Elevated C-Reactive Protein in Patients With Depression, Independent of Genetic, Health, and Psychosocial Factors: Results From the UK Biobank
Abstract
Objective:
The authors investigated the pathways (genetic, environmental, lifestyle, medical) leading to inflammation in major depressive disorder using C-reactive protein (CRP), genetic, and phenotypic data from the UK Biobank.
Methods:
This was a case-control study of 26,894 participants with a lifetime diagnosis of major depressive disorder from the Composite International Diagnostic Interview and 59,001 control subjects who reported no mental disorder and had not reported taking any antidepressant medication. Linear regression models of log CRP level were fitted to regress out the effects of age, sex, body mass index (BMI), and smoking and to test whether the polygenic risk score (PRS) for major depression was associated with log CRP level and whether the association between log CRP level and major depression remained after adjusting for early-life trauma, socioeconomic status, and self-reported health status.
Results:
CRP levels were significantly higher in patients with depression relative to control subjects (2.4 mg/L compared with 2.1 mg/L, respectively), and more case than control subjects had CRP levels >3 mg/L (21.2% compared with 16.8%, respectively), indicating low-grade inflammation. The PRS for depression was positively and significantly associated with log CRP levels, but this association was no longer significant after adjustment for BMI and smoking. The association between depression and increased log CRP level was substantially reduced, but still remained significant, after adjustment for the aforementioned clinical and sociodemographic factors.
Conclusions:
The data indicate that the “genetic” contribution to increased inflammation in depression is due to regulation of eating and smoking habits rather than an “autoimmune” genetic predisposition. Moreover, the association between depression and increased inflammation even after full adjustment indicates either the presence of yet unknown or unmeasured psychosocial and clinical confounding factors or that a core biological association between depression and increased inflammation exists independently from confounders.
Meta-analytical evidence has confirmed that patients with depression have higher inflammation than the general population, measured as proinflammatory cytokines and C-reactive protein (CRP), but the pathways underpinning this finding—genetic, environmental, lifestyle, medical—have yet to be clarified (1–3). Approximately 20%−30% of patients with depression, as well as 30%−45% of treatment-resistant or overweight depressed patients (4, 5), have CRP levels above 3 mg/L, indicating low-grade inflammation and increased cardiovascular risk, compared with around 10%−20% of healthy control subjects (1). Evidence from Mendelian randomization that CRP has a causal role in depression (6) and data showing that increased blood CRP level correlates well with brain (i.e., CSF) CRP level (7) indicate that this biomarker provides insights into the mechanisms by which increased inflammation may cause depression.
Genetic factors may play a role in the association between depression and inflammation. Depression is frequently comorbid with autoimmune disorders independently from levels of disability (8, 9), indicating that this comorbidity is not explained by generic disease status. Moreover, candidate gene studies, with all their limitations, have found that immune genes are associated with an increased risk of more severe and treatment-resistant forms of depression (10). Furthermore, of the genes identified in the major depressive disorder genome-wide association study (GWAS) by Wray et al. (11), OLFM4 and NEGR1 are associated with body mass index (BMI) and obesity, and LRFN5 is associated with neuroinflammation, while pathway analysis identified genes involved in immune response (11).
Patients with depression also show an excess of clinical and psychosocial risk factors for inflammation, from childhood trauma to medical comorbidities (12). For example, depression is associated with higher BMI, and BMI contributes to chronic low-grade inflammation (13, 14), although depression remains associated with inflammation even after adjusting for BMI (1, 5, 15). Furthermore, a history of early-life trauma is associated with increased inflammation, including CRP levels (16, 17). Finally, increased inflammation in depression frequently co-occurs with ill health, including cardiovascular disorders, diabetes, and cancer (18–22). Whether there is a direct association between depression and inflammation, even after adjusting for these potential confounding factors or other mechanisms, is unknown. Moreover, while some studies have suggested interactions between immune genes and early-life trauma in predicting the risk of depression (23, 24), no studies to our knowledge have investigated the interaction between genetics and early-life trauma in predicting levels of immune biomarkers.
In this study, we investigated the relationship between depression and CRP levels through the use of genetic and phenotypic data in approximately 86,000 participants from the UK Biobank. We addressed the following questions. First, can we replicate the association between depression and inflammation (CRP level) in the UK Biobank? Second, is there a genetic predisposition to inflammation in depression, as shown by a genetic correlation between depression and CRP level, and/or an association between the polygenic risk score (PRS) for major depression and CRP level? Third, can BMI, smoking, early-life trauma, socioeconomic status, and medical comorbidity fully explain the association between depression and CRP level? Fourth, is there a gene-by-environment interaction between the major depression PRS and childhood trauma in predicting CRP level?
Methods
This study analyzed data from the UK Biobank under application number 18177. All participants provided written consent.
Study Population
The UK Biobank is a health resource with more than 500,000 participants recruited from across the United Kingdom from 2006 to 2010. Participants completed health questionnaires and provided blood for biomarker analysis, including measures of serum CRP level. Approximately 150,000 participants also completed an online mental health questionnaire between 2016 and 2017 (25).
Participants included in our case-control study had completed the mental health questionnaire, had data available on CRP level, and had complete data available on age, sex, BMI, smoking status, socioeconomic status, and self-reported health status. When examining genetic data from the UK Biobank, we followed the quality control protocol used in our previous studies and analyzed only participants of European ancestry (26). These inclusion criteria yielded 85,895 participants for the study (see Figure S1 in the online supplement for a flow chart of the participant selection process). The participants’ characteristics are summarized in Table 1.
| Characteristic | Whole Sample (N=85,895) | Control Subjects (N=59,001; 68.7%) | Depression Case Subjects (N=26,894; 31.3%) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| C-reactive protein level (mg/L) | 2.22 | 3.94 | 2.11 | 3.84 | 2.44 | 4.13 |
| Age (years) | 56 | 7.7 | 56.8 | 7.6 | 54.3 | 7.5 |
| Body mass index | 26.7 | 4.5 | 26.4 | 4.15 | 27.2 | 5.0 |
| Socioeconomic status score | −1.84 | 2.77 | −2.00 | 2.66 | −1.47 | 2.95 |
| Trauma score | 1.15 | 1.89 | 0.83 | 1.45 | 1.87 | 2.47 |
| N | % | N | % | N | % | |
| Sex | ||||||
| Female | 46,993 | 54.7 | 28,550 | 48.4 | 18,443 | 68.6 |
| Male | 38,902 | 45.3 | 30,451 | 51.6 | 8,451 | 31.4 |
| Smoking status | ||||||
| Never smoked | 50,018 | 58.2 | 35,794 | 60.7 | 14,224 | 52.9 |
| Previous smoker | 30,002 | 35 | 19,845 | 33.6 | 10,157 | 37.8 |
| Current smoker | 5,875 | 6.8 | 3,362 | 5.7 | 2,513 | 9.3 |
| Health status | ||||||
| Healthy | 63,288 | 73.7 | 44,662 | 75.7 | 18,626 | 69.3 |
| Not healthy | 22,607 | 26.3 | 14,339 | 24.3 | 8,268 | 30.7 |
TABLE 1. Demographic and clinical characteristics of participants in a study of depression in the UK Biobank
Clinical and Psychosocial Phenotypes
Depression case subjects met lifetime criteria for major depressive disorder based on the short form of the Composite International Diagnostic Interview included in the mental health questionnaire (25); if participants also reported schizophrenia, psychosis, or bipolar disorder, they were excluded. Participants in the control group reported no mental disorder, had never been hospitalized for a mental disorder, and had never reported taking any antidepressant medication (27).
Early-life trauma was assessed in the mental health questionnaire using the Childhood Trauma Screener, a shorter version of the Childhood Trauma Questionnaire (25). It comprises five items, each rated from “never true” to “very often true”: “When I was growing up, … 1) I felt loved; 2) People in my family hit me so hard that it left me with bruises or marks; 3) I felt that someone in my family hated me; 4) Someone molested me (sexually); and 5) There was someone to take me to the doctor if I needed it.” Each answer was then recoded on a scale of 0 to 3, reflecting the severity of the item (0=no trauma, 1=mild trauma, 2=moderate trauma, 3=severe trauma), based on both the severity and the frequency of the trauma (see Table S1 in the online supplement for the recoding of the answer and Table 2 for the proportion of responses in each category). The recoded answers were then summed to generate a single trauma score for each participant (range=0–15; see Table S2 and Figure S2 in the online supplement). No “mild trauma” (i.e., a score of 1) code was given for sexual molestation, which was always coded as moderate (i.e., a score of 2) or severe (i.e., a score of 3), consistent with the approach used by other screening instruments for early-life trauma (28).
| Variable | Never True | Rarely True | Sometimes True | Often | Very Often True |
|---|---|---|---|---|---|
| Felt hated by a family member | 85.8% | 5.7% | 6.0% | 1.4% | 1.1% |
| Physically abused | 82.2% | 10.4% | 6.1% | 0.8% | 0.5% |
| Sexually molested | 91.8% | 4.4% | 2.9% | 0.5% | 0.4% |
| Felt loved | 1.2% | 4.1% | 15.0% | 25.8% | 53.9% |
| Had someone to take them to the doctor | 1.9% | 0.7% | 2.4% | 10.2% | 84.8% |
TABLE 2. Responses on a questionnaire measuring early-life trauma in the UK Biobank, showing the proportion of responses in each category in the total sample of 85,895 participants
At the baseline assessment visit, BMI was calculated from height and weight measurements; smoking status was self-defined as nonsmoker, previous smoker, and current smoker; frequency of alcohol consumption was self-defined on a 5-point scale ranging from “never” to “four or more times a week”; and socioeconomic status was assigned according to the Townsend deprivation index for the participant’s postal code of residence (25). Self-reported health status was defined as a binary variable for those who did, or did not, report at least one major health diagnosis; the list of diagnoses is based on the classification for being of standard or nonstandard risk in insurance underwriting and comprises a large number of disorders, including various cancers and cardiovascular, respiratory, gastrointestinal, neurological, hematological, immunological, infectious, and metabolic disorders (see the online supplement). In the original UK Biobank, approximately 70% of the sample are considered healthy, that is, with none of these disorders.
Genetic Analyses
PRSs were calculated for major depressive disorder (11), CRP level (14), BMI (29), smoking (30), and nine autoimmune disorders: celiac disease (31), rheumatoid arthritis (32), multiple sclerosis (33), systemic lupus erythematosus (34), biliary cirrhosis (35), ankylosing spondylitis (36), primary sclerosing cholangitis (37), Crohn’s disease, and ulcerative colitis (38) (see Table S4 in the online supplement). For major depressive disorder, participants from the UK Biobank were excluded from the summary statistics used to calculate PRSs. No other GWASs included UK Biobank participants.
All PRSs were calculated using standard pipelines with PRSice-2 (39). Prior to creation of the scores, clumping was used to obtain single-nucleotide polymorphisms in linkage disequilibrium with an R2 <0.1 within a 250 kb window. PRSs at a single p value threshold of 0.3 were used. A Bonferroni multiple testing correction for 24 tests (12 phenotypes, two models) was applied (p<0.0021). We calculated the genetic correlation between major depression and CRP levels from GWAS summary statistics for these traits (11, 14) using LD Score Regression (LDSC) (40, 41).
C-Reactive Protein (CRP)
The UK Biobank measured a variety of biomarkers; full information on storage and analysis is provided at the Biobank showcase. Serum CRP levels were measured by immunoturbidimetric high-sensitivity analysis on a Beckman Coulter AU5800. Fasting time (i.e., hours between last consumption of food or drink and blood sample collection) was also recorded. For our analyses, CRP level was log transformed to account for the highly skewed distribution (see Figures S3 and S4 in the online supplement).
Our initial investigation tested for phenotypes of age, sex, smoking status, and BMI, which have been associated with increased CRP levels. We performed linear regressions for each variable to confirm association with CRP level, controlling for fasting time and assessment center (see Table S3 in the online supplement). Two adjusted CRP measures were used as outcome variables in regression modeling: first, the effects of age, sex, and fasting time were regressed out of log CRP level, and, in a second model, BMI and smoking status were additionally regressed out. We decided to use this approach, as age, sex, BMI, and smoking status were strongly associated with CRP level (see Table S3 in the online supplement), and thus we wanted to regress out their effects in the same way across all analyses. Additional covariates were added to these models so that each covariate was handled only once.
Statistical Analyses
First, we used linear regression to test whether CRP levels were different between subjects with depression and control subjects. Then linear regression models were fitted to test, first, whether log CRP level was associated with the PRS for major depressive disorder, CRP level, and autoimmune disorders, and second, whether any association between log CRP level and depression status was partially or fully explained by adjusting for early-life trauma, socioeconomic status (measured using the Townsend deprivation index), and health status. Two outcome variables were used for the regression models: log CRP level after regressing out age, sex, and fasting time (model 1), and log CRP level after additionally regressing out BMI and smoking status (model 2). All models controlled for the assessment center that participants visited during recruitment, and models with PRSs additionally included six ancestry-informative principal components and the genotyping batch. Regression modeling was performed using the lm function in R (version 3.3.3/3.6.0).
Results
Association of Lifetime Depression With Increased CRP Levels and Increased Prevalence of Risk Factors for Inflammation
Our study had 85,895 participants (54.7% female; average age, 56 years, range=38–70); 31.3% of the participants were classified as having major depressive disorder. The mean CRP level was 2.22 mg/L (range=0.08–78.05), and the mean BMI was 26.7 (SD=4.5).
CRP levels were higher in case subjects with depression compared with control subjects (2.4 mg/L compared with 2.1 mg/L, respectively, p<10 × 10−10; Table 1); moreover, more control than case subjects had CRP levels <1 mg/L (47.0% compared with 42.6%, respectively), and more case than control subjects had CRP levels >3 mg/L (21.2% compared with 16.8%, respectively), an indication of low-grade inflammation (Table 1 and Figure 1). Case subjects were 2.5 years younger than control subjects, were more often women, had higher BMI, had lower socioeconomic status, had more history of trauma, were more likely to be smokers, and had more self-reported diseases. Not surprisingly, CRP levels were associated with age, sex, BMI, smoking status, and ill health (see Table S3 in the online supplement). Thus, as mentioned above, two outcome variables were used for the regression models: log CRP level after regressing out age and sex (model 1), and log CRP level after additionally regressing out BMI and smoking (model 2).
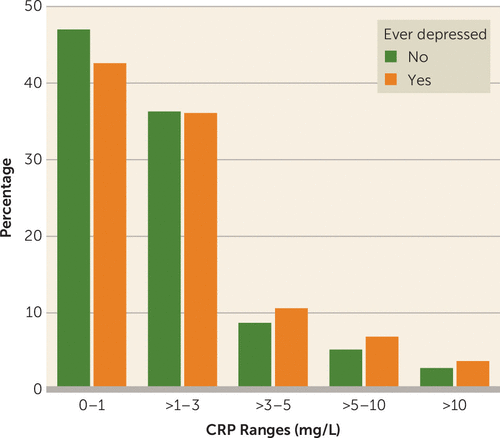
FIGURE 1. C-reactive protein (CRP) levels among subjects with and without depression in the UK Biobank
Association of Depression PRS With CRP Levels
The genetic correlation analysis between depression and CRP level, using summary statistics from the Psychiatric Genomics Consortium for depression (11) and from the CHARGE consortium for CRP (14), showed a positive correlation (rg=0.0977, SE=0.0353, p=0.0056), indicating some common genetic variation underpinning these traits.
We found that the PRS for major depression was strongly positively associated with log CRP level in the model regressing out age and sex (model 1; beta=0.017, 95% CI=0.01, 0.024, p=1.17 × 10−6); however, surprisingly, no association was detected in the model that additionally regressed out BMI and smoking (model 2; beta=0.003, 95% CI=−0.003, 0.009, p=0.324) (Table 3).
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Polygenic Risk Score | beta | 95% CI | p | beta | 95% CI | p |
| Major depressive disorder | 0.017 | 0.010, 0.024 | 1.17 × 10−6 | 0.003 | −0.003, 0.009 | 0.324 |
| BMI | 0.077 | 0.070, 0.084 | 5.88 × 10−4 | −0.024 | −0.030, –0.018 | 5.25 × 10−14 |
| Smoking status | 0.013 | 0.006, 0.020 | 3.70 × 10−4 | 0.0009 | −0.006, 0.007 | 0.79 |
| CRP | 0.171 | 0.164, 0.178 | <5 × 10−324 | 0.135 | 0.129, 0.141 | <5 × 10−324 |
| Ankylosing spondylitis | −0.021 | −0.028, –0.014 | 1.60 × 10−9 | −0.026 | −0.032, –0.02 | 1.71 × 10−16 |
| Biliary cirrhosis | 0.024 | 0.016, 0.031 | 3.99 × 10−10 | 0.018 | 0.012, 0.025 | 7.06 × 10−8 |
| Celiac disease | 0.003 | −0.004, 0.010 | 0.378 | 0.008 | 0.002, 0.015 | 0.013 |
| Crohn’s disease | 0.014 | 0.007, 0.021 | 7.28 × 10−5 | 0.013 | 0.006, 0.019 | 8.60 × 10−5 |
| Multiple sclerosis | 0.008 | 0.0005, 0.015 | 0.037 | 0.007 | 0.0006, 0.013 | 0.033 |
| Primary sclerosing cholangitis | −0.012 | −0.019, –0.005 | 0.00082 | −0.014 | −0.020, –0.007 | 2.44 × 10−5 |
| Rheumatoid arthritis | 0.017 | 0.010, 0.024 | 3.74 × 10−6 | 0.013 | 0.007, 0.019 | 6.10 × 10−5 |
| Systemic lupus erythematosus | −0.006 | −0.013, 0.001 | 0.094 | −0.007 | −0.013, –0.0004 | 0.037 |
| Ulcerative colitis | 0.001 | −0.006, 0.008 | 0.693 | 0.006 | 0.0003, 0.013 | 0.041 |
TABLE 3. Association of C-reactive protein (CRP) with polygenic risk scores (PRSs) for major depressive disorder, CRP level, and autoimmune disordersa
As expected, the CRP PRS was strongly positively associated with log CRP level across both models (Table 3). In contrast, and similar to the major depression PRS results, both BMI and smoking PRSs were positively correlated with CRP levels only in the model regressing out age and sex and not in the model that additionally regressed out BMI and smoking (Table 3).
To interpret the strength of the genetic association between the major depression PRS and log CRP values, we examined the association between immune disorder PRSs and CRP level (Table 3). After Bonferroni correction for 24 tests (p<0.0021), PRSs for biliary cirrhosis, Crohn’s disease, and rheumatoid arthritis were all positively associated with log CRP levels, even after regressing out BMI and smoking. In model 1 (with age and sex regressed out), the effect size for the major depression PRS (beta=0.017) was similar to that for the rheumatoid arthritis PRS (beta=0.017) and between those of the Crohn’s disease (beta=0.014) and biliary cirrhosis (beta=0.024) PRSs. This suggests that the association between the major depression PRS and CRP levels, when adjusted only for age and sex, was similar to the association of these three PRSs for autoimmune disorders and CRP levels. It is of note that, differently from the PRS for major depression, the associations between the three PRSs for autoimmune disorders and log CRP level remained significant even after adjusting for BMI and smoking.
Effects of Other Factors on the Association Between Depression and Increased CRP Level
As expected, based on the unadjusted comparison (Table 1), depression was strongly positively associated with log CRP level in the model that regressed out age and sex (model 1; beta=0.144, 95% CI=0.129, 0.158, p=5.84 × 10−80); moreover, the effect size of this association was reduced substantially, but remained significant, in the model that additionally regressed out BMI and smoking (model 2; beta=0.036, 95% CI=0.023, 0.05, p=1.02 × 10−7) (Table 4).
| Log CRP (Model 1; Adjusted for Age and Sex) | Log CRP (Model 2; Adjusted for Age, Sex, Smoking, and BMI) | |||||
|---|---|---|---|---|---|---|
| Added Covariate | beta | 95% CI | p | beta | 95% CI | p |
| None | 0.144 | 0.129, 0.158 | 5.84 × 10–80 | 0.036 | 0.023, 0.05 | 1.02 × 10–7 |
| Trauma score | 0.116 | 0.101, 0.131 | 1.48 × 10–49 | 0.029 | 0.015, 0.043 | 4.34 × 10–05 |
| Trauma and socioeconomic status scores | 0.109 | 0.094, 0.124 | 5.93 × 10–44 | 0.027 | 0.013, 0.041 | 1.35 × 10–04 |
| Trauma score, socioeconomic status score, and health status | 0.103 | 0.087, 0.118 | 3.79 × 10–39 | 0.024 | 0.010, 0.038 | 5.87 × 10–04 |
TABLE 4. Association of C-reactive protein (CRP) with depression after controlling for covariates, showing model estimates for depressiona
When early-life trauma was added as a covariate to the model, the association between depression and log CRP level was attenuated further but again remained significant (the beta value was reduced from 0.144 to 0.116 in model 1 and from 0.036 to 0.029 in model 2). Further adjusting for socioeconomic status and health status had a modest effect on the association, which remained significant in both models (Table 4).
Sensitivity analyses are presented in the online supplement. Our findings did not change after excluding participants with CRP levels >10 mg/L, those with autoimmune disorders, or those who were taking antidepressant or anti-inflammatory medication at the time of blood collection. In addition, there was no effect of time of onset of depression (before or after blood collection) or of alcohol consumption, and there was no moderation by sex.
Association of Early-Life Trauma With Higher CRP Levels
As expected, early-life trauma was significantly associated with log CRP level in both model 1 (beta=0.034, 95% CI=0.030, 0.037, p=3.57 × 10−74) and model 2 (beta=0.009, 95% CI=0.006, 0.012, p=7.94 × 10−8).
Because we had found an association between the major depression PRS and CRP levels (see above and Table 3), we examined whether there was a gene-by-environment interaction between the depression PRS and early-life trauma in predicting log CRP levels. The analysis showed no significant interaction in either model 1 (beta=0.003, 95% CI=−0.0006, 0.006, p=0.102) or model 2 (beta=0.003, 95% CI=−0.0005, 0.006, p=0.099).
Discussion
We wanted to identify the biopsychosocial pathways underpinning the increased inflammation seen in depression by examining the largest ever community-based sample with mental health, inflammation, GWAS, environmental, lifestyle, and general medical health information. We have two main findings. First, the PRS for major depressive disorder is positively associated with CRP levels, but this association is no longer significant after regressing out the effects of BMI and smoking, suggesting that the “genetic” contribution to the increased inflammation in depression is due to regulation of eating and smoking habits rather than an “autoimmune” genetic predisposition. Second, the increased inflammation in depression is only partially explained by clinical and sociodemographic factors such as age, sex, BMI, smoking, alcohol consumption, exposure to early-life trauma, socioeconomic status, and self-reported health status (including taking antidepressant or anti-inflammatory medication or having an autoimmune disorder). These findings suggest either the presence of unknown or unmeasured confounding effects or that a “core” biological association between depression and increased inflammation exists independently from confounding factors.
We found a prevalence of low-grade inflammation (i.e., above 3 mg/L) in 21% of patients with depression, which is similar to a meta-analysis estimate for community samples (24%) (1) and similar to our recent study in a clinical sample of patients who had a lifetime diagnosis of depression but who were not currently depressed (23%) (5). Moreover, our CRP distribution (Figure 1), with fewer depressed case than control subjects with levels <1 mg/L, and with more depressed case than control subjects with levels >3 mg/L, is consistent with a meta-analysis showing that increased inflammation in depression is not a feature of a subgroup but rather a result of a continuous distribution of increased CRP levels that is “shifted to the right” (3) (and indeed, our findings are not driven by participants with CRP levels above 10 mg/L). Finally, we found an association between depression and increased inflammation in the model that regressed out the effects of age, sex, BMI, and smoking, and Osimo et al. also found that CRP is elevated in depression independently from age, sex, BMI, and smoking (1, 3). The novelty of our study is that we were able to demonstrate these findings in a single cohort that has many more patients with depression than the total sum of depressed case subjects from these meta-analyses.
We also show here that the PRS for major depressive disorder is positively associated with CRP level: the more the genetic loading for depression, the higher the CRP level. Indeed, in the model with age and sex regressed out, the strength of the association (indicated by the beta values) is similar to the strengths of the association between three autoimmune PRSs (Crohn’s disease, rheumatoid arthritis, and biliary cirrhosis) and CRP level. However, and perhaps even more interestingly, the association between the major depression PRS and CRP level disappears when we additionally regress out the effects of BMI and smoking. This is consistent with previous evidence showing strong genetic relationships between major depressive disorder and both BMI and smoking. Specifically, two genes identified by a GWAS of depression, OLFM4 and NEGR1, have been associated with BMI and obesity; major depression is genetically correlated with both BMI (rg=0.09) and smoking (rg=0.29), and Mendelian randomization indicates that BMI and smoking have causal relationships with major depression (11, 42). Taken together, our findings confirm a genetic component underpinning increased inflammation in depression but suggest that this is due to genes regulating smoking, eating, and perhaps other behaviors, rather than through a genetic autoimmune predisposition. Indeed, epidemiological data strongly support a bidirectional relationship between autoimmune disorders and depression, but shared genetic susceptibility is not a major contributor to the increased risk, as shown by two recent large GWASs of major depressive disorder: Wray et al. (11) found no significant genetic correlations between major depression and nine autoimmune disorders, and only Crohn’s disease had a significant genetic correlation in the study by Howard et al. (43) (rg=0.088). Our genetic correlation between the PRSs for CRP and major depression (rg=0.098, SE=0.035) is much lower than a previous nonsignificant estimate (rg=0.21) using smaller genome-wide studies of both CRP and major depression (44).
In this study, we modeled the association between depression and CRP level while adding potential covariates that are independently associated with both depression and CRP level. A history of early-life trauma, socioeconomic status, and self-reported diseases was found to explain a large part—but not all—of the association between depression and increased CRP levels; however, a significant association between depression and CRP level remains even in the most conservative model (after regressing out the effects of age, sex, BMI, and smoking, and after the inclusion of early-life trauma, socioeconomic status, self-reported health status, and alcohol consumption as covariates). Because we know that any genetic effects (as captured by the PRS for major depression) are no longer significant once BMI and smoking are regressed out, what still drives the increased inflammation in depression that we detected?
Of course, unknown or unmeasured confounding factors could offer an explanation. First, although the UK Biobank is the largest and most complete data set on CRP levels, depression, and many psychosocial and clinical variables, the control of these confounders may be incomplete because of, for example, the retrospective and self-reporting ascertainment of these variables, at a single point in time, with no external validation. Second, factors that are not captured in this data set could potentially explain the remaining association between depression and increased inflammation, such as exposure to maternal depression in utero (45), unhealthy diet and exercise habits (46, 47), or severe infections (9). However, a third, nonmutually exclusive explanation for these findings is that, simply put, increased inflammation might be present after adjusting for potential confounders because it is a core biological component of the depressive phenotype, as part of the activation of stress-related molecular pathways (48–50).
There are important limitations to our study. First, lifetime depression was diagnosed 6–10 years after blood collection, although, reassuringly, results were confirmed in the subgroup who had onset of depression after the blood collection. Second, CRP was measured on a single occasion, and samples were not collected after the same fasting intervals or in the same center, although these two variables were included in all analyses. Third, even if the UK Biobank is a community sample, ours is a case-control study that uses only a subset of participants with all required data (approximately 86,000 individuals, compared with the full database of 500,000); this led to a proportion of case subjects with depression (approximately 31% of the sample) that is slightly higher than the overall prevalence in the UK Biobank (24%) (25), although this percentage is not much greater than other lifetime estimates for depression (e.g., 29% in a systematic review by Steel et al. [51]). However, notwithstanding these limitations, our findings are consistent with previous meta-analyses, and our new analyses have statistical power previously unreached, which offers strong reassurance that our results are valid. Nevertheless, replication of the same research questions in mega-analyses or in other longitudinal data sets with a similar wealth of data is needed.
In summary, we found that the association between depression and increased inflammation is reduced, but still remains significant, when taking into consideration the most recognized “candidate explanations,” including immune gene pleiotropy, an excess of unhealthy habits, a more adverse socioeconomic background, or more ill health. We suggest that the remaining association between depression and increased inflammation reflects, at least in part, a core biological process and thus possibly a crucial pathogenetic mechanism leading to the depressive phenotype.
1 , : Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med 2019; 49:1958–1970Crossref, Medline, Google Scholar
2 , : Absolute measurements of macrophage migration inhibitory factor and interleukin-1-β mRNA levels accurately predict treatment response in depressed patients. Int J Neuropsychopharmacol 2016; 19:1–10Crossref, Google Scholar
3 , : Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun 2020; 87:901–909Crossref, Medline, Google Scholar
4 , : A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 2013; 70:31–41Crossref, Medline, Google Scholar
5 , : Treatment-resistant depression and peripheral C-reactive protein. Br J Psychiatry 2019; 214:11–19Crossref, Medline, Google Scholar
6 , : Shared mechanisms between coronary heart disease and depression: findings from a large UK general population-based cohort. Mol Psychiatry 2020; 25:1477–1486Crossref, Medline, Google Scholar
7 , : What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry 2020; 25:1301–1311Crossref, Medline, Google Scholar
8 , : Effects of immunomodulatory drugs on depressive symptoms: a mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol Psychiatry 2020; 25:1275–1285Crossref, Medline, Google Scholar
9 , : Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry 2013; 70:812–820Crossref, Medline, Google Scholar
10 : Genetic contributions of inflammation to depression. Neuropsychopharmacology 2017; 42:81–98Crossref, Medline, Google Scholar
11 , : Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 2018; 50:668–681Crossref, Medline, Google Scholar
12 , : Major depressive disorder. Nat Rev Dis Primers 2016; 2:16065Crossref, Medline, Google Scholar
13 , : Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999; 282:2131–2135Crossref, Medline, Google Scholar
14 , : Genome analyses of >200,000 individuals identify 58 loci for chronic inflammation and highlight pathways that link inflammation and complex disorders. Am J Hum Genet 2018; 103:691–706Crossref, Medline, Google Scholar
15 , : Metabolic and inflammatory markers: associations with individual depressive symptoms. Psychol Med 2018; 48:1102–1110Crossref, Medline, Google Scholar
16 , : Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry 2016; 21:642–649Crossref, Medline, Google Scholar
17 : The devastating clinical consequences of child abuse and neglect: increased disease vulnerability and poor treatment response in mood disorders. Am J Psychiatry 2020; 177:20–36Link, Google Scholar
18 , : Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med 2013; 11:129Crossref, Medline, Google Scholar
19 , : Inflammation associated with coronary heart disease predicts onset of depression in a three-year prospective follow-up: a preliminary study. Brain Behav Immun 2019; 81:659–664Crossref, Medline, Google Scholar
20 , : Insufficient glucocorticoid signaling and elevated inflammation in coronary heart disease patients with comorbid depression. Brain Behav Immun 2015; 48:8–18Crossref, Medline, Google Scholar
21 , : Depressive symptoms, health behaviors, and subsequent inflammation in patients with coronary heart disease: prospective findings from the heart and soul study. Am J Psychiatry 2011; 168:913–920Link, Google Scholar
22 , : Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry 2001; 158:1252–1257Link, Google Scholar
23 , : Interaction between childhood maltreatment on immunogenetic risk in depression: discovery and replication in clinical case-control samples. Brain Behav Immun 2018; 67:203–210Crossref, Medline, Google Scholar
24 , : Effects of IL1B single nucleotide polymorphisms on depressive and anxiety symptoms are determined by severity and type of life stress. Brain Behav Immun 2016; 56:96–104Crossref, Medline, Google Scholar
25 , : Mental health in UK Biobank: development, implementation and results from an online questionnaire completed by 157 366 participants: a reanalysis. BJPsych Open 2020; 6:e18Crossref, Medline, Google Scholar
26 , : Genome-wide gene-environment analyses of major depressive disorder and reported lifetime traumatic experiences in UK Biobank. Mol Psychiatry 2020; 25:1430–1446Crossref, Medline, Google Scholar
27 , : Prevalence and characteristics of probable major depression and bipolar disorder within UK Biobank: cross-sectional study of 172,751 participants. PLoS One 2013; 8:e75362Crossref, Medline, Google Scholar
28 , : The childhood experience of care and abuse questionnaire (CECA.Q): validation in a community series. Br J Clin Psychol 2005; 44:563–581Crossref, Medline, Google Scholar
29 , : Genetic studies of body mass index yield new insights for obesity biology. Nature 2015; 518:197–206Crossref, Medline, Google Scholar
30 , : Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet 2010; 42:441–447Crossref, Medline, Google Scholar
31 , : Multiple common variants for celiac disease influencing immune gene expression. Nat Genet 2010; 42:295–302Crossref, Medline, Google Scholar
32 , : Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 2014; 506:376–381Crossref, Medline, Google Scholar
33 , : Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 2011; 476:214–219Crossref, Medline, Google Scholar
34 , : Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet 2015; 47:1457–1464Crossref, Medline, Google Scholar
35 , : International genome-wide meta-analysis identifies new primary biliary cirrhosis risk loci and targetable pathogenic pathways. Nat Commun 2015; 6:8019Crossref, Medline, Google Scholar
36 , : Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet 2013; 45:730–738Crossref, Medline, Google Scholar
37 , : Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat Genet 2017; 49:269–273Crossref, Medline, Google Scholar
38 , : Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 2015; 47:979–986Crossref, Medline, Google Scholar
39 : PRSice-2: polygenic risk score software for biobank-scale data. Gigascience 2019; 8:giz082Crossref, Medline, Google Scholar
40 , : LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet 2015; 47:291–295Crossref, Medline, Google Scholar
41 , : An atlas of genetic correlations across human diseases and traits. Nat Genet 2015; 47:1236–1241Crossref, Medline, Google Scholar
42 , : Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. Psychol Med 2020; 50:2435–2443Crossref, Medline, Google Scholar
43 , : Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci 2019; 22:343–352Crossref, Medline, Google Scholar
44 , : Genetic correlations among psychiatric and immune-related phenotypes based on genome-wide association data. Am J Med Genet B Neuropsychiatr Genet 2018; 177:641–657Crossref, Medline, Google Scholar
45 , : Prenatal maternal depression is associated with offspring inflammation at 25 years: a prospective longitudinal cohort study. Transl Psychiatry 2016; 6:e936Crossref, Medline, Google Scholar
46 : The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol 2005; 45:1563–1569Crossref, Medline, Google Scholar
47 : Dietary protein and changes in biomarkers of inflammation and oxidative stress in the Framingham Heart Study Offspring cohort. Curr Dev Nutr 2019; 3:nzz019Crossref, Medline, Google Scholar
48 : The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2016; 16:22–34Crossref, Medline, Google Scholar
49 : When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 2003; 160:1554–1565Link, Google Scholar
50 , : From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9:46–56Crossref, Medline, Google Scholar
51 , : The global prevalence of common mental disorders: a systematic review and meta-analysis 1980–2013. Int J Epidemiol 2014; 43:476–493Crossref, Medline, Google Scholar



