874 Crossref, Medline, Google Scholar
25 : Neural substrates of depression and resilience. Neurotherapeutics 2017; 14:677–686 Crossref, Medline, Google Scholar
26 : Ventral tegmental area GABA neurons mediate stress-induced blunted reward-seeking in mice. Nat Commun 2021; 12:3539 Crossref, Medline, Google Scholar
27 : Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci 2010; 30:16453–16458 Crossref, Medline, Google Scholar
28 : Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 2006; 311:864–868 Crossref, Medline, Google Scholar
29 : Essential role of mesolimbic brain-derived neurotrophic factor in chronic social stress-induced depressive behaviors. Biol Psychiatry 2016; 80:469–478 Crossref, Medline, Google Scholar
30 : Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 2013; 493:532–536 Crossref, Medline, Google Scholar
31 : Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. Am J Psychiatry 2018; 175:1111–1120 Link, Google Scholar
32 : Dysfunctional reward processing in depression. Curr Opin Psychol 2015; 4:114–118 Crossref, Medline, Google Scholar
33 : Reward processing in adolescent depression across neuroimaging modalities. Z Kinder Jugendpsychiatr Psychother 2019; 47:535–541 Crossref, Medline, Google Scholar
34 : Prefrontal cortex and depression. Neuropsychopharmacology 2022; 47:225–246 Crossref, Medline, Google Scholar
35 : Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 2009; 166:702–710 Link, Google Scholar
36 : Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry 2006; 47:1031–1040 Crossref, Medline, Google Scholar
37 : The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord 2013; 151:531–539 Crossref, Medline, Google Scholar
38 : Characterizing anhedonia: a systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cogn Affect Behav Neurosci 2020; 20:816–841 Crossref, Medline, Google Scholar
39 : Abnormal temporal difference reward-learning signals in major depression. Brain 2008; 131:2084–2093 Crossref, Medline, Google Scholar
40 : Impaired reward prediction error encoding and striatal-midbrain connectivity in depression. Neuropsychopharmacology 2018; 43:1581–1588 Crossref, Medline, Google Scholar
41 : Expected value and prediction error abnormalities in depression and schizophrenia. Brain 2011; 134:1751–1764 Crossref, Medline, Google Scholar
42 : Blunted medial prefrontal cortico-limbic reward-related effective connectivity and depression. Brain 2020; 143:1946–1956 Crossref, Medline, Google Scholar
43 : Anhedonia reduction and the association between left ventral striatal reward response and 6-month improvement in life satisfaction among young adults. JAMA Psychiatry 2019; 76:958–965 Crossref, Medline, Google Scholar
44 : Reward versus nonreward sensitivity of the medial versus lateral orbitofrontal cortex relates to the severity of depressive symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging 2021; 6:259–269 Crossref, Medline, Google Scholar
45 : Association of neural and emotional impacts of reward prediction errors with major depression. JAMA Psychiatry 2017; 74:790–797 Crossref, Medline, Google Scholar
46 : Neural mechanisms of reinforcement learning in unmedicated patients with major depressive disorder. Brain 2017; 140:1147–1157 Crossref, Medline, Google Scholar
47 : Altered neural reward and loss processing and prediction error signalling in depression. Soc Cogn Affect Neurosci 2015; 10:1102–1112 Crossref, Medline, Google Scholar
48 : Abnormal reward valuation and event-related connectivity in unmedicated major depressive disorder. Psychol Med 2021; 51:795–803 Crossref, Medline, Google Scholar
49 : Mapping anhedonia-specific dysfunction in a transdiagnostic approach: an ALE meta-analysis. Brain Imaging Behav 2016; 10:920–939 Crossref, Medline, Google Scholar
50 : Anhedonia and general distress show dissociable ventromedial prefrontal cortex connectivity in major depressive disorder. Transl Psychiatry 2016; 6:e810 Crossref, Medline, Google Scholar
51 : Blunted neural response to anticipation, effort, and consummation of reward and aversion in adolescents with depression symptomatology. J Psychopharmacol 2017; 31:303–311 Crossref, Medline, Google Scholar
52 : Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry 2009; 166:64–73 Link, Google Scholar
53 : Ventral striatum functional connectivity as a predictor of adolescent depressive disorder in a longitudinal community-based sample. Am J Psychiatry 2017; 174:1112–1119 Link, Google Scholar
54 : The brain’s response to reward anticipation and depression in adolescence: dimensionality, specificity, and longitudinal predictions in a community-based sample. Am J Psychiatry 2015; 172:1215–1223 Link, Google Scholar
55 : Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiol Dis 2013; 52:66–74 Crossref, Medline, Google Scholar
56 : Corticostriatal pathways contribute to the natural time course of positive mood. Nat Commun 2015; 6:10065 Crossref, Medline, Google Scholar
57 : Impaired reward-related learning signals in remitted unmedicated patients with recurrent depression. Brain 2019; 142:2510–2522 Crossref, Medline, Google Scholar
58 : Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J Affect Disord 2012; 136:1126–1134 Crossref, Medline, Google Scholar
59 : Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry 2010; 67:380–387 Crossref, Medline, Google Scholar
60 : Major depression in mothers predicts reduced ventral striatum activation in adolescent female offspring with and without depression. J Abnorm Psychol 2014; 123:298–309 Crossref, Medline, Google Scholar
61 : Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry 2008; 165:90–98 Link, Google Scholar
62 : Ketamine normalizes subgenual cingulate cortex hyper-activity in depression. Neuropsychopharmacology 2020; 45:975–981 Crossref, Medline, Google Scholar
63 : Neural correlates of change in major depressive disorder anhedonia following open-label ketamine. J Psychopharmacol 2015; 29:596–607 Crossref, Medline, Google Scholar
64 : Dopaminergic enhancement of striatal response to reward in major depression. Am J Psychiatry 2017; 174:378–386 Link, Google Scholar
65 : Machine learning identifies large-scale reward-related activity modulated by dopaminergic enhancement in major depression. Biol Psychiatry Cogn Neurosci Neuroimaging 2020; 2:163–172 Google Scholar
66 : Association between childhood anhedonia and alterations in large-scale resting-state networks and task-evoked activation. JAMA Psychiatry 2019; 76:624–633 Crossref, Medline, Google Scholar
67 : Longitudinal trajectory of the link between ventral striatum and depression in adolescence. Am J Psychiatry 2022; 179:470–481 Link, Google Scholar
68 : White matter microstructure alterations of the medial forebrain bundle in melancholic depression. J Affect Disord 2014; 155:186–193 Crossref, Medline, Google Scholar
69 : Link between structural connectivity of the medial forebrain bundle, functional connectivity of the ventral tegmental area, and anhedonia in unipolar depression. Neuroimage Clin 2022; 34:102961 Crossref, Medline, Google Scholar
70 : Neuroanatomical prediction of anhedonia in adolescents. Neuropsychopharmacology 2017; 42:2087–2095 Crossref, Medline, Google Scholar
71 : Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci 2019; 22:343–352 Crossref, Medline, Google Scholar
72 : Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol 2006; 74:658–670 Crossref, Medline, Google Scholar
73 : Positive affect treatment for depression and anxiety: a randomized clinical trial for a core feature of anhedonia. J Consult Clin Psychol 2019; 87:457–471 Crossref, Medline, Google Scholar
74 : Reward network modulation as a mechanism of change in behavioral activation. Behav Modif 2020; 44:186–213 Crossref, Medline, Google Scholar
75 : Pharmacological interventions targeting anhedonia in patients with major depressive disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry 2019; 92:109–117 Crossref, Medline, Google Scholar
76 : Kappa opioid receptor antagonists as potential therapeutics for stress-related disorders. Annu Rev Pharmacol Toxicol 2020; 60:615–636 Crossref, Medline, Google Scholar
77 : Kappa-opioid antagonists for psychiatric disorders: from bench to clinical trials. Depress Anxiety 2016; 33:895–906 Crossref, Medline, Google Scholar
78 : kappa-Opioid receptor signaling and brain reward function. Brain Res Rev 2009; 62:127–146 Crossref, Medline, Google Scholar
79 : Tracking down the molecular substrates of stress: new roles for p38α mapk and kappa-opioid receptors. Neuron 2011; 71:383–385 Crossref, Medline, Google Scholar
80 : The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010; 210:121–135 Crossref, Medline, Google Scholar
81 : Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacol Ther 2009; 123:334–343 Crossref, Medline, Google Scholar
82 : Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology 2006; 31:1241–1248 Crossref, Medline, Google Scholar
83 : Antidepressant-like effects of the novel kappa opioid antagonist MCL-144B in the forced-swim test. Pharmacology 2008; 81:229–235 Crossref, Medline, Google Scholar
84 : Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem 2004; 90:1258–1268 Crossref, Medline, Google Scholar
85 : U50,488, a kappa opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci Lett 1994; 181:57–60 Crossref, Medline, Google Scholar
86 : A randomized proof-of-mechanism trial applying the “fast-fail” approach to evaluating κ-opioid antagonism as a treatment for anhedonia. Nat Med 2020; 26:760–768 Crossref, Medline, Google Scholar
87 : Selective kappa-opioid antagonism ameliorates anhedonic behavior: evidence from the Fast-Fail Trial in Mood and Anxiety Spectrum Disorders (FAST-MAS). Neuropsychopharmacology 2020; 45:1656–1663 Crossref, Medline, Google Scholar
88 : Neurobiology of resilience. Nat Neurosci 2012; 15:1475–1484 Crossref, Medline, Google Scholar
89 : KCNQ channel openers reverse depressive symptoms via an active resilience mechanism. Nat Commun 2016; 7:11671 Crossref, Medline, Google Scholar
90 : Effects of the KCNQ channel opener ezogabine on functional connectivity of the ventral striatum and clinical symptoms in patients with major depressive disorder. Mol Psychiatry 2020; 25:1323–1333 Crossref, Medline, Google Scholar
91 : The integration of negative affect, pain, and cognitive control in the cingulate cortex. Nat Rev Neurosci 2011; 12:154–167 Crossref, Medline, Google Scholar
92 : Impact of the KCNQ2/3 channel opener ezogabine on reward circuit activity and clinical symptoms in depression: results from a randomized controlled trial. Am J Psychiatry 2021; 178:437–446 Link, Google Scholar
93 : The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry 2018; 175:150–158 Link, Google Scholar
94 : Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 2018; 23:801–811 Crossref, Medline, Google Scholar
95 : Neurobiology of the rapid-acting antidepressant effects of ketamine: impact and opportunities. Biol Psychiatry 2021; 90:85–95 Crossref, Medline, Google Scholar
96 : A translational perspective on the anti-anhedonic effect of ketamine and its neural underpinnings. Mol Psychiatry 2022; 27:81–87 Crossref, Medline, Google Scholar
97 : Changes in symptoms of anhedonia in adults with major depressive or bipolar disorder receiving IV ketamine: results from the Canadian Rapid Treatment Center of Excellence. J Affect Disord 2020; 276:570–575 Crossref, Medline, Google Scholar
98 : Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry 2014; 4:e469 Crossref, Medline, Google Scholar
99 : Ketamine modulates fronto-striatal circuitry in depressed and healthy individuals. Mol Psychiatry 2021; 26:3292–3301 Crossref, Medline, Google Scholar
100 : Fractionating blunted reward processing characteristic of anhedonia by over-activating primate subgenual anterior cingulate cortex. Neuron 2019; 101:307–320.e6 Crossref, Medline, Google Scholar
101 : The first implementation of the NIMH FAST-FAIL approach to psychiatric drug development. Nat Rev Drug Discov 2018; 18:82–84 Crossref, Medline, Google Scholar
102 : How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov 2010; 9:203–214 Crossref, Medline, Google Scholar
103 : The NIMH experimental medicine initiative. World Psychiatry 2015; 14:151–153 Crossref, Medline, Google Scholar
104 : Translational assessments of reward and anhedonia: a tribute to Athina Markou. Biol Psychiatry 2018; 83:932–939 Crossref, Medline, Google Scholar
105 : Affective biases and their interaction with other reward-related deficits in rodent models of psychiatric disorders. Behav Brain Res 2019; 372:112051 Crossref, Medline, Google Scholar
106 : Probabilistic reinforcement learning and anhedonia. Curr Top Behav Neurosci (Online ahead of print, April 19, 2022) Google Scholar
107 : Vigor, effort-related aspects of motivation and anhedonia. Curr Top Behav Neurosci (Online ahead of print, May 4, 2022) Google Scholar
108 : Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry 2005; 57:319–327 Crossref, Medline, Google Scholar
109 : Translational assessments of reward responsiveness in the marmoset. Int J Neuropsychopharmacol 2021; 24:409–418 Crossref, Medline, Google Scholar
110 : Empirical validation of a touchscreen probabilistic reward task in rats. Transl Psychiatry 2020; 10:285 Crossref, Medline, Google Scholar
111 : Assessment of reward responsiveness in the response bias probabilistic reward task in rats: implications for cross-species translational research. Transl Psychiatry 2013; 3:e297 Crossref, Medline, Google Scholar
112 : Association between nicotine withdrawal and reward responsiveness in humans and rats. JAMA Psychiatry 2014; 71:1238–1245 Crossref, Medline, Google Scholar
113 : Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology (Berl) 2008; 196:221–232 Crossref, Medline, Google Scholar
114 : Corticotropin-releasing hormone receptor type 1 (CRHR1) genetic variation and stress interact to influence reward learning. J Neurosci 2011; 31:13246–13254 Crossref, Medline, Google Scholar
115 : Investigating dopamine and glucocorticoid systems as underlying mechanisms of anhedonia. Psychopharmacology (Berl) 2018; 235:3103–3113 Crossref, Medline, Google Scholar
116 : Effects of dopamine modulation on chronic stress-induced deficits in reward learning. Cogn Affect Behav Neurosci (Online ahead of print, April 8, 2022) Google Scholar
117 : Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res 2008; 43:76–87 Crossref, Medline, Google Scholar
118 : A cross-species assay demonstrates that reward responsiveness is enduringly impacted by adverse, unpredictable early-life experiences. Neuropsychopharmacology 2022; 47:767–775 Crossref, Medline, Google Scholar
119 : Frontostriatal and dopamine markers of individual differences in reinforcement learning: a multi-modal investigation. Cereb Cortex 2018; 28:4281–4290 Crossref, Medline, Google Scholar
120 : Individual differences in reinforcement learning: behavioral, electrophysiological, and neuroimaging correlates. Neuroimage 2008; 42:807–816 Crossref, Medline, Google Scholar
121 : Pretreatment reward sensitivity and frontostriatal resting-state functional connectivity are associated with response to bupropion after sertraline nonresponse. Biol Psychiatry 2020; 88:657–667 Crossref, Medline, Google Scholar
122 : Baseline reward processing and ventrostriatal dopamine function are associated with pramipexole response in depression. Brain 2020; 143:701–710 Crossref, Medline, Google Scholar
123 : Mapping disease course across the mood disorder spectrum through a Research Domain Criteria framework. Biol Psychiatry Cogn Neurosci Neuroimaging 2021; 6:706–715 Medline, Google Scholar
124 : Depression genetic risk score is associated with anhedonia-related markers across units of analysis. Transl Psychiatry 2019; 9:236 Crossref, Medline, Google Scholar
125 : Clinical and preclinical assessments of anhedonia in psychiatric disorders. Curr Top Behav Neurosci (Online ahead of print, April 19, 2022) Google Scholar
126 : A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry 1995; 167:99–103 Crossref, Medline, Google Scholar
127 : Assessment of anhedonia in adults with and without mental illness: a systematic review and meta-analysis. JAMA Netw Open 2020; 3:e2013233 Crossref, Medline, Google Scholar
128 : Development and validation of the Dimensional Anhedonia Rating Scale (DARS) in a community sample and individuals with major depression. Psychiatry Res 2015; 229:109–119 Crossref, Medline, Google Scholar
129 : Anticipatory and consummatory components of the experience of pleasure: a scale development study. J Res Pers 2006; 40:1086–1102 Crossref, Google Scholar
130 : The Positive Valence Systems Scale: development and validation. Assessment 2020; 27:1045–1069 Crossref, Medline, Google Scholar
131 : Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010; 167:748–751 Link, Google Scholar
132 Pizzagalli DA: Anhedonia: preclinical, translational, and clinical integration. Springer Nature Switzerland AG, Springer, 2022Google Scholar
133 : Virtual reality reward training for anhedonia: a pilot study. Front Psychol 2021; 11:613617 Crossref, Medline, Google Scholar
134 : Superolateral medial forebrain bundle deep brain stimulation in major depression: a gateway trial. Neuropsychopharmacology 2019; 44:1224–1232 Crossref, Medline, Google Scholar
135 : Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry 2016; 3:472–480 Crossref, Medline, Google Scholar
136 : The compensation and capitalization models: a test of two approaches to individualizing the treatment of depression. Behav Res Ther 2012; 50:699–706 Crossref, Medline, Google Scholar
137 : Toward a quantification of anhedonia: unified matching law and signal detection for clinical assessment and drug development. Perspect Behav Sci 2021; 44:517–540 Crossref, Medline, Google Scholar



