Functional Connectome Prediction of Anxiety Related to the COVID-19 Pandemic
Abstract
Objective:
Increased anxiety in response to the COVID-19 pandemic has been widely noted. The purpose of this study was to test whether the prepandemic functional connectome predicted individual anxiety induced by the pandemic.
Methods:
Anxiety scores from healthy undergraduate students were collected during the severe and remission periods of the pandemic (first survey, February 22–28, 2020, N=589; second survey, April 24 to May 1, 2020, N=486). Brain imaging data and baseline (daily) anxiety ratings were acquired before the pandemic. The predictive performance of the functional connectome on individual anxiety was examined using machine learning and was validated in two external undergraduate student samples (N=149 and N=474). The clinical relevance of the findings was further explored by applying the connectome-based neuromarkers of pandemic-related anxiety to distinguish between individuals with specific mental disorders and matched healthy control subjects (generalized anxiety disorder, N=43; major depression, N=536; schizophrenia, N=72).
Results:
Anxiety scores increased from the prepandemic baseline to the severe stage of the pandemic and remained high in the remission stage. The prepandemic functional connectome predicted pandemic-related anxiety and generalized to the external sample but showed poor performance for predicting daily anxiety. The connectome-based neuromarkers of pandemic-related anxiety further distinguished between participants with generalized anxiety and healthy control subjects but were not useful for diagnostic classification in major depression and schizophrenia.
Conclusions:
These findings demonstrate the feasibility of using the functional connectome to predict individual anxiety induced by major stressful events (e.g., the current global health crisis), which advances our understanding of the neurobiological basis of anxiety susceptibility and may have implications for developing targeted psychological and clinical interventions that promote the reduction of stress and anxiety.
The COVID-19 pandemic widely and rapidly spread around the world, and it had a profound impact on public mental health (1–3). During the COVID-19 crisis, people were exposed to an unprecedented environment of threats and uncertainties. Facing physical and social isolation (as most people were under stay-at-home orders), the uncertainty of infection, and other stressors, people may be especially susceptible to anxiety-related symptoms (3–6) and exhibit individualized behavioral and emotional responses in the face of the pandemic-induced changes and restrictions, which were unlikely in daily life before the pandemic. In addition, there may be great individual differences in levels of anxiety related to the pandemic. In these circumstances, it is helpful to explore brain-based predictors of anxiety, which would advance the understanding of the neural basis of anxiety and may have implications for clinical practice.
The functional connectome is like a fingerprint of the brain and shows highly individualized functional connectivity profiles that successfully distinguish individuals with high accuracy (7). Emerging evidence based on machine-learning approaches suggests that the individualized functional connectome can be used to predict individual differences in cognition (8), personality (9), and mental disorder symptoms (10). However, results from recent studies have been inconsistent on the prediction of the functional connectome on individual anxiety (11, 12), partly because individual differences in anxiety may not be well measured in the absence of large stressful events and threatening stimuli in daily life. Instead, researchers have tended to provoke anxiety in individuals through experimental manipulation, such as exposure to aversive stimuli (13). Compared with traditional experimental manipulation, the unprecedented uncertainties and stresses caused by this global health crisis may have higher ecological validity that amplified individual differences in anxiety during the pandemic (14), which has enabled us to use a considerable number of participants to investigate the neural correlates of both pandemic-related anxiety and daily anxiety, as well as to further identify potential connectome-based neuromarkers. Thus, we hypothesized that the prepandemic functional connectome could predict individuals’ pandemic-related anxiety but may show poor performance for predicting daily anxiety. Moreover, to investigate the continuous effect of the pandemic on mental health and further examine the reliability of the neuromarkers of pandemic-related anxiety, we utilized data from a longitudinal cohort study with brain imaging data and daily anxiety scores collected before the pandemic and pandemic-related anxiety scores collected during the severe and remission periods of the pandemic.
The acute and persistent panic and fear caused by this crisis also resulted in some psychopathology symptoms (15), and recent national cohort studies have reported that people are more likely to screen positive for mental disorders (e.g., pathological anxiety) during the pandemic (6, 16). It is possible that the identified connectome-based neural correlates of pandemic-related anxiety have the potential to be used for risk assessment of common mental disorders. To explore this possibility, three independent clinical data sets were applied to investigate whether the neuromarkers of pandemic-related anxiety could be generalized to participants with generalized anxiety disorder, major depression, or schizophrenia.
Methods
Participants
Three independent undergraduate student data sets (main data set: N=589; two validation data sets: N=474 and N=149) were included in this study (Table 1). The main data set comprises individuals in our ongoing project, the Behavioral Brain Research Project of Chinese Personality (BBP), which was used to investigate the predictive performance of the functional connectome on daily anxiety and pandemic-related anxiety, as well as to identify the connectome-based neuromarkers of pandemic-related anxiety. Two other undergraduate student data sets were used to validate the prediction results. We also included three independent mental disorder data sets (generalized anxiety disorder data set: N=43; major depression data set: N=536; schizophrenia data set: N=72) to examine the clinical relevance of the neuromarkers of pandemic-related anxiety (Table 1).
| Male | Age (Years) | ||||
|---|---|---|---|---|---|
| Data set | N | N | % | Mean | SD |
| BBP | |||||
| First pandemic survey | 589 | 173 | 29.37 | 19.35 | 0.87 |
| Second pandemic survey | 486 | 137 | 28.19 | 19.34 | 0.86 |
| Validation sample | 149 | 22 | 14.77 | 19.25 | 0.62 |
| SLIM | 474 | 207 | 43.67 | 20.02 | 1.28 |
| Generalized anxiety disorder | |||||
| Anxious group | 25 | 10 | 40 | 16.84 | 0.47 |
| Healthy control group | 18 | 9 | 50 | 16.72 | 0.89 |
| Major depressive disorder | |||||
| Depressed group | 282 | 99 | 35.11 | 38.74 | 13.65 |
| Healthy control group | 254 | 88 | 34.65 | 39.65 | 15.80 |
| Schizophrenia | |||||
| Schizophrenia group | 26 | 22 | 84.62 | 31.92 | 12.35 |
| Healthy control group | 46 | 33 | 71.74 | 32.65 | 10.67 |
TABLE 1. Demographic and clinical characteristics of three undergraduate student data sets in China before and during the COVID-19 pandemica
Specifically, a total of 901 undergraduates from the BBP were recruited via mobile telephone text message to complete the first online pandemic questionnaire survey from February 22 to 28, 2020. Among these participants, 604 had completed prepandemic brain scanning from September to December 2019, as well as a self-reported anxiety measurement immediately after the scanning (considered as the baseline or daily anxiety), in which eight participants were missing baseline anxiety scores and seven were excluded because of excessive head motion. We adopted a widely used criterion of head motion to exclude participants if the number of volumes with a framewise displacement >0.5 mm was more than 10% of the total number of volumes to ensure that head motion artifacts were not driving observed effects (17–19). Thus, for the first pandemic survey of the BBP, 589 undergraduates with brain imaging data, baseline anxiety scores, and pandemic-related anxiety scores were included in prediction analysis of whether the prepandemic functional connectome could predict pandemic-related anxiety. Some participants (N=486) had also completed the second online pandemic questionnaire survey from April 24 to May 1, 2020. In addition, a validation sample included 149 undergraduates who had completed an online pandemic survey from February 21 to 28, 2020, and brain scanning from June to October 2019, in which no one was excluded because of excessive head motion. The validation sample and anxiety scores from the second pandemic survey of the BBP were used to examine the reliability of the neural correlates of pandemic-related anxiety identified in the first pandemic survey.
Another validation sample was included from our former project (Southwest University Longitudinal Imaging Multimodal Project [SLIM]), comprising individuals whose self-reported anxiety scores were collected immediately after brain scanning (the data were collected before the COVID-19 pandemic) (20), which were used to validate the predictive performance of the functional connectome on daily anxiety. The present study included 474 undergraduates from SLIM after excluding 12 participants with excessive head motion. Details of the SLIM cohort are presented in the online supplement, as well as in our data description study (20). For the three undergraduate data sets, strict procedures were applied to ensure that all participants had no psychiatric illness or physical health problems and met the requirements for MRI scanning (for further details, see the online supplement). Data collection period and anxiety score information for the three undergraduate data sets are presented in Table 2.
| Data Collection Period | STAI-T | STAI-S | ||||||
|---|---|---|---|---|---|---|---|---|
| Data set | Anxiety Data | Brain Imaging Data | Mean | SD | Range | Mean | SD | Range |
| BBP | ||||||||
| Baseline (N=589) | September– December 2019c | September– December 2019 | 43.27 | 7.35 | 23–71 | —b | —b | —b |
| First pandemic survey (N=589) | February 22–28, 2020 | Same as baseline | 44.76 | 8.78 | 20–73 | 41.76 | 9.71 | 20–68 |
| Second pandemic survey (N=486) | April 24–May 1, 2020 | Same as baseline | 45.54 | 8.98 | 20–77 | 42.75 | 10.35 | 20–69 |
| Validation sample (N=149) | February 21–28, 2020 | June–October 2019 | 46.08 | 9.48 | 28–70 | 46.17 | 9.98 | 22–70 |
| SLIM (N=474) | November 2011– January 2013c | November 2011– January 2013 | 40.62 | 7.99 | 22–79 | 35.45 | 8.32 | 20–65 |
TABLE 2. Data collection period and anxiety scores for three undergraduate data sets in China before and during the COVID-19 pandemica
We further explored the clinical relevance by linking the neuromarkers of pandemic-related anxiety to different mental disorders. Details on clinical diagnosis and symptom information are provided in the online supplement, as well as in previous studies (19, 21, 22). Briefly, the generalized anxiety disorder data set was collected from the Second Xiangya Hospital of Central South University, which included 25 participants with generalized anxiety disorder and 18 healthy control subjects (21). The major depression data set was collected from the First Affiliated Hospital of Chongqing Medical School and Southwest University, which included 282 participants with major depression and 254 healthy control subjects (19). The schizophrenia data set was obtained from the Center of Biomedical Research Excellence, which included 26 participants with schizophrenia and 46 healthy control subjects. A flow diagram with details of participants’ screening is provided in Figure S1 in the online supplement. All research projects were approved by the local institutional review boards, and written informed consent was obtained from each participant in accordance with the Declaration of Helsinki. The participants’ demographic characteristics are summarized in Table 1 (see also Tables S1 and S2 in the online supplement).
Anxiety Scoring
Participants’ anxiety scores from the three undergraduate data sets were assessed using the State-Trait Anxiety Inventory (STAI) (23), which consists of 20 items that assess an individual’s feelings over the past week (the state scale of STAI [STAI-S]) and 20 other items that assess an individual’s general feelings (the trait scale of STAI [STAI-T]) based on a 4-point Likert scale. Participants from the BBP and SLIM completed the STAI immediately after brain scanning, which was considered baseline or daily anxiety compared with pandemic-related anxiety. Notably, given that the BBP was originally designed to investigate the neural basis of Chinese personality and trait-like behaviors, participants of the BBP only completed the STAI-T at baseline. In addition, at the first pandemic survey, we used two open questions to assess individual concerns about the COVID-19 infection using a 5-point Likert scale: “How likely do you think you are to be infected with the COVID-19 coronavirus?” and “How likely do you think your family is to be infected with the COVID-19 coronavirus?”
Neuroimaging Data Preprocessing and Functional Network Construction
The three undergraduate data sets and the major depression data set were collected using the same scanner at the Brain Imaging Center of Southwest University. The generalized anxiety disorder and schizophrenia data sets were collected at two other sites. Resting-state functional MRI (fMRI) data from the different data sets were preprocessed independently (18). The preprocessed data were parcellated using the Brainnetome Atlas, which includes 210 cortical regions and 36 subcortical regions (24). The representative time series of each region was obtained for each individual by averaging the time series over all the voxels in that region. Pearson’s correlation between the time courses of each pair of nodes was calculated, and Fisher’s z transformation was performed to improve normality, which resulted in a 246 × 246 symmetric functional connectivity matrix with 30,135 edges for each participant. Further details are presented in the online supplement.
Predictive Analysis
We adopted relevance vector regression to examine the predictive performance of the functional connectome on daily anxiety and pandemic-related anxiety (first pandemic survey) in the BBP and SLIM data sets (Figure 1). Relevance vector regression is a sparse kernel multivariate regression method that uses Bayesian inference to obtain parsimonious solutions that can generalize well and provide inferences at low computational cost (25). Notably, relevance vector regression has no algorithm-specific parameter and does not require extra computational resources to estimate optimal algorithm-specific parameters. By using 10-fold cross-validation (10F-CV), participants of the BBP and SLIM were randomly divided into 10 subsets; nine folds (90% of participants) were used as the training set, and the remaining fold (10% of participants) was used as the testing set. Feature selection was performed in the training set by correlating anxiety scores with whole-brain functional connectivity using Pearson’s partial correlation after controlling for sex, age, and head motion. Daily anxiety scores were used as an additional covariate for feature selection of pandemic-related anxiety. According to a specific p value, some functional connectivity with a high correlation coefficient was retained. To avoid the arbitrariness of a single threshold and to further explore the optimal predictive performance, we applied several common thresholds (uncorrected p values: 1 × 10−4, 5 × 10−4, 1 × 10−3, 5 × 10−3, 1 × 10−2, and 5 × 10−2) for feature selection (26). After feature selection, each selected functional connectivity was linearly scaled to the range of 0 to 1 across the training set to exclude the influence from features with greater numeric ranges that may dominate those with smaller numeric ranges (27). The scaling parameters were further applied to scale the testing set. A regression model was built using relevance vector regression to fit the selected functional connectivity and the anxiety scores in the training set. The testing set was then fed into the model to generate the predicted anxiety scores. After all folds were completed, we obtained the predicted scores for each participant. Because each random division results in different testing sets and training sets, we repeated the above prediction pipeline 20 times to generate 20 predicted scores for each participant and further averaged these predicted scores to obtain robust estimates. Pearson’s correlation coefficients and mean absolute error between the predicted and actual scores were computed to provide final estimates of prediction performance. We randomly shuffled the anxiety scores 1,000 times and ran the above prediction pipeline for each time to obtain a null distribution of correlation coefficients between the predicted and actual scores to assess the significance (permutation test, ppt<0.05). In addition, two other regression methods, elastic net regression and support vector regression, were applied to validate the reliability of the prediction results. Because the two methods have algorithm-specific parameters, we therefore used nested 10F-CV, with the outer 10F-CV estimating the generalizability of the model and the inner 10F-CV determining the optimal parameters (27) (for further details, see the online supplement).
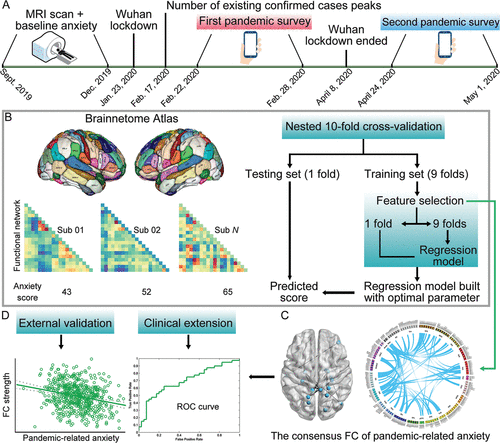
FIGURE 1. Schematic overview of the main analysis of three undergraduate student data sets in China before and during the COVID-19 pandemica
a Panel A shows the time axis of the data acquisition of the main data set (ongoing project titled Behavioral Brain Research Project of Chinese Personality [BBP]) and three important events during the COVID-19 pandemic in China. Before the pandemic (September–December 2019), participants of the BBP had completed an anxiety measurement immediately after brain scanning, which was considered baseline or daily anxiety compared with pandemic-related anxiety. On January 23, 2020, China imposed a lockdown in Wuhan to quarantine the center of an outbreak of COVID-19. On February 17, 2020, the number of existing confirmed cases reached the peak in China. The authors’ first online pandemic questionnaire survey was performed during the severe period of the pandemic (February 22–28, 2020). On April 8, 2020, the Wuhan lockdown officially ended. The authors’ second online pandemic questionnaire survey was performed during the remission period of the pandemic (April 24–May 1, 2020). Panel B shows how to use the functional connectome to predict an individual’s anxiety score (daily anxiety and pandemic-related anxiety) based on a machine-learning framework. Nested 10-fold cross-validation (10F-CV) was used for the elastic net regression and support vector regression, with the outer 10F-CV estimating the generalizability of the model and the inner 10F-CV determining the optimal parameter. Notably, the relevance vector regression has no algorithm-specific parameter, thus traditional 10F-CV (no inner 10F-CV) was used for relevance vector regression. Panel C shows the consensus functional connectivity (FC) that appeared in all cross-validation rounds (i.e., 200 rounds, because 10F-CV was repeated 20 times). Panel D shows the external validation and clinical extension of the identified consensus FC. For external validation, Pearson’s partial correlation was used to examine the relationship between the connectivity strength of the consensus FC (by summing the FC strength) and pandemic-related anxiety in an independent sample, as well as the pandemic-related anxiety ratings that were collected from the second online survey in the BBP. For the clinical extension, binary logistic regression was used to examine whether the consensus FC could be used to distinguish between specific mental disorders (including generalized anxiety disorder, major depression, and schizophrenia) and matched healthy control subjects. All data in the figure are simulated data rather than actual data. ROC=receiver operating characteristic.
Selecting Consensus Functional Connectivity for External Validation
Each round of cross-validation generated slightly different functional connectivity. According to the optimal prediction performance of the functional connectome on pandemic-related anxiety, we retained the functional connectivity that appeared in all rounds (i.e., 200 rounds, because 10F-CV was repeated 20 times), referred to as the consensus functional connectivity (28, 29). To examine the reliability of the consensus functional connectivity, we calculated the Pearson’s partial correlation between the connectivity strength of the consensus functional connectivity (by summing the functional connectivity strength) and the anxiety scores that were collected from the second pandemic survey of the BBP after controlling for sex, age, head motion, and baseline STAI-T scores. We also extracted the consensus functional connectivity from the validation sample and calculated the Pearson’s partial correlation between the functional connectivity strengths and pandemic-related anxiety after controlling for sex, age, and head motion. No baseline anxiety measures were available for the validation sample.
Clinical Extension
Finally, we used binary logistic regression to examine whether the consensus functional connectivity of pandemic-related anxiety could be used to distinguish between specific mental disorders and matched healthy control subjects. To overcome the overfitting problem that may be caused by a set of features, we applied an L2-regularization technique to address this issue, which minimizes the sum of the squares of regression coefficients and retains all features in the model (27, 30). We applied nested 10F-CV, with the outer 10F-CV estimating the generalizability of the model and the inner 10F-CV determining the optimal regularization parameter (for further details, see the online supplement). The area under the receiver operating characteristic curve (AUC) and classification accuracy were used to assess the classification performance. Because each random division results in a different testing set and training set, we repeated nested 10F-CV 20 times and averaged the classification results to generate the overall accuracy. We performed two types of permutation tests to ensure that the classification results were significantly better than random. For the first type of permutation test (pt1), we retained the consensus functional connectivity but randomly shuffled the diagnostic label 1,000 times and ran the prediction pipeline to obtain a null distribution of classification performance. For the second type of permutation test (pt2), we retained the diagnostic label but randomly selected the same number of consensus functional connectivity 1,000 times and ran the prediction pipeline to obtain a null distribution of classification performance.
Code and Data Availability
The Pattern Recognition for Neuroimaging Toolbox (http://www.mlnl.cs.ucl.ac.uk/pronto) was used to implement relevance vector regression (31), the Glmnet toolbox (https://web.stanford.edu/∼hastie/glmnet_matlab) was used to implement elastic net regression and binary logistic regression, and LIBSVM (https://www.csie.ntu.edu.tw/∼cjlin/libsvm) was used to implement support vector regression (32). The SLIM cohort (http://fcon_1000.projects.nitrc.org/indi/retro/southwestuni_qiu_index.html), major depression data set (http://rfmri.org/REST-meta-MDD), and schizophrenia data set (http://fcon_1000.projects.nitrc.org/indi/retro/cobre.html) are available online. The code used in this study is also available online (https://osf.io/rxnvs), and the data are available from the authors on request.
RESULTS
Behavioral Data
A spatial map of COVID-19 case information and the sample size of the BBP during our pandemic survey are presented in Figure S2 in the online supplement. The case data were obtained from the National Health Commission of the People’s Republic of China. The number of existing, confirmed COVID-19 cases (N=58,016) peaked on February 17, 2020. The pandemic remained severe during our first online questionnaire collection period from February 22 to 28, 2020, when the number of existing, confirmed cases ranged from 39,919 to 54,965. After this, the peak of the initial COVID-19 outbreak was passed in China, with a gradually decreasing number of confirmed cases. The number of existing, confirmed cases ranged from 557 to 838 during our second online questionnaire collection period from April 24 to May 1, 2020. One-way repeated-measures analysis of variance showed that the STAI-T scores for the BBP increased from baseline to the severe (first pandemic survey) and remission (second pandemic survey) stages of the pandemic (F=24.70, df=2, 970, p<0.001, η2=0.048; Figure 2). Bonferroni post hoc tests showed that the STAI-T scores at the severe (p<0.001, 95% CI=0.63, 2.16) and remission (p<0.001, 95% CI=1.49, 3.22) stages of the pandemic were higher than the prepandemic baseline scores and that the STAI-T scores at the remission stage were higher than those at the severe stage (p=0.012, 95% CI=0.16, 1.75), even though the pandemic had greatly improved in China during our second pandemic survey.
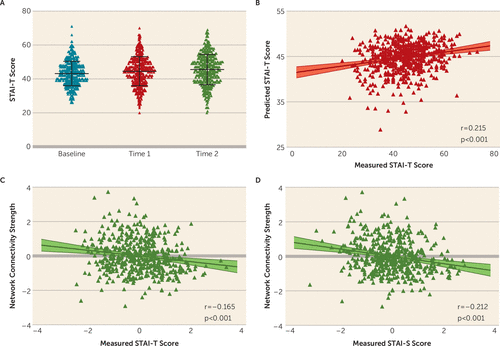
FIGURE 2. Anxiety score changes and prediction of pandemic-related anxiety from the prepandemic functional connectomea
a Panel A shows that scores on the trait scale of the State-Trait Anxiety Inventory (STAI-T) for participants in the Behavioral Brain Research Project of Chinese Personality increased from baseline to the severe stage of the pandemic and to the remission stage of the pandemic. Panel B shows the optimal Pearson’s correlation between the predicted and measured STAI-T scores during the severe period of the pandemic (first pandemic survey) using relevance vector regression. Panels C and D show the Pearson’s partial correlation between the connectivity strength of the consensus functional connectivity (by summing the functional connectivity strength) and anxiety scores (STAI-T and state scale of the STAI [STAI-S]) that were collected from the second pandemic survey. Standardized residuals are shown in the two scatterplots after controlling for sex, age, head motion, and prepandemic baseline anxiety.
Because we lacked baseline STAI-S scores, we used paired-sample t tests, which showed that the STAI-S scores at the second pandemic survey were higher than those at the first pandemic survey (t=2.71, df=485, p=0.007; 95% CI=0.29, 1.81; Cohen’s d=0.112). We collected two open questions about the concerns of COVID-19 infection during the first pandemic and found that individual anxiety scores were positively correlated with self-concern (STAI-T: r=0.140, p<0.001; STAI-S: r=0.156, p<0.001) and family concern (STAI-T: r=0.159, p<0.001; STAI-S: r=0.162, p<0.001).
Prediction of the Individualized Anxiety Score
According to the optimal prediction performance, the prepandemic functional connectome predicted individuals’ STAI-T and STAI-S scores during the severe period of the pandemic using relevance vector regression (STAI-T: r=0.215, ppt<0.001, mean absolute error=6.97, Figure 2B; STAI-S: r=0.185, ppt<0.001, mean absolute error=7.93; see also Figure S3 in the online supplement). Two other methods showed similar prediction results, as illustrated in Figures S4 and S5 in the online supplement (elastic net regression: STAI-T: r=0.191, ppt<0.001, mean absolute error=6.96; STAI-S: r=0.195, ppt<0.001, mean absolute error=7.84; support vector regression: STAI-T: r=0.215, ppt<0.001, mean absolute error=6.93; STAI-S: r=0.186, ppt<0.001, mean absolute error=7.81).
However, the functional connectome showed poor performance for predicting individuals’ daily anxiety, even though anxiety scores were collected immediately after brain scanning. According to the optimal prediction performance, there was no close correlation between the predicted and measured daily anxiety in the BBP data set (STAI-T: r=0.016, ppt>0.05, mean absolute error=6.34; see Figure S3 in the online supplement). The SLIM cohort further confirmed the poor prediction of the functional connectome on daily anxiety (STAI-T: r=0.104, ppt>0.05, mean absolute error=6.57; STAI-S: r=0.048, ppt>0.05, mean absolute error=7.07; see Figure S3 in the online supplement). Similarly, poor predictive performance was also found using elastic net regression and support vector regression (see Figures S4 and S5 in the online supplement).
Consensus Functional Connectivity and External Validation
Given that the functional connectome had a greater advantage in predicting the STAI-T score during the severe period of the COVID-19 pandemic, subsequent analyses focused on the connections at the optimal threshold of feature selection (i.e., p=5 × 10−4; see Figure S3 in the online supplement). Only 32 connections occurred in all rounds and were defined as the consensus functional connectivity that also survived correction for multiple comparisons across the whole-brain functional connectivity using the full BBP data set (false discovery rate corrected p<0.05). The correlation coefficients between the consensus functional connectivity and the pandemic-related STAI-T scores ranged from −0.223 to −0.180 after controlling for sex, age, head motion, and baseline STAI-T scores (see Table S3 in the online supplement). Figure 3A shows that the consensus functional connectivity primarily included connections between the prefrontal cortex, insula, anterior cingulate cortex, and subcortical nuclei (e.g., thalamus and putamen), as well as the connections between the insula, thalamus, hippocampus, parahippocampal gyrus, and sensorimotor cortex. To generate a simple interpretation of the importance of each node within the consensus network, we used a common measure (i.e., node strength). Node strength is the sum of the weights of links connected to the node (33). The correlation coefficients between the consensus functional connectivity and anxiety scores were used as the weights of links, and then node strength was computed by summing the absolute value of the correlation coefficients (because all correlation coefficients have negative values; see Table S3 in the online supplement). Regions with higher node strength were mainly located in the insula, thalamus, prefrontal cortex, anterior cingulate cortex, hippocampus, and parahippocampal gyrus (Figure 3B).
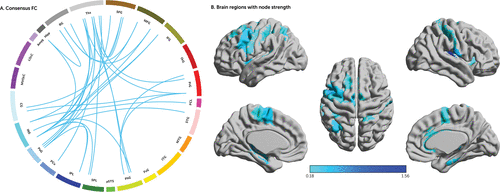
FIGURE 3. Consensus functional connectivity and node strength within the consensus networka
a Panel A shows the consensus functional connectivity (FC) in a circle plot with 246 nodes that are grouped into 24 macroscale brain regions. According to the optimal prediction performance of the functional connectome on pandemic-related anxiety, only 32 connections occurred in all rounds and were defined as the consensus FC related to pandemic anxiety. Panel B shows the node strength of the consensus FC. The correlation coefficients between the consensus FC and anxiety scores were used as the weights of links, and then node strength was computed by summing the absolute value of the correlation coefficients (because all correlation coefficients have negative values, see also Table S3 in the online supplement). Amyg=amygdala; BG=basal ganglia; CG=cingulate gyrus; FuG=fusiform gyrus; Hipp=hippocampus; IFG=inferior frontal gyrus; INS=insular gyrus; IPL=inferior parietal lobule; ITG=inferior temporal gyrus; LOcC=lateral occipital cortex; MFG=middle frontal gyrus; MTG=middle temporal gyrus; MVOcC=medioventral occipital cortex; OrG=orbital gyrus; PCL=paracentral lobule; Pcun=precuneus; PhG=parahippocampal gyrus; PoG=postcentral gyrus; PrG=precentral gyrus; pSTS=posterior superior temporal sulcus; STG=superior temporal gyrus; SFG=superior frontal gyrus; SPL=superior parietal lobule; Tha=thalamus.
The strength of the consensus functional connectivity correlated with individuals’ anxiety scores during the remission period of the pandemic (second pandemic survey) after controlling for sex, age, head motion, and baseline anxiety (STAI-T: r=−0.165, p<0.001; STAI-S: r=−0.212, p<0.001; Figure 2). Moreover, we extracted the consensus functional connectivity from the validation sample and also found that the strength of the consensus functional connectivity correlated with the pandemic-related STAI-S score (r=−0.179, p=0.034) but not significantly with the STAI-T score (r=−0.045, p>0.05).
Mental Disorder Classification
Finally, we tested the clinical relevance of the consensus functional connectivity of pandemic-related anxiety in generalized anxiety disorder, major depression, and schizophrenia. We extracted the consensus functional connectivity from the three clinical data sets and performed nested 10F-CV using binary logistic regression with L2 regularization. The classifier achieved a significant classification performance that distinguished participants with generalized anxiety disorder and matched healthy control subjects (AUC=0.720, ppt1=0.025, ppt2=0.012; accuracy=68.72%, ppt1=0.025, ppt2=0.014; sensitivity=71.40%, specificity=65.00%). However, the classifier showed poor classification performance for major depression (AUC=0.537, ppt1>0.05, ppt2>0.05; accuracy=53.68%, ppt1>0.05, ppt2>0.05; sensitivity=72.20%, specificity=33.13%) and schizophrenia (AUC=0.596, ppt1>0.05, ppt2>0.05; accuracy=59.10%, ppt1>0.05, ppt2>0.05; sensitivity=32.88%, specificity=73.91%).
Discussion
The unexpected pandemic rapidly spread to the entire world and had a pervasive effect on public mental health, especially generally increased feelings of anxiety. We conducted a prospective cohort study and collected anxiety ratings for a considerable number of participants during the severe and remission periods of the COVID-19 pandemic, and these participants also completed brain scanning and baseline anxiety measures before the pandemic. This study provides evidence that the prepandemic individualized functional connectome predicted pandemic-induced anxiety but showed poor performance for predicting daily anxiety. Moreover, the identified consensus functional connectivity of pandemic-related anxiety could distinguish between participants with generalized anxiety disorder and matched healthy control subjects but was not sensitive to the identification of other mental disorders, such as major depression and schizophrenia. These findings suggest that individual anxiety was highly susceptible to the great stress and uncertainty during this unprecedented period, which contributes to identifying reliable neuromarkers of anxiety and could inform particular psychological intervention and clinical practice.
People experienced increased feelings of anxiety during the pandemic, as shown by comparing individuals’ anxiety scores that were collected before and during the severe and remission periods. Differently from everyday life situations, the uncertainty of infection, social and physical isolation, and other stressors caused by COVID-19 aggravated public anxiety and amplified individual differences during these extraordinary circumstances (3, 14, 34, 35). Although the number of COVID-19 cases dropped sharply between our two surveys and the pandemic had greatly improved, people’s anxiety levels did not decline but slightly increased, suggesting that the pandemic has sustained adverse effects on mental health and that we need to pay attention to the long-term mental health consequences of the COVID-19 pandemic (15). The amplified individual differences in anxiety also provide a rare opportunity to construct reliable brain-behavior associations to deepen our knowledge of the neural basis of anxiety.
Connectome-based prediction analyses revealed that the prepandemic functional connectome predicted individuals’ anxiety scores during the severe period of the pandemic, and the identified consensus functional connectivity could further capture significant variance of the anxiety scores that were collected during the remission period and was also validated on an independent sample. However, the functional connectome showed poor performance for predicting daily anxiety, even though the anxiety scores were collected immediately after brain scanning. These findings indicate that the intrinsic functional network is more sensitive as a marker of the severity of anxiety induced by real major stressful events, such as this global health crisis, and provide a reasonable explanation in the current debate that the prediction of functional networks on anxiety is not stable (11, 12). The latter may be caused by the fact that the lack of large and unexpected stressors in daily life weakens the ability to capture actual individual differences in stress-related emotional consequences, such as anxiety.
We further demonstrated that the connectome-based neuromarkers of pandemic-related anxiety distinguished between participants with generalized anxiety disorder and healthy control subjects. The specific clinical extension expands recent behavioral findings that the population prevalence of clinically significant levels of anxiety greatly increased during the pandemic (6), revealing a link between the neural correlates of pandemic-related anxiety and pathological anxiety, and suggests that the consensus functional connectivity is likely to be a core component of the neural basis of pathological anxiety. However, the consensus functional connectivity was not sensitive to the identification of other mental disorders, such as major depression and schizophrenia. Although previous studies have revealed that there are common and unique neural circuits across the spectrum of depression and anxiety disorders (36, 37), the clinical extension from the neural correlates of pandemic-related anxiety identified in people without psychiatric illness to those with major depression may be limited because of the different types of psychopathology symptoms in anxiety and depression and also because of sample heterogeneity. Also, the identified neural correlates of pandemic-related anxiety may not involve the connections from the default mode network that are found in the neural circuits of major depression (38). The classification analysis confirms the potential clinical relevance of the neuromarkers of pandemic-related anxiety and also highlights the higher susceptibility to mental distress (e.g., pathological anxiety) during the pandemic. The anxiety-specific neuromarkers may provide a special contribution to novel clinical practice for stress and anxiety reduction, such as defining connectome-based targets for neuromodulation (39, 40).
The consensus functional connectivity mainly comprised connections across multiple areas and primarily included two distributed neural circuits. The first neural circuit included the connections between the prefrontal cortex, insula, anterior cingulate cortex, and subcortical nuclei (e.g., thalamus and putamen). The weaker connectivity between the prefrontal cortex and the thalamus, as well as that between the prefrontal cortex, anterior cingulate cortex, and insula, may reflect lower effects of top-down executive control mechanisms (41) that thereby are unable to successfully inhibit or regulate somatic and autonomic bodily states (42, 43) and the hyperactivity caused by detecting and filtering salient stressful events (44), which may account for increased signs of pandemic-related anxiety. The second neural circuit also included weaker connectivity involving the insula and the thalamus but additionally involving the hippocampus, parahippocampal gyrus, and sensorimotor cortex associated with later higher pandemic-related anxiety. This suggests that the interaction between somatic arousal, interoceptive awareness, and adverse event memory may also be predisposing factors for anxiety in stress-provoking situations, such as the COVID-19 pandemic. Additionally, recent findings have revealed that cognitive-behavioral therapy modulated the activity of some core regions (e.g., the prefrontal cortex and anterior cingulate cortex) in our identified neural circuit during panic-related semantic processing in patients with panic disorder (45), as well as during the processing of cognitive reappraisal of negative self-beliefs in patients with social anxiety disorder (46). This evidence emphasizes that individuals with high pandemic-related anxiety may benefit from evidence-based therapeutic approaches that promote stress reduction and from cognitive-behavioral strategies that reduce anxiety.
There are several limitations to this study. Anxiety scores were collected from undergraduate students and may not be representative of the general public. Further work will be useful to assess the predictive validity in healthy individuals in the general population, as well as in specific groups such as frontline health care workers, who are fully exposed to anxiety-provoking situations such as COVID-19 and may experience more mental distress (47). Although we used strict inclusion criteria to ensure that participants from the three undergraduate student data sets had no psychiatric illness or major physical health problems before brain scanning and that no one reported having been diagnosed with COVID-19, the lack of diagnostics during the pandemic may have some potential impact on the inference of our predictive results. In addition, the heterogeneity of the three clinical data sets (e.g., different scanning sites and unmatched demographic information) may be regarded as a limitation to understanding our results for clinical extension. Additionally, people have shown varying degrees of changes in psychopathology symptoms from before the pandemic to during the pandemic (6, 16). Also, many symptoms will subside for some people but persist for others as the pandemic wanes in the coming months (35). The examination of which brain features determine individual anxiety changes from baseline to when the outbreak ended is another promising direction for future studies using long-term follow-up.
Conclusions
Combining multiple data sets and a machine-learning predictive framework, this longitudinal cohort study demonstrates that the prepandemic functional connectome predicted pandemic-related anxiety but showed poor performance in predicting daily anxiety. The consensus functional connectivity identified from the predictive model of pandemic-related anxiety could further specifically detect participants with generalized anxiety disorder but was not sensitive to the identification of major depression and schizophrenia. These findings advance our understanding of the neurobiological basis of anxiety susceptibility and may have implications for developing particular psychological and clinical interventions for individuals who are at risk for high anxiety induced by major stressful events, such as COVID-19.
1 , : Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry 2020; 7:547–560Crossref, Medline, Google Scholar
2 , : Mental health response to the COVID-19 outbreak in China. Am J Psychiatry 2020; 177:574–575Link, Google Scholar
3 , : The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet 2020; 395:912–920Crossref, Medline, Google Scholar
4 : Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA 2020; 323:2133–2134Crossref, Medline, Google Scholar
5 : Suicide mortality and coronavirus disease 2019: a perfect storm? JAMA Psychiatry (Epub ahead of print April 10, 2020) Google Scholar
6 : U.S. Census Bureau-assessed prevalence of anxiety and depressive symptoms in 2019 and during the 2020 COVID-19 pandemic. Depress Anxiety 2020; 37:954–956Crossref, Medline, Google Scholar
7 , : Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci 2015; 18:1664–1671Crossref, Medline, Google Scholar
8 , : Functional connectivity predicts changes in attention observed across minutes, days, and months. Proc Natl Acad Sci USA 2020; 117:3797–3807Crossref, Medline, Google Scholar
9 : Intersubject similarity of personality is associated with intersubject similarity of brain connectivity patterns. Neuroimage 2019; 186:56–69Crossref, Medline, Google Scholar
10 , : Large-scale functional brain network architecture changes associated with trauma-related dissociation. Am J Psychiatry 2021; 178:165–173Link, Google Scholar
11 : Toward robust anxiety biomarkers: a machine learning approach in a large-scale sample. Biol Psychiatry Cogn Neurosci Neuroimaging 2020; 5:799–807Crossref, Medline, Google Scholar
12 , : Brain connectome mapping of complex human traits and their polygenic architecture using machine learning. Biol Psychiatry 2020; 87:717–726Crossref, Medline, Google Scholar
13 , : Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science 2011; 334:1151–1153Crossref, Medline, Google Scholar
14 : Virtual treatment and social distancing. Lancet Psychiatry 2020; 7:388–389Crossref, Medline, Google Scholar
15 , : Changes in network centrality of psychopathology symptoms between the COVID-19 outbreak and after peak. Mol Psychiatry 2020; 25:3140–3149Crossref, Medline, Google Scholar
16 , : Mental health before and during the COVID-19 pandemic: a longitudinal probability sample survey of the UK population. Lancet Psychiatry 2020; 7:883–892Crossref, Medline, Google Scholar
17 , : Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012; 59:2142–2154Crossref, Medline, Google Scholar
18 , : DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 2016; 14:339–351Crossref, Medline, Google Scholar
19 , : Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain 2016; 139:3296–3309Crossref, Medline, Google Scholar
20 , : Longitudinal test-retest neuroimaging data from healthy young adults in southwest China. Sci Data 2017; 4:170017Crossref, Medline, Google Scholar
21 , : Decreased intrinsic functional connectivity in first-episode, drug-naive adolescents with generalized anxiety disorder. Front Hum Neurosci 2019; 12:539Crossref, Medline, Google Scholar
22 , : Functional imaging of the hemodynamic sensory gating response in schizophrenia. Hum Brain Mapp 2013; 34:2302–2312Crossref, Medline, Google Scholar
23 :
24 , : The human Brainnetome Atlas: a new brain atlas based on connectional architecture. Cereb Cortex 2016; 26:3508–3526Crossref, Medline, Google Scholar
25 : Sparse Bayesian learning and the relevance vector machine. J Mach Learn Res 2001; 1:211–244Google Scholar
26 , : Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protoc 2017; 12:506–518Crossref, Medline, Google Scholar
27 : The effect of machine learning regression algorithms and sample size on individualized behavioral prediction with functional connectivity features. Neuroimage 2018; 178:622–637Crossref, Medline, Google Scholar
28 , : Prediction of individual brain maturity using fMRI. Science 2010; 329:1358–1361Crossref, Medline, Google Scholar
29 , : Connectome-based individualized prediction of temperament trait scores. Neuroimage 2018; 183:366–374Crossref, Medline, Google Scholar
30 : Regularization and variable selection via the elastic net. J R Stat Soc Series B Stat Methodol 2005; 67:301–320Crossref, Google Scholar
31 , : PRoNTo: pattern recognition for neuroimaging toolbox. Neuroinformatics 2013; 11:319–337Crossref, Medline, Google Scholar
32 : LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol 2011; 2:27Crossref, Google Scholar
33 : Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010; 52:1059–1069Crossref, Medline, Google Scholar
34 , : Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Network Open 2020; 3(3):e203976-eCrossref, Google Scholar
35 , : A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behav Immun 2020; 87:40–48Crossref, Medline, Google Scholar
36 : Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry 2011; 168:968–978Link, Google Scholar
37 , : Distinctive and common neural underpinnings of major depression, social anxiety, and their comorbidity. Soc Cogn Affect Neurosci 2015; 10:552–560Crossref, Medline, Google Scholar
38 , : Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc Natl Acad Sci USA 2019; 116:9078–9083Crossref, Medline, Google Scholar
39 : Opportunities of connectomic neuromodulation. Neuroimage 2020; 221:117180Crossref, Medline, Google Scholar
40 , : Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry 2020; 177:435–446Link, Google Scholar
41 : Frontal cortex and the hierarchical control of behavior. Trends Cogn Sci 2018; 22:170–188Crossref, Medline, Google Scholar
42 : Brain state dependent activity in the cortex and thalamus. Curr Opin Neurobiol 2015; 31:133–140Crossref, Medline, Google Scholar
43 : Functions of the anterior insula in taste, autonomic, and related functions. Brain Cogn 2016; 110:4–19Crossref, Medline, Google Scholar
44 : Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 2015; 16:55–61Crossref, Medline, Google Scholar
45 , : Effect of CBT on biased semantic network in panic disorder: a multicenter fMRI study using semantic priming. Am J Psychiatry 2020; 177:254–264Link, Google Scholar
46 , : Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA Psychiatry 2013; 70:1048–1056Crossref, Medline, Google Scholar
47 , : The mental health effects of COVID-19 on health care providers in China. Am J Psychiatry 2020; 177:635–636Link, Google Scholar



