The Interaction of Parental History of Suicidal Behavior and Exposure to Adoptive Parents' Psychiatric Disorders on Adoptee Suicide Attempt Hospitalizations
Abstract
Objective:
The authors examined the risk of suicide attempt or other psychiatric hospitalization among adoptees whose biological parents died from or were hospitalized for suicidal behavior (BPSB) relative to adoptees whose biological parents had a psychiatric hospitalization but never for suicide attempt (BPPH). The authors examined whether risk was moderated by having an adoptive parent who had a psychiatric hospitalization during the adoptee's childhood or adolescence.
Method:
This retrospective cohort study used national longitudinal population-based Swedish registry data from 1973 to 2003 to identify 2,516 adoptees with BPSB and 5,875 adoptees with BPPH. Cox regression models compared the risk for suicide attempt and other psychiatric hospitalization in the two groups.
Results:
The interaction of BPSB with adoptive mothers' psychiatric hospitalization while the adoptee was younger than 18 years old increased the risk for an adoptee's suicide attempt. Neither BPSB nor psychiatric hospitalization among adoptive mothers alone placed adoptees at greater risk for suicide attempt hospitalizations. The interaction results were specific to adoptee suicide attempt.
Conclusions:
Exposure to the hospitalization of an adoptive mother because of a psychiatric disorder amplified an adoptee's risk for suicide attempt hospitalization among those adoptees at high genetic risk of suicide or suicide attempt. These results imply that suicide attempts among those at biological risk might be prevented with the early recognition and care of parental psychiatric illness.
With over 1 million suicides per year worldwide, suicide is a major public health problem. It is well established that suicide has a heterogeneous and multidetermined etiology. Family studies, including adoption, twin, and high-risk designs, have shown familial transmission of suicidal behavior. In a case-control study of 57 adoptee suicides from the Danish Adoption Register, Schulsinger et al. (1) observed a sixfold increase in suicide risk in the biological relatives of adoptees who died from suicide as compared with the biological relatives of living adoptee comparison subjects. This finding supports a genetic rather than an environmental effect on offspring suicide risk; however, the study did not specifically focus on the child-rearing environment as it included family members other than parents. Twin studies have shown that approximately 43% of the variability of suicidal behavior is attributable to biological or genetic factors and 57% is attributable to environmental factors. The concordance of suicidal behaviors among monozygotic twins is not 100%, which also supports environmental contributions (2–5).
High-risk studies, a variant of a family study design that compares the offspring of affected and unaffected parents, have found that the timing of parental suicide is relevant. Parental suicide during the offspring's childhood or adolescence, but not in young adulthood, increases the risk of suicide among offspring (6). This evidence could provide support for the developmental or environmental impact of parental suicide by implying stronger environmental risk after losing a primary caregiver during a critical developmental period. However, greater suicide risk among children who lost a parent to suicide during early childhood may also imply a stronger genetic risk, as their parents may have had severe early-onset mental disorders. High-risk studies cannot disentangle genetic and environmental influences on the transmission of suicidal behaviors.
One challenge in this area of research is how to parse out the unique contributions of the genetic and environmental impact of growing up in the context of parental suicidal behaviors from that of growing up in the context of parental psychiatric illness. Because 90% of suicide attempters and completers had at least one psychiatric disorder at the time of death or attempt (7, 8), the majority of offspring of suicidal parents are also exposed to parental psychiatric illness. Few, if any, life events are more traumatic for a child than experiencing parental suicide, especially if they witnessed the suicide or found the body. Children adopted early in life may not have directly experienced their biological parents' suicidal behaviors or psychiatric disorders. An adoption study design comparing the adopted offspring of biological parents who had suicidal behaviors (BPSB; defined as suicide or suicide attempt hospitalizations) with the adopted offspring of biological parents who had nonsuicidal psychiatric hospitalizations (BPPH) can help to address the challenges mentioned above.
In this study, we examined the long-term risk of suicide attempt and other psychiatric hospitalizations among adoptees with BPSB and BPPH. We also investigated the influence of an environmental factor (psychiatric hospitalizations of adoptive parents before the adoptees were 18 years old) on the risk for psychiatric hospitalizations of adoptees with BPSB and BPPH. We had the unique opportunity to study how psychiatric impairment in adoptive parents that was severe enough to warrant hospitalization affected adoptee outcomes and to distinguish the risk above and beyond that conferred by having a BPSB. We hypothesized that family environment, indexed as adoptive parents' psychopathology, would interact with genetic risk for suicidal behavior to increase the adoptee's risk for suicide attempt hospitalizations.
Method
Data Sources
We conducted a retrospective cohort study of all adoptees in Sweden from 1973 to 2003 based on linked data from multiple Swedish national registers using the personal identification number assigned to all individuals at birth or after being granted permanent residency. The Multi-Generation Register, which indicates biological and adoptive parent-child relations for all persons who either were born in Sweden or received permanent citizenship since 1932 (currently 13 million people), was used to identify biological and adoptive parent-adoptee pairs. The Cause of Death Register includes information on 99% of all deaths in Sweden, with cause of death classified according to the code revisions in ICD-6 through ICD-10. Suicide and suicide attempt hospitalizations were defined using the following ICD codes: from ICD-6 and ICD-7, E970–E979; from ICD-8 and ICD-9, E950–E959 and E980–E989; and from ICD-10, X60–X84 and Y10–Y34. These ICD codes indicate a definite or uncertain verdict of suicide or suicide attempt by a board-certified physician, pathologist, or forensic pathologist. Data on parent and adoptee psychiatric hospitalizations were obtained from the National Inpatient Register, which recorded ICD diagnoses on all individuals admitted to any hospital in Sweden for assessment or treatment from 1973 to 2003 (reporting is mandatory for all health care providers, including private hospitals). Admissions for the following psychiatric disorders were used to define “any psychiatric disorder” for parents and, along with suicide and hospitalizations for suicide attempt, hospitalizations for the first four psychiatric disorders listed below were examined as adoptee outcomes: unipolar depression (ICD-8: 296.0 and 300.4; ICD-9: 296b, 296x, 300e, and 311; ICD 10: F32–F39); alcohol abuse or dependence (ICD-8: 303; ICD-9: 303 and 305a; ICD 10: F10 except x.5); drug abuse or dependence (ICD-8: 304; ICD-9: 304 and 305x; ICD-10: F11–F19 except x.5); personality disorders (ICD-8 and ICD-9: 301; ICD-10: F60–F62); any nonorganic psychotic disorders (ICD-8: 291 and 295–299; ICD-9: 291–292 and 295–298; ICD-10: F20–F29 and x.5 in F10–F19); schizophrenia (ICD-8: 295; ICD-9: 295; ICD-10: F20); bipolar disorders (ICD-8: 296.1–296.9; ICD-9: 296a, 296c–296e, and 296w; ICD-10: F30–F31); anxiety disorders (ICD-8 and ICD-9: 300; ICD-10: F40–F48).
Study Population
Swedish registry data from 1973 to 2003 were used to gather data for 2,516 adoptees who had at least one biological parent with certain suicide (N=531), uncertain suicide (N=116), certain suicide attempt hospitalizations (N=1,361), or uncertain suicide attempt hospitalizations (N=508). These adoptees had at least one biological parent with suicidal behavior (BPSB). The comparison group comprised adoptees whose biological parents did have at least one psychiatric hospitalization (BPPH) but were not suicidal (N=5,875). All adoptions were domestic, and we defined the study groups based on suicide and suicide attempt hospitalizations in the biological parents. Adoptions by relatives were also included. Data were not available on the age of offspring at the time of adoption or the circumstances surrounding the adoption. Private adoptions are prohibited by Swedish law, so children were taken into institutional care by the municipalities shortly after birth to be adopted at a mean age of 6 months, with few adoptions after age 12 months (9, 10). No formal policy was in place to match the characteristics of adoptive offspring with the characteristics of adoptive parents, but this cannot be ruled out in individual cases. The proportion of domestic adoptions in Sweden since the 1960s has steadily declined as a result of access to contraceptives.
Statistical Analysis
The adoptee outcomes we investigated were suicide and hospitalizations for suicide attempt; unipolar depression; and alcohol, drug, and personality disorders. The main exposure variables were biological parents' certain or uncertain death by suicide or biological parents' certain or uncertain hospitalization for suicide attempt. We combined parental suicide and suicide attempt hospitalization because family studies have shown that the familial phenotype for suicidal behavior includes both completed and attempted suicide (11). First, we examined the sociodemographic characteristics of the adoptees and the biological and adoptive parents. We then used Cox proportional hazard models to compare the risk for outcomes among adoptees with BPSB and BPPH. In psychiatric hospitalizations, adoptees with BPSB were more similar to those with BPPH than to the entire group of adoptees without BPSB (which included adoptees with and without BPPH). Because we were concerned about residual confounding (i.e., adjusting for parental psychiatric hospitalization may not adequately control for psychiatric hospitalizations among biological parents), we conducted an analysis directly comparing adoptees with BPSB with adoptees with BPPH. We also examined whether psychiatric hospitalizations among adoptive parents when the offspring were under 18 years old increased the risk for psychiatric hospitalizations of the adoptee. We then examined whether the risk for psychiatric hospitalization in adoptees with BPSB and BPPH differed by adoptive mother's and father's psychiatric status before the adoptee was 18 years old by including interaction terms. For all analyses, we defined the study entry time as when the adoptee was 5 years old or as 1973 (when data on psychiatric hospitalizations became available). Analyses were restricted to the date of first occurrence of each outcome. Because it was possible for the adoptees to receive inpatient care on multiple occasions and for multiple disorders, we selected the date of first hospitalization for each disorder for which they received inpatient care. Adoptees were censored administratively on December 31, 2003, or when they died if their death preceded this date. In all models, we further adjusted for adoptee birth cohort (before 1950, 1950–1959, 1960–1969, or 1970 and later). Since BPSB were more likely to have lifetime alcohol or drug use hospitalizations relative to BPPH (results not shown), we adjusted for biological mothers' alcohol and drug use disorders. Adoptees with BPSB and BPPH did not differ in the proportion of adoptive parents who were psychiatrically hospitalized while the adoptees were under age 18 or the diagnosis on hospitalization; however, the number of hospitalizations for anxiety disorders was higher in the adoptive mothers of BPPH offspring compared with the adoptive mothers of BPSB offspring (1.3% and 0.8%, respectively). We went back to the full population of adoptees to check for differential placement of adoptees with BPSB who also had adoptive parents with psychiatric hospitalizations (i.e., whether adoptees with BPSB [N=2,516] were more likely than those without BPSB [N=25,688] to be placed with adoptive parents with psychiatric hospitalizations). We did not find evidence of differential placement: 1.5% of adoptees with and without BPSB had adoptive mothers with psychiatric hospitalizations while the adoptees were under age 18. Adjusting for adoptive parent's death before age 18 did not affect our findings, so the more parsimonious models were reported.
The proportional-hazard assumption for adoptee birth cohort was violated in models examining adoptee hospitalization for alcohol use disorder, drug use disorder, and unipolar depression, which suggests that adoptees represented in specific age cohorts had different risk trajectories. For these outcomes, we allowed the models to have unique baseline hazards with respect to this covariate. Twenty-three percent of adoptees with BPSB and 20% with BPPH were biological siblings. The analyses employed robust standard errors to account for nonindependence of observations.
All analyses were performed using Stata, version 10 (12). The institutional review boards of the Johns Hopkins School of Medicine and the Karolinska Institutet approved the study.
Results
Table 1 summarizes the characteristics of the adoptees with BPSB and those with BPPH. Most adoptees were born in Sweden (99% in both groups). The sex representation was almost balanced (52% male in the BPPH group and 51% male in the BPSB group). Adoptees with BPPH were slightly older on average than those with BPSB. The proportion of adoptees with BPSB and BPPH with lifetime psychiatric hospitalizations was similar (17% and 16%, respectively).
| All Adoptees of Biological Parents Without Suicidal Behavior (N=25,688) | Adoptees of Biological Parents With Psychiatric Hospitalization (N=5,875) | Adoptees of Biological Parents With Suicidal Behaviors (N=2,516) | ||||
|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % |
| Sex | ||||||
| Male | 13,460 | 52 | 3,035 | 52 | 1,271 | 51 |
| Female | 12,228 | 48 | 2,840 | 48 | 1,245 | 49 |
| Country of birtha | ||||||
| Sweden | 25,434 | 99 | 5,825 | 99.1 | 2,489 | 99 |
| Other (Scandinavian) | 147 | 0.7 | 33 | 0.6 | 14 | 0.5 |
| Other (non-Scandinavian) | 79 | 0.3 | 12 | 0.2 | 11 | 0.4 |
| Adoptive parent's death before age 18 | 1,426 | 6 | 319 | 5 | 122 | 5 |
| Mean | SD | Mean | SD | Mean | SD | |
| Mean year of birth | 1957 | 10 | 1959 | 10 | 1964 | 11 |
| Lifetime psychiatric hospitalizationb | 3,196 | 12 | 925 | 16 | 434 | 17 |
TABLE 1. Characteristics of Adoptees With and Without a History of Suicidal Behaviors in Biological Parents From Swedish Population-Based Registries, 1973–2003 (N=28,204)
Table 2 summarizes the characteristics of the biological and adoptive parents. The majority of parents were born in Sweden. Adoptive parents were older than biological parents, and lifetime psychiatric hospitalizations were higher in biological than in adoptive parents. For a large proportion of adoptees, data on biological fathers were missing (29% among adoptees with BPPH and 46% among those with BPSB). The biological mothers of adoptees with data missing on biological fathers were not younger than biological mothers with data available on biological fathers. Among the adoptees with data missing for biological fathers, there were few differences between adoptees with BPSB and BPPH, except that those with BPSB were younger.
| Biological Parents | Adoptive Parents | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | All Adoptees of Biological Parents Without Suicidal Behavior (N=25,688) | Adoptees of Biological Parents With Psychiatric Hospitalization (N=5,875) | Adoptees of Biological Parents With Suicidal Behaviors (N=2,516) | All Adoptees of Biological Parents Without Suicidal Behavior (N=25,688) | Adoptees of Biological Parents With Psychiatric Hospitalization (N=5,875) | Adoptees of Biological Parents with Suicidal Behaviors (N=2,516) | ||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Country of birthb | ||||||||||||
| Mother | ||||||||||||
| Sweden | 20,641 | 89 | 4,965 | 90 | 2,151 | 88 | 24,210 | 94 | 5,553 | 95 | 2,345 | 94 |
| Other (Scandinavian) | 1,866 | 8 | 444 | 8 | 220 | 9 | 902 | 4 | 216 | 4 | 120 | 5 |
| Other (non-Scandinavian) | 689 | 3 | 116 | 2 | 65 | 3 | 385 | 2 | 76 | 1 | 39 | 1 |
| Father | ||||||||||||
| Sweden | 12,238 | 90 | 3,733 | 90 | 1,629 | 88 | 24,457 | 97 | 5,626 | 97 | 2,382 | 95 |
| Other (Scandinavian) | 722 | 5 | 249 | 6 | 140 | 8 | 527 | 2 | 114 | 2 | 74 | 3 |
| Other (non-Scandinavian) | 594 | 5 | 161 | 4 | 80 | 4 | 341 | 1 | 74 | 1 | 47 | 2 |
| Lifetime psychiatric hospitalizationc | ||||||||||||
| Mother | 3,680 | 15 | 3,680 | 66 | 1,458 | 59 | 2,140 | 8 | 523 | 9 | 165 | 7 |
| Father | 2,731 | 20 | 2,731 | 66 | 917 | 49 | 1,470 | 6 | 344 | 6 | 126 | 5 |
| Psychiatric hospitalization before adoptees were age 18c | ||||||||||||
| Mother | 1,242 | 5 | 1,242 | 22 | 832 | 34 | 395 | 2 | 111 | 2 | 38 | 2 |
| Father | 948 | 7 | 948 | 23 | 505 | 27 | 234 | 1 | 69 | 1 | 35 | 1 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Birth year | ||||||||||||
| Mother | 1934 | 12 | 1935 | 11 | 1940 | 11 | 1924 | 12 | 1926 | 12 | 1931 | 13 |
| Father | 1930 | 13 | 1931 | 12 | 1936 | 12 | 1921 | 13 | 1923 | 13 | 1928 | 13 |
TABLE 2. Characteristics of Biological and Adoptive Parents of Adoptees From Swedish Population-Based Registries, 1973–2003a
When we examined the risk of suicide and psychiatric hospitalization among adoptees with BPSB relative to adoptees with BPPH, those with BPSB were not at greater risk for any of the outcomes, except for a marginally higher risk for suicide and for drug use disorder hospitalizations (p<0.10 and p>0.05) (Table 3), after adjusting for adoptee birth cohort and biological mothers' alcohol and drug use disorders. We also compared the risk of adoptee psychiatric hospitalization among those with and without adoptive parents who were psychiatrically hospitalized when adoptees were under age 18. Adopted offspring of psychiatrically hospitalized adoptive parents were at greater risk for hospitalization for unipolar depression (adjusted hazard ratio=1.87, 95% CI=1.10–3.16) and alcohol use disorder (adjusted hazard ratio=1.87, 95% CI=1.19–2.95) (Table 3, Figure 1).
| Biological Parent's Suicidal Behavior | Adoptive Parent's Psychiatric Hospitalization | Adoptive Mother Without Psychiatric Hospitalization | Adoptive Mother With Psychiatric Hospitalization | Adoptive Father Without Psychiatric Hospitalization | Adoptive Father With Psychiatric Hospitalization | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Offspring Outcomes | AHR | 95% CI | AHR | 95% CI | AHR | 95% CI | AHR | 95% CI | AHR | 95% CI | AHR | 95% CI |
| Suicideb | 1.66 | 0.99–2.77* | 0.61 | 0.08–4.41 | – | – | – | – | ||||
| Suicide attempt | 1.18 | 0.96–1.45 | 0.99 | 0.57–1.73 | 1.15 | 0.93–1.41 | 4.19 | 1.27–13.77** | 1.17 | 0.95–1.44 | 2.10 | 0.30–14.69 |
| Unipolar depression | 1.16 | 0.92–1.47 | 1.87 | 1.10–3.16** | 1.16 | 0.91–1.46 | 2.06 | 0.60–7.09 | 1.16 | 0.91–1.46 | 1.62 | 0.37–7.02 |
| Alcohol use disorder | 1.11 | 0.91–1.35 | 1.87 | 1.19–2.95*** | 1.10 | 0.90–1.35 | 2.08 | 0.77–5.67 | 1.10 | 0.90–1.34 | 2.36 | 0.49–11.27 |
| Drug use disorder | 1.24 | 0.97–1.58* | 1.50 | 0.85–2.64 | 1.20 | 0.93–1.54 | 4.29 | 1.17–15.71** | 1.23 | 0.96–1.57 | 1.65 | 0.37–7.31 |
| Personality disorder | 1.22 | 0.93–1.60 | 1.59 | 0.86–2.94 | 1.21 | 0.92–1.60 | 1.94 | 0.47–8.11 | 1.22 | 0.93–1.60 | 1.37 | 0.23–8.08 |
TABLE 3. Risk of Psychiatric Outcomes in Adopted Offspring of Biological Parents With Suicidal Behavior, With and Without Stratification by Adoptive Parent's Psychiatric Status, From Swedish Population-Based Registries, 1973–2003a
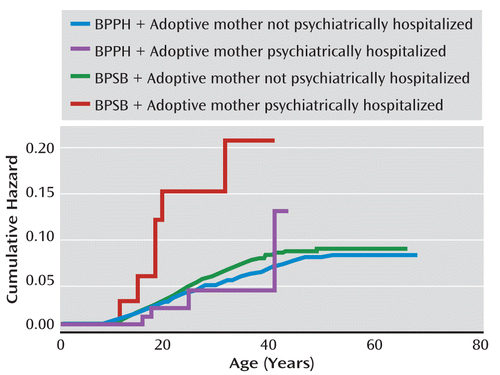
FIGURE 1. Cumulative Risk for Hospitalization of Suicide Attempta
a BPPH=adopted offspring of biological parents who had nonsuicidal psychiatric hospitalizations; BPSB=adopted offspring of biological parents who had suicidal behaviors.
Among those with an adoptive mother who was psychiatrically hospitalized when the adoptees were younger than 18, adoptees with BPSB were at greater risk for suicide attempt hospitalizations relative to adoptees with BPPH (adjusted hazard ratio=4.19, 95% CI=1.27–13.77; interaction p=0.04). In contrast, no difference was observed between offspring of BPSB and offspring of BPPH when the adoptive mother was not psychiatrically hospitalized (adjusted hazard ratio=1.15, 95% CI=0.93–1.41). A similar trend was noted for drug use disorder hospitalizations (interaction p=0.06). Adoptees with a psychiatrically hospitalized adoptive mother and BPSB had a greater risk for drug use disorder hospitalization than those with a psychiatrically hospitalized adoptive mother and BPPH (adjusted hazard ratio=4.29; 95% CI=1.17–15.71). However, no difference was observed between offspring of BPSB and BPPH whose adoptive mother was not psychiatrically hospitalized when the adoptees were under age 18 (adjusted hazard ratio=1.20; 95% CI=0.93–1.54).
Discussion
This study found that genetic risk for suicidal behaviors, indicated by having a BPSB, on adoptee's risk for suicide attempt hospitalization was conditional upon the adoptive mother's psychiatric hospitalization during the adoptee's childhood or adolescence. Neither a parental history of suicidal behaviors nor an adoptive mother's psychiatric hospitalization alone placed adoptees at greater risk for suicide attempt hospitalizations, but the interaction of the two factors increased the risk for adoptee suicide attempt more than fourfold. The interaction results were specific to adoptee suicide attempt hospitalizations.
We found marginal statistical significance in the interaction effect of BPSB by adoptive mothers' psychiatric hospitalization on adoptee hospitalizations for drug use disorder, even after adjusting for hospitalizations for drug use disorder in biological mothers. Adoptees who grew up in a home in which an adoptive parent had a psychiatric hospitalization were at greater risk for hospitalization for depression and alcohol use disorder than those adoptees whose adoptive parents were never hospitalized.
These findings should be interpreted in light of some limitations. The most prominent is the registers' inclusion of only those suicide attempts and psychiatric disorders that were severe enough to require hospitalization. We could not examine suicide attempts and psychiatric problems that were untreated, that were treated in outpatient facilities, or that occurred before 1973. Therefore, the estimation of the impact of BPSB relative to BPPH on adopted offspring outcomes is subject to some degree of error and likely reflects the operation of these risk factors only for those who display the most severe psychopathology. We did not have data on parent income, education level, employment status, and marital status in our database, and these variables were not adjusted for in the analyses. Our results are limited by the lack of information on specific suicide susceptibility genes or pathways of genetic effect. Only 38 adoptees had both risk factors (BPSB and an adoptive mother with a psychiatric hospitalization while the adoptee was younger than 18), resulting in broad confidence intervals for the interaction term. Generalizability may be limited to the Western world, since the Swedish population is primarily Caucasian and has a relatively high socioeconomic status and universal access to health care. Studies that have examined gene-environment correlation have found that risk can be reciprocal in that the behavioral and psychiatric characteristics of offspring may contribute to parental psychiatric status. We did not examine this correlation in this study.
Limitations of the adoption design include the possibility of range restriction in the adoptive parents because adoptive parents are screened and may enjoy greater financial security and better mental health than the general population. It has been suggested that gene-environment interactions may exist only at the extremes of genetic and environmental variation, and thus our findings may actually be an underestimate of the impact of environmental risk (13). We do not know the age of the children at the time of adoption, nor do we have data on their circumstances before the adoption. The possibility of selective placement cannot be ruled out in some individual instances. We are missing data on a large proportion of biological fathers, which indicates that a large proportion of mothers (29% among adoptees with BPPH and 46% among adoptees with BPSB) were unwed. This may have resulted in more conservative estimates; some adoptees with BPPH with missing data on their biological fathers may have had fathers with suicidal behaviors. We do not have data on whether adoptees were aware of their biological parents' suicidal behaviors and psychiatric disorders. We did not exclude offspring adopted by relatives, but adoptees with BPSB and BPPH did not differ in the proportion of adoptive parents with psychiatric hospitalizations.
In terms of counterbalancing strengths, this study used a large and nationally representative sample of adoptees. The proposed research was conducted in the context of a 30-year longitudinal and prospective adoption design to disentangle the complex interplay between genes and the environment. The constructs were not biased by self-report, and there were minimal selection, ascertainment, and diagnostic biases. In contrast to many other studies in this domain, we had detailed information on psychiatric hospitalizations of the biological and adoptive parents. We had the ability to stratify by maternal and paternal adoptive parents' psychiatric hospitalizations. This is a major strength, as the impact of fathers' psychiatric status on offspring outcomes is rarely studied. Although we did not observe an impact of adoptive fathers' psychiatric hospitalizations on offspring outcomes, a recent review (14) found that father engagement positively affects offspring social, behavioral, psychological, and cognitive outcomes.
Family studies have shown that suicidal behaviors cluster in families and are transmitted within families independently of the transmission of psychiatric disorders. Impulsive aggression, defined as the tendency to respond to provocation or frustration with hostility or aggression, appears to be transmitted independently of psychiatric disorders and may be an intermediate phenotype for early-onset suicidal behavior (15). As our comparison group was characterized by previous psychiatric hospitalizations in biological parents, our findings agree with the literature that suicidal behaviors are transmitted partially independently of psychiatric illness (16).
Studies of other phenotypes have found that family environment, indexed as adoptive mother's psychopathology, interacts with genetic risk to predict adolescent problem behaviors and adult conduct problems and depression (17–19). In contrast, low levels of environmental risk have been found to protect against high genetic risk for substance abuse in a prospective sample of adolescent twins (20).
One implication of this study is that our studied environmental factor is modifiable and can be targeted for intervention. Weissman et al. (21) reported that remission of maternal major depression following antidepressant treatment was associated with reduced diagnoses and symptoms in their children and that these reductions were sustained 1 year after the initiation of the mothers' treatment (22). The children of mothers whose depression remitted early showed substantial decreases in externalizing symptoms and improved psychosocial functioning (23). A report from a randomized trial of interpersonal psychotherapy also showed benefits to offspring depressive symptoms after their mothers were treated (24). These studies suggest that a reduction in stress or in adverse early experiences associated with maternal remission may ameliorate symptoms in children who are psychiatrically vulnerable; however, these treatment studies do not have the ability to separate environmental and genetic impact.
There is accumulating evidence indicating that adverse childhood experiences have long-term consequences (25). Epigenetic processes, such as DNA methylation, might mediate the effects of the environment during childhood on gene expression, which might then persist into adulthood and influence vulnerability to suicidal behaviors through effects on hypothalamic-pituitary-adrenal (HPA) activity (26). Animal studies have shown that the quality of maternal parenting influences the development of individual differences in offspring behaviors and stress responses (27, 28) possibly through epigenetic mechanisms (29). A pilot intervention targeting parenting (e.g., monitoring, consistent discipline, and positive reinforcement) of maltreated preschool children immediately after their placement in a new foster home improved child functioning and altered HPA activity as assessed by salivary cortisol measures (30). Given the strong relationships between parental and offspring psychiatric disorders, and the benefit of treatment and remission of maternal psychiatric illness on offspring psychiatric symptoms and disorders (21–24), maternal psychiatric illness is an important modifiable environmental factor.
The study of gene-environment interactions has been cited as one of the most important goals of genetic epidemiology (31) and may further our understanding of how to prevent suicidal behaviors. The results of this study imply that biology is not destiny. We have identified a subgroup more likely to express vulnerability to suicide attempt hospitalization (i.e., those with BPSB who grow up in the context of maternal psychiatric hospitalizations), suggesting that facilitating the early identification and treatment of psychiatric disorders among mothers could be a critical step in the prevention of the generational transmission of suicidal behaviors.
1. : A family study of suicide, in Origin, Prevention, and Treatment of Affective Disorders. Edited by Schou MStromgren E. New York, Academic Press, 1979, pp 277–287Google Scholar
2. : Suicidal behavior: an epidemiological and genetic study. Psychol Med 1998; 28:839–855Crossref, Medline, Google Scholar
3. : Suicide attempts in an adolescent female twin sample. J Am Acad Child Adolesc Psychiatry 2001; 40:1300–1307Crossref, Medline, Google Scholar
4. : What can psychiatric genetics offer suicidology? Crisis 2001; 22:61–65Crossref, Medline, Google Scholar
5. : A twin study of genetic and environmental influences on suicidality in men. Psychol Med 2002; 32:11–24Crossref, Medline, Google Scholar
6. : Psychiatric morbidity, violent crime, and suicide among children and adolescents exposed to parental death. J Am Acad Child Adolesc Psychiatry 2010; 49:514–523Crossref, Medline, Google Scholar
7. : Psychological autopsy studies of suicide: a systematic review. Psychol Med 2003; 33:395–405Crossref, Medline, Google Scholar
8. : Prevalence and comorbidity of mental disorders in persons making serious suicide attempts: a case-control study. Am J Psychiatry 1996; 153:1009–1014Link, Google Scholar
9. : Adopted Children and Their Families: A Follow-Up Study of Adopted Children, Their Background, Environment, and Adjustment. Stockholm, Proprius, 1970Google Scholar
10. : Familial factors and suicide: an adoption study in a Swedish national cohort. Psychol Med 2011; 41:749–758Crossref, Medline, Google Scholar
11. : What family studies teach us about suicidal behavior: implications for research, treatment, and prevention. Eur Psychiatry 2010; 25:260–263Crossref, Medline, Google Scholar
12. Stata Reference Manual: Release 10. College Station, Tex, StataCorp, 2007Google Scholar
13. : Genetic and environmental influences on human behavioral differences. Annu Rev Neurosci 1998; 21:1–24Crossref, Medline, Google Scholar
14. : Fathers' involvement and children's developmental outcomes: a systematic review of longitudinal studies. Acta Paediatr 2008; 97:153–158Crossref, Medline, Google Scholar
15. : Candidate endophenotypes for genetic studies of suicidal behavior. Biol Psychiatry 2009; 65:556–563Crossref, Medline, Google Scholar
16. : Family history of suicide among suicide victims. Am J Psychiatry 2003; 160:1525–1526Link, Google Scholar
17. : Depression spectrum disease, I: the role of gene-environment interaction. Am J Psychiatry 1996; 153:892–899Link, Google Scholar
18. : Genetic-environmental interaction in the genesis of aggressivity and conduct disorders. Arch Gen Psychiatry 1995; 52:916–924Crossref, Medline, Google Scholar
19. : Biology-environment interaction and evocative biology-environment correlation: contributions of harsh discipline and parental psychopathology to problem adolescent behaviors. Behav Genet 2003; 33:205–220Crossref, Medline, Google Scholar
20. : Searching for interactive effects in the etiology of early-onset substance use. Behav Genet 1999; 29:433–444Crossref, Medline, Google Scholar
21.
22. : Children of depressed mothers 1 year after the initiation of maternal treatment: findings from the STAR*D-Child study. Am J Psychiatry 2008; 165:1136–1147Link, Google Scholar
23. : Children of depressed mothers 1 year after remission of maternal depression: findings from the STAR*D-Child study. Am J Psychiatry 2011; 168:593–602Link, Google Scholar
24. : Brief interpersonal psychotherapy for depressed mothers whose children are receiving psychiatric treatment. Am J Psychiatry 2008; 165:1155–1162Link, Google Scholar
25. : Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA 2009; 301:2252–2259Crossref, Medline, Google Scholar
26. : Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 2009; 12:342–348Crossref, Medline, Google Scholar
27. : Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci 2001; 24:1161–1192Crossref, Medline, Google Scholar
28. : CSF monoamine metabolite concentrations vary according to age, rearing, and sex, and are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacology (Berl) 1991; 103:551–556Crossref, Medline, Google Scholar
29. : Stress, genes, and the mechanism of programming the brain for later life. Neurosci Biobehav Rev 2005; 29:271–281Crossref, Medline, Google Scholar
30. : Preventive intervention for maltreated preschool children: impact on children's behavior, neuroendocrine activity, and foster parent functioning. J Am Acad Child Adolesc Psychiatry 2000; 39:1356–1364Crossref, Medline, Google Scholar
31. : Genomic priorities and public health. Science 2003; 302:599–601Crossref, Medline, Google Scholar



