Association of Prospective Risk for Chronic PTSD Symptoms With Low TNFα and IFNγ Concentrations in the Immediate Aftermath of Trauma Exposure
Abstract
Objective:
Although several reports have documented heightened systemic inflammation in posttraumatic stress disorder (PTSD), few studies have assessed whether inflammatory markers serve as prospective biomarkers for PTSD risk. The present study aimed to characterize whether peripheral immune factors measured in blood samples collected in an emergency department immediately after trauma exposure would predict later chronic development of PTSD.
Methods:
Participants (N=505) were recruited from a hospital emergency department and underwent a 1.5-hour assessment. Blood samples were drawn, on average, about 3 hours after trauma exposure. Follow-up assessments were conducted 1, 3, 6, and 12 months after trauma exposure. Latent growth mixture modeling was used to identify classes of PTSD symptom trajectories.
Results:
Three distinct classes of PTSD symptom trajectories were identified: chronic (N=28), resilient (N=160), and recovery (N=85). Multivariate analyses of covariance revealed a significant multivariate main effect of PTSD symptom trajectory class membership on proinflammatory cytokines. Univariate analyses showed a significant main effect of trajectory class membership on plasma concentrations of proinflammatory tumor necrosis factor α (TNFα) and interferon-γ (IFNγ). Concentrations of proinflammatory TNFα and IFNγ were significantly lower in individuals in the chronic PTSD class compared with those in the recovery and resilient classes. There were no significant differences in interleukin (IL) 1β and IL-6 concentrations by PTSD symptom trajectory class. Anti-inflammatory and other cytokines, as well as chemokines and growth factor concentrations, were not associated with development of chronic PTSD.
Conclusions:
Overall, the study findings suggest that assessing the proinflammatory immune response to trauma exposure immediately after trauma exposure, in the emergency department, may help identify individuals most at risk for developing chronic PTSD in the aftermath of trauma.
Posttraumatic stress disorder (PTSD) is a DSM-5 psychiatric syndrome that develops after exposure to a traumatic event (1). PTSD is a heterogeneous disorder often characterized by an assortment of hyperarousal, reexperiencing, and avoidance/numbing symptoms, as well as negative alterations in cognition and mood (1). Epidemiological reports indicate that up to 70% of the general population will experience a traumatic event during their lifetime, and 7% of those individuals will go on to develop PTSD (2). Rates of trauma exposure and lifetime PTSD are higher in military samples (3) and in urban environments (4). Because the prevalence of PTSD and the adverse psychological and economic consequences of PTSD continue to climb, it is imperative that prospective biomarkers be characterized so that individuals at risk for developing PTSD in the aftermath of trauma exposure can be identified. Early identification will allow earlier treatment with psychological and pharmacological interventions that have been shown to be effective in attenuating PTSD development (5, 6).
One biological system that may confer individual risk for development of PTSD is the immune system (7). Increased basal concentrations of proinflammatory cytokines, including interleukin (IL) 1β, IL-6, and interferon-γ (IFNγ), have consistently been found to be associated with PTSD (8). Higher concentrations of chemokines and immune system growth factors have also been associated with trauma exposure (9) and chronic PTSD (10, 11). Additionally, resilience to traumatic events (12) and PTSD are associated with higher concentrations of anti-inflammatory cytokines, such as IL-10 (11, 13). Basic and translational data show that proinflammatory cytokines can directly affect the CNS, as demonstrated by measures of neurochemistry (14) and brain circuitry implicated in the pathophysiology of PTSD, including the amygdala (15, 16) and the prefrontal cortex (17), to facilitate the expression of the cardinal psychiatric symptoms of PTSD. While many studies have demonstrated an association between a proinflammatory state and PTSD, there is a paucity of evidence on whether inflammatory markers can serve as prospective risk factors for the development of PTSD.
To date, only one study has addressed the question of whether heightened systemic inflammation prior to trauma exposure is a risk factor for the development of PTSD in the aftermath of trauma (18). That data, from the Marine Resiliency Study, showed that higher predeployment concentrations of C-reactive protein, a systemic inflammatory marker, increased risk for postdeployment development of PTSD in a predominantly male sample of marines (18). Although the study was of interest in showing that heightened immune system activation confers individual risk for PTSD development, the difficulty in securing biological samples before trauma exposure in large numbers of individuals precludes the adaptability of such measures to assessment of prospective risk for PTSD. Thus, the present study was designed to characterize immune factors that predict chronic PTSD development in blood samples collected in the immediate aftermath of trauma exposure in an emergency department setting. Given preexisting data showing that a proinflammatory state is associated with greater PTSD symptom severity (8), we hypothesized that higher concentrations of proinflammatory cytokines, including IL-1β, IL-6, tumor necrosis factor α (TNFα), and IFNγ, and higher concentrations of chemokines and growth factors in the acute posttraumatic period, would be associated with increased risk for development of chronic PTSD. Furthermore, we hypothesized that lower concentrations of anti-inflammatory cytokines would be associated with prospective development of PTSD.
Methods
Participants
Participants (N=505) between the ages of 18 and 65 years were recruited between June 2012 and January 2017 from the emergency department at Grady Memorial Hospital in Atlanta after experiencing a DSM-IV criterion A trauma within the past 24 hours (19). Other inclusion criteria were ability to provide informed consent, ability to understand and speak English, provision of blood samples (obtained by emergency department care staff), and access to a telephone to allow contact for follow-up appointment scheduling. Individuals were excluded if they had a current or past history of mania, schizophrenia, or other psychoses; prominent suicidal ideation in the past month; intoxication, severe pain, active labor, or respiratory distress; anticipation of immediate surgery or admission to the intensive care unit; medical instability; or hemodynamic compromise.
Eligible trauma survivors were approached by trained evaluators in the emergency department after initial medical evaluation, appropriate laboratory testing, and medical clearance had occurred. After providing informed consent, participants underwent a bedside assessment that lasted 1.5 hours. Study participants returned for follow-up visits at 1, 3, 6, and 12 months for assessment of the development of PTSD symptoms. Participants were compensated $50 at each of the assessments for their participation.
All study procedures were reviewed and approved by the Emory University Institutional Review Board and the Grady Hospital Research Oversight Committee. All study data were recorded and managed electronically using the REDCap platform (20).
Measures
A standardized trauma interview was administered in the emergency department to collect sociodemographic data (e.g., sex, age, race, income, body mass index [BMI]) and characteristics of the presenting trauma exposure, including the type of trauma (interpersonal or noninterpersonal) (21). The PTSD Symptom Scale (22) was used to assess the development of PTSD symptoms at the 1-, 3-, 6-, and 12-month follow-ups, as described previously (23); interrater reliability was 97%.
PTSD Symptom Trajectories
Latent growth mixture modeling was applied using Mplus, version 7, to determine how many distinct latent classes best describe the trajectories of PTSD symptom severity based on PTSD Symptom Scale scores in the study data set when available (only one assessment, 15.6%; two assessments, 13.3%; three or more assessments, 71.0%). To identify the best-fitting number of trajectory classes, we started with up to six possible classes and examined linear and quadratic slope to identify the best-fitting trajectory shape. We used a nested-model approach to test a progressive number of classes until the model fit indices no longer favored the addition of any more classes. Relevant criteria for determining the number of classes included the reduction in the Bayesian information criterion, sample-size-adjusted Bayesian information criterion, Akaike information criterion indices, and significance indicated by the Vuong-Lo-Mendell-Rubin likelihood and the bootstrap likelihood, together with parsimony and interpretability. Clarity of class specification was determined by entropy. Trajectory analyses were run on the sample of participants who had at least one follow-up assessment (N=274). Participants were assigned to one of the three identified trajectories (chronic, recovery, or resilient) on the basis of their most likely trajectory class membership (highest posterior probability).
Blood Sample Collection and Cytokine Measurements
Venous blood samples were collected in EDTA tubes in the emergency department after trauma exposure (mean time elapsed between trauma and blood sampling, 201 minutes [SEM=12.8]) by medical staff using standard techniques. Within 6 hours of collection, EDTA tubes were centrifuged at 4°C and the plasma was aliquoted into 500 μL samples and frozen at −80°C until time of assay. Twenty-seven cytokines, chemokines, and growth factors were quantified in plasma available from 274 participants with at least one follow-up assessment using a commercially available human multiplex assay (M500KCAF0Y, Bio-Rad, Hercules, Calif.) on a MAGPIX instrument (Luminex, Austin, Tex.).
Samples were run on four plates by an analyst who was blind to PTSD outcomes. Three samples were run on every plate to control for interplate variability (coefficient of variation, 0.7%−16%) and intraplate variability (coefficient of variation, 4.5%−11.2%). Assays were checked for quality control to fit the standard curve. One participant (assigned to the resilient class) was excluded as an outlier because of aberrantly high values across the board. Proinflammatory cytokines assessed were IL-1β, IL-6, TNFα, and IFNγ. Anti-inflammatory factors assayed were IL-10 and IL-1RA. Other cytokines (IL-5, IL-15, IL-2, IL-13, IL-4, IL-9, IL-12, and IL-17) were also assessed. Chemokines measured were eotaxin/CCL11, MIP-1α/CCL3, IP-10/CCL10, MCP-1/CCL2, IL-8, MIP-1β/CCL4, and RANTES/CCL5. Growth factors assayed were granulocyte macrophage-colony stimulating factor (GM-CSF), basic fibroblast growth factor (bFGF), IL-7, and platelet-derived growth factor BB (PDGF-BB). G-CSF and VEGF were not detected. Because of the low concentrations of IFNγ and the Bio-Rad kit’s lower assay sensitivity of 6.1 pg/mL, we confirmed the values in a subset of the participants (N=84) with a magnetic Luminex performance assay, human IFNγ high sensitivity (LHSCM285B, R&D Systems), to ensure that the low concentration values of IFNγ were reproducible with a different assay (data not shown).
Statistical Analysis
The data were analyzed using SPSS, version 24 (IBM, Armonk, N.Y.) and summarized as mean values and standard error of the mean. The alpha level was set at 0.05 for statistical significance. One-way analyses of variance and chi-square tests were used to assess baseline sociodemographic differences in PTSD trajectory class (chronic, recovery, and resilient). Multivariate analyses of covariance (MANCOVAs) were used to test our hypotheses that peritraumatic concentrations of proinflammatory and anti-inflammatory factors and cytokines, chemokines, and growth factors would be associated with increased risk for chronic PTSD symptom development, controlling for sex, BMI, time elapsed between trauma exposure and blood sampling, time of day of blood sampling, and incident and premorbid interpersonal trauma exposure.
Results
Latent growth mixture modeling analyses assigned 28 individuals to the chronic PTSD class, 85 to the recovery class, and 160 to the resilient class (Table 1). There were no PTSD trajectory class group differences in age, sex, BMI, race, income, and time elapsed between trauma exposure and blood sampling. Individuals in the chronic PTSD class were exposed to significantly higher rates of interpersonal trauma compared with individuals in the recovery and resilient classes.
| PTSD Symptom Trajectory Class | ||||||||
|---|---|---|---|---|---|---|---|---|
| Chronic | Recovery | Resilient | Analysis | |||||
| Mean | SEM | Mean | SEM | Mean | SEM | F | p | |
| Age (years) | 35.4 | 2.08 | 35.5 | 1.39 | 36.8 | 1.03 | 0.33 | 0.72 |
| Body mass index | 28.8 | 1.25 | 29.0 | 0.91 | 28.6 | 0.52 | 0.077 | 0.93 |
| Time between trauma exposure and blood sampling (minutes) | 177.2 | 35.3 | 225.6 | 22.5 | 193.3 | 17.3 | 0.86 | 0.43 |
| N | % | N | % | N | % | χ2 | p | |
| Sex | 5.55 | 0.062 | ||||||
| Male | 11 | 4 | 38 | 14 | 92 | 34 | ||
| Female | 17 | 6 | 47 | 17 | 68 | 25 | ||
| Trauma type | 13.0 | 0.001 | ||||||
| Interpersonal | 8 | 3 | 21 | 8 | 15 | 5 | ||
| Noninterpersonal | 20 | 7 | 65 | 24 | 145 | 53 | ||
| Premorbid interpersonal trauma | 12.1 | 0.002 | ||||||
| No | 5 | 2 | 28 | 10 | 77 | 28 | ||
| Yes | 23 | 8 | 58 | 21 | 83 | 31 | ||
| Race | 9.91 | 0.27 | ||||||
| Black | 23 | 8 | 70 | 25 | 107 | 39 | ||
| White | 3 | 1 | 11 | 4 | 35 | 13 | ||
| Other | 2 | 1 | 4 | 2 | 18 | 7 | ||
| Monthly income ($) | 12.3 | 0.14 | ||||||
| 0–249 | 3 | 1 | 6 | 3 | 12 | 5 | ||
| 250–499 | 0 | 0 | 7 | 3 | 9 | 3 | ||
| 500–999 | 5 | 2 | 18 | 7 | 14 | 5 | ||
| 1000–1999 | 6 | 3 | 20 | 8 | 33 | 12 | ||
| 2000 or more | 13 | 5 | 33 | 12 | 87 | 31 | ||
TABLE 1. Associations between sociodemographic variables, trauma variables, and PTSD symptom trajectory classes in a study of peripheral immune factors measured in an emergency department immediately after trauma exposure
Association of Chronic PTSD With Lower Concentrations of TNFα and IFNγ
MANCOVA analyses found a multivariate main effect of trajectory class membership on proinflammatory cytokine concentrations (F=2.07, p=0.037) (Table 2). Subsequent univariate analyses showed a main effect of class membership on concentrations of TNFα (F=3.24, p=0.041) and IFNγ (F=4.27, p=0.015). Posttraumatic concentrations of TNFα were significantly lower in participants in the chronic PTSD class compared with those in the recovery (p=0.013) and resilient classes (p=0.022) (Figure 1A). Posttraumatic concentrations of TNFα did not differ significantly between participants in the recovery and resilient classes (p=0.68) (Figure 1A). Similarly, posttraumatic concentrations of IFNγ were significantly lower in individuals in the chronic PTSD class compared with those in the recovery (p=0.008) and resilient classes (p=0.005) (Figure 1B). Posttraumatic concentrations of IFNγ did not differ significantly between those in the recovery and resilient classes (p=0.91) (Figure 1B). Lastly, there were no significant differences in IL-1β and IL-6 concentrations between the three trajectory classes (p values >0.05) (Table 2).
| PTSD Symptom Trajectory Class | ||||||||
|---|---|---|---|---|---|---|---|---|
| Chronic | Recovery | Resilient | Analysis | |||||
| Marker | Mean | SEM | Mean | SEM | Mean | SEM | F | p |
| Proinflammatory cytokines | ||||||||
| IL-1β | 8.50 | 0.76 | 8.53 | 0.43 | 8.29 | 0.32 | 0.11 | 0.90 |
| IL-6 | 2.23 | 0.52 | 2.28 | 0.29 | 2.78 | 0.22 | 1.10 | 0.33 |
| TNFα | 27.8 | 2.02 | 33.6 | 1.16 | 33.0 | 0.85 | 3.24 | 0.041 |
| IFNγ | 2.40 | 0.38 | 3.56 | 0.22 | 3.59 | 0.16 | 4.27 | 0.015 |
| Anti-inflammatory factors | ||||||||
| IL-10 | 8.72 | 1.29 | 8.39 | 0.73 | 9.32 | 0.54 | 0.52 | 0.59 |
| IL-1RA | 257.1 | 135.9 | 485.8 | 77.6 | 400.3 | 56.8 | 1.15 | 0.32 |
| Other cytokines | ||||||||
| IL-5 | 9.09 | 1.21 | 10.3 | 0.69 | 11.0 | 0.50 | 1.19 | 0.31 |
| IL-15 | 23.9 | 5.05 | 32.9 | 2.88 | 32.7 | 2.11 | 1.41 | 0.25 |
| IL-2 | 6.31 | 0.68 | 6.62 | 0.39 | 7.12 | 0.29 | 0.87 | 0.42 |
| IL-13 | 2.34 | 0.25 | 2.67 | 0.14 | 2.52 | 0.11 | 0.77 | 0.47 |
| IL-4 | 1.54 | 0.13 | 1.71 | 0.075 | 1.76 | 0.055 | 1.17 | 0.31 |
| IL-9 | 103.8 | 8.02 | 111.6 | 4.57 | 114.9 | 3.35 | 1.86 | 0.16 |
| IL-12 | 2.73 | 0.33 | 3.17 | 0.19 | 3.41 | 0.14 | 1.17 | 0.31 |
| IL-17 | 30.3 | 2.32 | 34.2 | 1.32 | 33.9 | 0.97 | 0.84 | 0.43 |
| Chemokines | ||||||||
| Eotaxin | 31.9 | 4.70 | 35.1 | 2.68 | 35.7 | 1.97 | 0.26 | 0.77 |
| MIP-1α | 1.51 | 4.92 | 4.34 | 2.81 | 7.16 | 2.06 | 0.69 | 0.50 |
| RANTES | 14,750 | 3,501 | 9,062 | 1,998 | 10,854 | 1,463 | 1.04 | 0.36 |
| IL-8 | 6.13 | 6.01 | 7.57 | 3.43 | 11.3 | 2.51 | 0.54 | 0.58 |
| MCP-1 | 13.1 | 2.69 | 18.1 | 1.53 | 18.1 | 1.12 | 1.54 | 0.22 |
| MIP-1 β | 87.3 | 4.36 | 93.9 | 2.49 | 92.9 | 1.82 | 0.89 | 0.41 |
| IP-10 | 297.5 | 111.0 | 430.2 | 63.4 | 368.3 | 46.4 | 0.64 | 0.53 |
| Growth factors | ||||||||
| GM-CSF | 1.44 | 0.15 | 1.73 | 0.084 | 1.71 | 0.062 | 1.60 | 0.20 |
| IL-7 | 12.5 | 1.63 | 14.8 | 0.94 | 15.7 | 0.69 | 1.58 | 0.21 |
| PDGF-BB | 1,451.7 | 377.1 | 1,467.3 | 215.2 | 1,597.1 | 157.6 | 0.14 | 0.87 |
| bFGF | 34.1 | 2.41 | 34.1 | 1.38 | 35.1 | 1.01 | 0.20 | 0.82 |
TABLE 2. Concentrations of inflammatory markers in a study of peripheral immune factors measured in an emergency department immediately after trauma exposurea
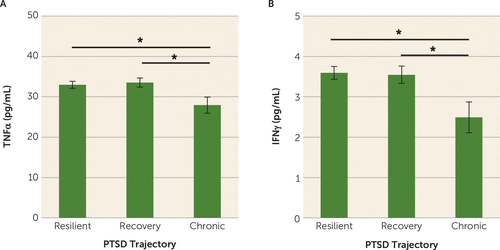
FIGURE 1. Mean TNFα and IFNγ concentrations in the overall sample in a study of peripheral immune factors measured in an emergency department immediately after trauma exposurea
a Asterisks denote significantly lower posttraumatic concentrations of tumor necrosis factor α (TNFα; panel A) and interferon-γ (IFNγ; panel B) in participants in the chronic PTSD trajectory class compared with those in the recovery and resilient trajectory classes (all p values <0.05). Error bars indicate standard error of the mean.
Because the number of individuals in the chronic PTSD class was disproportionately lower than in the recovery and resilient classes, we matched our 28 chronic PTSD case subjects to 28 recovery and 28 resilient control subjects on sociodemographic variables (age, sex, BMI, race, and income) and trauma-relevant variables (type of trauma and time elapsed between trauma exposure and blood sampling). Similar to the overall sample analysis, MANOVA analysis showed a multivariate main effect of trajectory class membership on proinflammatory cytokine concentrations (F=2.28, p=0.025). Posttraumatic concentrations of TNFα (F=3.23, p=0.045) and IFNγ (F=4.38, p=0.016) were significantly influenced by trajectory class (Figure 2). More specifically, posttraumatic concentrations of TNFα were significantly lower for individuals in the chronic PTSD class compared with those in the resilient class (p=0.015), but not those in the recovery class (p=0.14) (Figure 2A). Posttraumatic concentrations of TNFα did not differ significantly between participants in the recovery and resilient classes (p=0.29). Similarly, posttraumatic concentrations of IFNγ were significantly lower in individuals in the chronic PTSD class compared with those in the recovery (p=0.008) and resilient classes (p=0.02) (Figure 2B). Posttraumatic concentrations of IFNγ did not differ significantly between individuals in the recovery and resilient classes (p=0.72). Similar to the larger group analyses, there were no significant differences between the three trajectory classes in concentrations of IL-1β (F=0.067, p=0.80) and IL-6 (F=1.20, p=0.28).
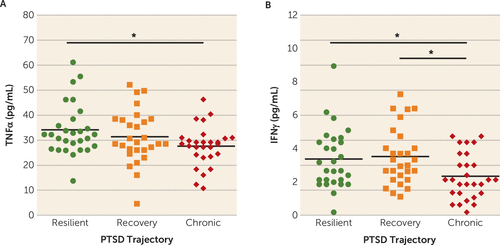
FIGURE 2. Mean TNFα and IFNγ concentrations in the matched analysis in a study of peripheral immune factors measured in an emergency department immediately after trauma exposurea
a In the matched analysis, the 28 chronic PTSD case subjects were matched to 28 recovery and 28 resilient control subjects on sociodemographic and trauma-relevant variables. In panel A, posttraumatic concentrations of tumor necrosis factor α (TNFα) were significantly lower in participants in the chronic PTSD trajectory class compared with those in the resilient trajectory class (p=0.015), but not those in the recovery trajectory class (p=0.14). In panel B, posttraumatic concentrations of interferon-γ (IFNγ) were significantly lower in participants in the chronic PTSD trajectory class compared with those in the recovery (p=0.008) and resilient trajectory classes (p=0.02).
No Association of Anti-Inflammatory Factors or Other Cytokine, Chemokine, and Growth Factor Concentrations With Development of Chronic PTSD
MANCOVA analyses showed no main effect of trajectory class membership on the anti-inflammatory factors assayed (F=0.87, p=0.48) (Table 2). Similarly, MANCOVA revealed no main effect of class membership on the other cytokines (F=0.81, p=0.67), chemokines (F=0.98, p=0.47), and growth factors (F=0.71, p=0.68) that were assayed (Table 2).
Discussion
Our results indicate that lower plasma concentrations of the proinflammatory markers TNFα and IFNγ in the immediate aftermath of trauma are associated with greater risk for developing chronic PTSD symptoms. To our knowledge, this is the first prospective examination of the association between immunological activity in response to trauma exposure and subsequent risk for PTSD development. Although our results suggest that lower TNFα and IFNγ concentrations may confer individual risk for the development of chronic PTSD, further studies are necessary to characterize the potential mechanisms by which immunological changes following trauma exposure influence the development of PTSD. Overall, our results highlight the feasibility and applicability of assessing immune signals in the acute aftermath of trauma to assess prospective PTSD risk in recently traumatized individuals, while also highlighting the importance of sample collection timing for assessing prospective PTSD risk (e.g., before or immediately after trauma exposure).
Because of the practical difficulties in obtaining resting-state blood samples in large numbers of individuals before they are exposed to trauma, we designed our study specifically to assess whether inflammatory signals collected in an emergency department setting immediately after trauma exposure predict chronic PTSD development. Based on the literature linking a proinflammatory state to increased cross-sectional risk for PTSD in traumatized individuals (8) and to increased prospective risk for PTSD development in a pre- and postdeployment study (18), we had hypothesized that heightened inflammation in the immediate posttraumatic period would increase risk for the development of PTSD. Our data, however, showed that lower TNFα and IFNγ concentrations in the immediate aftermath of trauma were predictive of greater PTSD symptom development. These results, unexpected on the basis of the existing literature, are reminiscent of when it was shown that individuals with PTSD show enhanced, as opposed to diminished, glucocorticoid negative feedback inhibition of the hypothalamic-pituitary-adrenal (HPA) axis (24). The most likely explanation for the discrepancy between previous findings and our results in the present study is the timing of the blood sample collection, as previous studies assessed resting-state, unprovoked concentrations of inflammatory markers in individuals with PTSD (25–27). However, our study design does not allow us to rule out the possibility that individuals at risk for PTSD may have lower baseline concentrations of TNF and IFNγ prior to trauma exposure.
Blood samples in this study were collected, on average, about 3 hours after exposure to a traumatic event, reflecting a state of innate immune system activation. Exposure to a stressor results in the activation of the sympathetic nervous system and the release of norepinephrine, which in turn induces nuclear factor-kappa B (NF-κB) activation and subsequent stimulation of proinflammatory cytokine production from the immune system (28). In the context of stress-induced activation of inflammatory responses, the lower concentrations of TNFα and IFNγ in the present study may reflect a blunted activation of the immune system in the aftermath of trauma, similar to previous studies showing that blunted cortisol responses to trauma exposure confer increased risk for PTSD development in survivors of sexual assault (29). Previous studies in humans indicate that blunted immune activation in response to stressor exposure is associated with greater cortisol reactivity to a stressor (30) or habituation to repeated exposure to stressors (31, 32), both of which may predispose to later PTSD responses. Similarly, findings from rodent studies show that immune deficiency following a stress exposure results in higher posttraumatic anxiety and startle responses (33, 34). Our data also underscore the possibility that diminished glucocorticoid negative feedback inhibition of the HPA axis acutely in the aftermath of trauma may contribute to the low concentrations of TNFα and IFNγ seen in participants who went on to develop chronic PTSD in this study. Taken together, these data suggest that low inflammation may have adverse consequences on mental health outcomes, a notion that is supported by findings showing that anti-inflammatory treatment for depression is effective in individuals with preexisting high levels of inflammation, whereas such treatment can increase affective symptoms in individuals with lower baseline levels (35).
We also assessed whether posttraumatic concentrations of anti-inflammatory cytokines, chemokines, or immune system growth factors were associated with risk for chronic PTSD symptom development. Previous studies have shown that higher concentrations of anti-inflammatory cytokines confer resilience to PTSD (11, 13) and that higher concentrations of chemokines and immune system growth factors are associated with chronic PTSD (10, 11). However, in this study, we did not find any associations between concentrations of anti-inflammatory factors, other cytokines, chemokines, and immune system growth factors assessed about 3 hours after trauma exposure and chronic PTSD symptom development. These null findings do not discount the possibility that increases in these cytokines, chemokines, and growth factors may occur on a different time course in the aftermath of trauma exposure and contribute to the development of PTSD. Moreover, a few studies have implicated lower chemokine production with increased stress reactivity (36), and GM-CSF administration in survivors of acute respiratory distress syndrome has been found to result in increased PTSD symptoms (37). Thus, further studies assessing the role of anti-inflammatory cytokines, chemokines, and immune system growth factors over time in the development of PTSD are warranted.
In summary, this study is the first prospective account linking concentrations of proinflammatory cytokines measured in the immediate aftermath of trauma exposure to increased risk for the development of chronic PTSD symptoms in civilian trauma survivors. The findings suggest that assessing immunological changes in response to trauma exposure in the emergency department may help identify patients who are most at risk for developing chronic PTSD symptoms in the aftermath of trauma. Such individuals may benefit from immediate psychological and pharmacological interventions that have been shown to be effective in attenuating PTSD development (5, 6). It is worth considering that altered immune activity in the context of trauma exposure may also serve as a prognostic indicator of treatment response, similar to what has been described in depression (38). While our results suggest a promising link between blunted immunological changes in the immediate aftermath of trauma exposure and chronic PTSD risk, further studies are needed to replicate and extend the generalizability of the findings.
1 : Diagnostic and Statistical Manual of Mental Disorders, 5th ed, DSM-5. Washington, DC, American Psychiatric Association, 2013Crossref, Google Scholar
2 : Post-traumatic stress disorder: definition, prevalence, and risk factors, in Post-Traumatic Stress Disorder: Basic Science and Clinical Practice. Edited by Shiromani PJ, Keane TM, LeDoux JE. New York, Humana Press, 2009, pp 1–19Crossref, Google Scholar
3 : Posttraumatic stress disorder in veterans and military personnel: epidemiology, screening, and case recognition. Psychol Serv 2012; 9:361–382Crossref, Medline, Google Scholar
4 : Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry 2009; 31:505–514Crossref, Medline, Google Scholar
5 : Morphine use after combat injury in Iraq and post-traumatic stress disorder. N Engl J Med 2010; 362:110–117Crossref, Medline, Google Scholar
6 : Early intervention following trauma may mitigate genetic risk for PTSD in civilians: a pilot prospective emergency department study. J Clin Psychiatry 2014; 75:1380–1387Crossref, Medline, Google Scholar
7 : Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 2017; 42:254–270Crossref, Medline, Google Scholar
8 : Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2015; 2:1002–1012Crossref, Medline, Google Scholar
9 : Long-term immune alterations accompanying chronic posttraumatic stress disorder following exposure to suicide bomb terror incidents during childhood. Neuropsychobiology 2017; 76:130–135Crossref, Medline, Google Scholar
10 : Serum concentrations of chemokines (CCL-5 and CXCL-12), chemokine receptors (CCR-5 and CXCR-4), and IL-6 in patients with posttraumatic stress disorder and avoidant personality disorder. Pharmacol Rep 2015; 67:1251–1258Crossref, Medline, Google Scholar
11 : Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety 2009; 26:447–455Crossref, Medline, Google Scholar
12 : Resilience to traumatic events related to urban violence and increased IL10 serum levels. Psychiatry Res 2017; 250:136–140Crossref, Medline, Google Scholar
13 : Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pac J Trop Med 2012; 5:323–325Crossref, Medline, Google Scholar
14 : Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 2012; 37:137–162Crossref, Medline, Google Scholar
15 : Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry 2009; 66:415–422Crossref, Medline, Google Scholar
16 : Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage 2012; 59:3222–3226Crossref, Medline, Google Scholar
17 : Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry 2009; 66:407–414Crossref, Medline, Google Scholar
18 : Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry 2014; 71:423–431Crossref, Medline, Google Scholar
19 : Diagnostic and Statistical Manual of Mental Disorders, 4th ed, DSM-IV. Washington, DC, American Psychiatric Association, 2000Google Scholar
20 : Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381Crossref, Medline, Google Scholar
21 : Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:617–627Crossref, Medline, Google Scholar
22 : Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress 1993; 6:459–473Crossref, Google Scholar
23 : Coping strategies as mediators in relation to resilience and posttraumatic stress disorder. J Affect Disord 2018; 225:153–159Crossref, Medline, Google Scholar
24 : Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry 1995; 52:583–593Crossref, Medline, Google Scholar
25 : Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am J Psychiatry 2015; 172:353–362Link, Google Scholar
26 : Increased pro-inflammatory milieu in combat related PTSD: a new cohort replication study. Brain Behav Immun 2017; 59:260–264Crossref, Medline, Google Scholar
27 : Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res 2007; 41:744–752Crossref, Medline, Google Scholar
28 : A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA 2003; 100:1920–1925Crossref, Medline, Google Scholar
29 : Effect of previous trauma on acute plasma cortisol level following rape. Am J Psychiatry 1995; 152:1675–1677Link, Google Scholar
30 : Stronger hypothalamus-pituitary-adrenal axis habituation predicts lesser sensitization of inflammatory response to repeated acute stress exposures in healthy young adults. Brain Behav Immun 2017; 61:228–235Crossref, Medline, Google Scholar
31 : HPA-axis and inflammatory reactivity to acute stress is related with basal HPA-axis activity. Psychoneuroendocrinology 2017; 78:168–176Crossref, Medline, Google Scholar
32 : Response and habituation of pro- and anti-inflammatory gene expression to repeated acute stress. Brain Behav Immun 2015; 46:237–248Crossref, Medline, Google Scholar
33 : Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J Neurobiol 2006; 66:552–563Crossref, Medline, Google Scholar
34 : Reducing post-traumatic anxiety by immunization. Brain Behav Immun 2008; 22:1108–1114Crossref, Medline, Google Scholar
35 : A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 2013; 70:31–41Crossref, Medline, Google Scholar
36 : Effects of acute psychological stress on glucose metabolism and subclinical inflammation in patients with post-traumatic stress disorder. Horm Metab Res 2010; 42:746–753Crossref, Medline, Google Scholar
37 : Psychiatric symptoms in survivors of acute respiratory distress syndrome: effects of age, sex, and immune modulation. Ann Am Thorac Soc 2017; 14:960–967Crossref, Medline, Google Scholar
38 : Defective inflammatory pathways in never-treated depressed patients are associated with poor treatment response. Neuron 2018; 99:914–924.e3, e913Crossref, Medline, Google Scholar




