Association of Cerebral Emboli With Accelerated Cognitive Deterioration in Alzheimer's Disease and Vascular Dementia
Abstract
Objective:
Spontaneous cerebral emboli occur frequently in patients with Alzheimer's disease and vascular dementia. The authors investigated the effect of cerebral emboli on cognitive and functional decline in both Alzheimer's disease and vascular dementia patients over a 2-year period.
Method:
Cerebral emboli entering the middle cerebral arteries were counted at baseline and every 6 months over the following 18 months using transcranial Doppler ultrasonography in 144 patients with dementia (Alzheimer's disease, N=84; vascular dementia, N=60). Deterioration in cognition was measured every 6 months over 2 years using the Alzheimer's Disease Assessment Scale-Cognitive (ADAS-Cog), the Mini-Mental State Examination (to assess cognition), the Interview for Deterioration in Daily Living Activities in Dementia (to assess function), and the Neuropsychiatric Inventory (to assess behavioral and psychological symptoms). The relationship between cerebral emboli and progression of dementia was analyzed using longitudinal regression modeling, adjusted for age, sex, diagnostic subtype, age at onset of dementia, and significant vascular risk factors.
Results:
Spontaneous cerebral emboli were detected in 63 (44%) dementia patients, 36 (43%) Alzheimer's disease patients, and 27 (45%) vascular dementia patients. ADAS-Cog scores revealed faster deterioration in cognitive functioning in patients with cerebral emboli over 2 years, with a mean increase in score of 15.4 in these patients, compared with only 6.0 for those without cerebral emboli. Similarly, Interview for Deterioration in Daily Living Activities in Dementia scores revealed more rapid deterioration in those with cerebral emboli, with a mean increase in score of 59.0 in these patients, compared with 17.9 for those without cerebral emboli. Neuropsychiatric Inventory scores also indicated faster decline in patients with cerebral emboli, with a mean increase in score of 12.0 in these patients, compared with a mean decrease in score of –3.8 for patients in whom no emboli were detected.
Conclusions:
Spontaneous cerebral emboli predict more rapid progression of dementia over 2 years in both Alzheimer's disease and vascular dementia. Clinical trials on the inhibition of cerebral emboli in the prevention and treatment of dementia are needed.
Dementia affects more than 24 million people worldwide, with the prevalence increasing as a result of the rising elderly population (1). Vascular risk factors, such as hypertension, diabetes, obesity, hypercholesterolemia, and stroke, are associated with an increased risk of both Alzheimer's disease and vascular dementia (2). However, the precise mechanisms of brain damage remain unknown. Irrespective of whether cerebrovascular disease plays a causative or additive role in the onset of clinical dementia (3), the treatment of vascular risk factors and elucidation of mechanisms of brain damage are considered important in the prevention and treatment of both Alzheimer's disease and vascular dementia (4).
Asymptomatic cerebral emboli, detected by transcranial Doppler ultrasonography of the middle cerebral arteries, were associated with the risk of stroke in patients with carotid artery disease (5, 6) and with the risk of postoperative cognitive decline in patients who underwent open-heart surgery, such as valve replacement or coronary artery bypass graft (7, 8). The middle cerebral arteries supply blood to the frontal, temporal, and parietal areas of the brain, which are considered critical in dementia. In a study of 170 patients with dementia, we detected spontaneous cerebral emboli in the middle cerebral arteries, during a single hour of transcranial Doppler monitoring, in 40% of patients with Alzheimer's disease and in 37% with vascular dementia, compared with only 14% of age- and sex-matched comparison subjects (9). We now present the findings of a prospective study of whether spontaneous cerebral emboli predict a more rapid progression of dementia over 2 years in patients with Alzheimer's disease and vascular dementia.
Method
Patients
Patients satisfying DSM-IV criteria for dementia were recruited through a network of community and outpatient geriatric psychiatry clinics in Greater Manchester (United Kingdom). Consultant geriatric psychiatrists explained the study details, including the inclusion/exclusion criteria and study assessments, to the patients and referred those patients who they thought would be able to adhere to the study method. Over a 2-year period, we recruited 156 patients with dementia, of whom 117 (75%) had participated in our previous case-control study (9). Alzheimer's disease, “probable” or “possible,” was diagnosed using the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association criteria (10). Vascular dementia was diagnosed using the National Institute of Neurological Disorders and Stroke/Association Internationale pour la Recherche et l'Enseignement en Neurosciences criteria (11). Three geriatric psychiatrists reviewed the clinical data, which included results from computerized tomography scans as well as magnetic resonance imaging (MRI), to reach a consensus diagnosis of Alzheimer's disease (N=90) or vascular dementia (N=66), with an independent psychiatrist making the final diagnosis. Patients were excluded from participation in the study if they had aphasia, severe dementia (Mini-Mental State Examination [MMSE] score <10 [12]), or an inadequate transtemporal window for transcranial Doppler insonation of the middle cerebral artery or if they were receiving anticoagulant medication. Patients were examined at baseline and then every 6 months for 24 months. The ethics committees of the National Health Service and the University of Manchester Senate approved the study. All patients with dementia and their caregivers provided written informed consent.
Cardiovascular Risk Factors and Carotid Disease
Cardiovascular risk factors were recorded at the interview with the patients and their caregivers and confirmed by reviewing case notes. Smoking status (current/former/nonsmoker), heavy alcohol use (>14 units/week for women and >21 units/week for men), drug history, and a detailed history of cardiovascular events and risk factors were recorded on purpose-designed data entry forms. Each patient's blood pressure was measured manually by sphygmomanometer after a 5-minute resting period, and a venous blood sample was taken to analyze total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglyceride levels as well as apolipoprotein E (APOE) genotype. Carotid artery disease was imaged by a color duplex ultrasound scanner (ATL Ultramark 9, Advanced Technology Laboratories, Inc., Bothell, Wash.), with the peak systolic velocity in the internal carotid artery used to calculate the severity of stenosis using established criteria (13).
The potential for paradoxical embolization from venous blood into the arterial circulation, usually through a patent foramen ovale, was investigated using a standardized transcranial Doppler method (14). The middle cerebral artery was insonated using transcranial Doppler, and an ultrasound contrast consisting of an air and saline emulsion was injected intravenously. The number of air microemboli entering the middle cerebral artery was counted and the severity of venous-to-arterial circulation was recorded using established criteria, with a significant venous-to-arterial circulation shunt equating to a patent foramen ovale and a major venous-to-arterial circulation shunt equating to a substantial right-to-left shunt across a patent foramen ovale (15, 16). The use of microbubbles as an ultrasound contrast medium to detect patent foramen ovale is routine in cardiology and entirely safe. The minimally invasive technique we used is comparatively sensitive and specific to patent foramen ovale detected by transesophageal echocardiography (14–16).
Spontaneous Cerebral Emboli
Patients attended the Vascular Studies Unit at the University Hospital of South Manchester every 6 months (for 2 years) and were independently assessed by vascular scientists who were blinded to dementia diagnoses and outcome measures for the progression of dementia. The method used to detect spontaneous cerebral emboli has been described in detail elsewhere (9) and is based on international consensus criteria (17). Briefly, cerebral emboli were detected by insonating the middle cerebral artery through the temporal window with the best signal by transcranial Doppler (EME Pioneer 4040, Überlingen, Germany) using a 2-MHz pulsed-wave Doppler probe. The insonation depth was set individually and ranged from 40 mm to 55 mm, and the sample volume length was set at 13 mm for all patients. Cerebral emboli were investigated over two separate 1-hour transcranial Doppler sessions at baseline and at 6-month intervals over the next 18 months. Patients were observed throughout each session in a manner that would allow movement artifacts to be recorded on digital tapes of the transcranial Doppler output for subsequent blind analyses by two vascular technologists. Each recording was played back through the same transcranial Doppler system, with a 128-point fast Fourier transform and an overlap of >50%. Embolic signals were identified using standard consensus criteria (17). If a patient was cerebral emboli positive in our original case-control study (9), he or she was considered cerebral emboli positive in the present study.
Outcome Measures
The following measures of cognition, function, and psychological symptoms were used to assess patients at baseline and at 6-month intervals to measure the progression of dementia: the Alzheimer's Disease Assessment Scale-Cognitive (ADAS-Cog [18]), the Interview for Deterioration in Daily Living Activities in Dementia (19), and the Neuropsychiatric Inventory (20).
Cognitive function (primary outcome measure).
The primary endpoint was deterioration in cognitive functioning as indicated by ADAS-Cog scores over 2 years of follow-up assessment. The ADAS-Cog is a widely used and validated psychometric assessment that evaluates attention, memory, orientation, language, and praxis (18). Scores range from 0 to 70 and are determined by the number of errors made on the test. An increase in score over time indicates a decline in cognitive function. Cognitive decline was also assessed using the MMSE. Scores on the MMSE range from 0 to 30, and a drop in score indicates cognitive decline.
Functional status.
The Interview for Deterioration in Daily Living Activities in Dementia is known to be reproducible and valid (19). This scale encompasses 33 activities from daily life, such as washing, dressing, eating, shopping, writing, and answering the telephone. Both the initiative to perform these activities and the performance itself are assessed. Any difficulties in the performance of each activity over the previous month are scored on a 7-point scale, and increasing scores over time indicate deterioration in functioning.
Behavioral and psychological symptoms.
Behavioral and psychological symptoms of dementia were assessed using the Neuropsychiatric Inventory, evaluating 12 behavioral disturbances based on symptoms over the previous month (20). If the answer to a screening question is positive, the severity and frequency of the behavior are scored and multiplied to yield a score for that particular symptom. The sum score of all symptom scores is a valid and reliable indicator, with higher scores indicating more severe symptoms.
Power Calculation and Statistical Analyses
We assumed that the overall decline in cognitive functioning as indicated by ADAS-Cog scores over 2 years would be similar in Alzheimer's disease and vascular dementia patients (21) and that ADAS-Cog scores would increase by approximately 7 points (10%) per year among patients with Alzheimer's disease (22). Our study was powered to detect whether spontaneous cerebral emboli would double the rate of progression of dementia from 10% to 20% each year (20% to 40% over 2 years). To achieve a 90% power to detect a doubling in the rate of cognitive deterioration stimulated by cerebral emboli separately in patients with Alzheimer's disease and vascular dementia, assuming a standard deviation of 20% and spontaneous cerebral emboli detection in 25% of patients, a total of 180 patients (accommodating a 30% attrition rate) would need to be examined. We were able to recruit 156 patients, and 144 with outcome data were included in all analyses. The attrition rate was higher than we anticipated, with 99 patients completing follow-up assessments up to 24 months.
Differences in baseline characteristics between patients with Alzheimer's disease and vascular dementia and between those with and without cerebral emboli were assessed using simple t test and chi-squared analysis as appropriate. Changes over the 24-month follow-up period (incorporating information from each of the assessments) in scores on the ADAS-Cog, MMSE, Interview for Deterioration in Daily Living Activities in Dementia, and Neuropsychiatric Inventory were analyzed by longitudinal regression modeling using generalized estimating equations with exchangeable correlation structure. This method enabled data up to the last recorded assessment for those patients who were lost to follow-up assessment before 24 months to be included in the statistical analysis.
The progression in dementia severity over 2 years was compared statistically between patients who had cerebral emboli detected at any time over the first 18 months (emboli positive) with those in whom cerebral emboli were never detected (emboli negative), using generalized estimating equations with exchangeable correlation structure to assess the significance of the interaction between time and presence of cerebral emboli. Covariates in the regression model included age, sex, type of dementia, age at onset, and vascular risk factors found to be related to the rate of progression of dementia at the 10% level of significance.
For each patient, the mean number of emboli detected over the study was calculated as the total number of emboli detected divided by the number of follow-up sessions attended. A ranking score was then derived based on the mean number of emboli detected, and the association between this score and the change in scores on outcome measures over 24 months was assessed using nonparametric Spearman correlations. Analyses were performed using SPSS, version 15 (SPSS, Inc., Chicago), and Stata, version 10 (StataCorp, College Station, Tex.).
Results
Patients
A total of 144 of the 156 recruited patients with dementia were included in the analyses (Alzheimer's disease, N=84 [63 “probable” and 21 “possible”]; vascular dementia, N=60) (Figure 1). The mean age of the included patients was 75.2 years (SD=7.3), and 48% were women. Not all patients completed each assessment at all time points, with data on outcome up to 24 months available for 58/84 (69%) of the Alzheimer's disease patients and 41/60 (65%) of the vascular dementia patients.
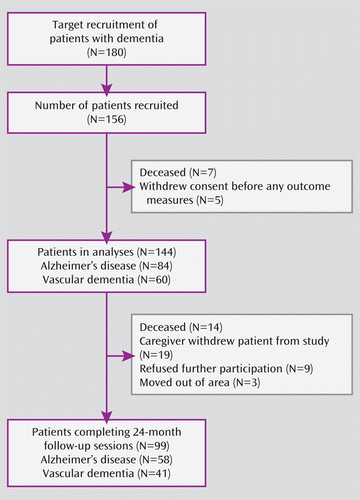
FIGURE 1. CONSORT Flow Chart of Patient Recruitment
Patients contributing data to the generalized estimating equation analysis for a time period shorter than 24 months, compared with those with 24-month data, were older (77.3 years [SD=5.8] versus 73.8 years [SD=7.8]) and had lower mean scores on the MMSE (18.6 [SD=6.8] versus 22.2 [SD=5.6]) and higher mean scores on the ADAS-Cog (28.2 [SD=15.6] versus 21.8 [SD=11.7]) and Interview for Deterioration in Daily Living Activities in Dementia (94.8 [SD=44.6] versus 76.8 [SD=40.4]) as well as slightly higher scores on the Neuropsychiatric Inventory (20.1 [SD=12.4] versus 16.9 [SD=11.8]). There was no difference between the two groups with respect to cardiovascular risk factors. The percentage of patients with spontaneous cerebral emboli at baseline was 34% in those with incomplete follow-up data and 39% in those who were followed for the entire 24-month study period.
Vascular dementia patients were more likely (>50% [p=0.03]) to have a history of stroke (p<0.001), transient ischemic attacks (p<0.001), hypertension (p<0.05), and internal carotid artery stenosis than Alzheimer's disease patients (Table 1, Table 2). Patients with Alzheimer's disease were more likely to be receiving acetylcholinesterase inhibitors (p<0.001), while those with vascular dementia were more likely to be receiving antiplatelet medication (p<0.001).
| Patient Groups | |||||||
|---|---|---|---|---|---|---|---|
| Risk Factor | All Dementia (N=144) | Alzheimer's Disease (N=84) | Vascular Dementia (N=60) | Analysis | |||
| N | % | N | % | N | % | pb | |
| Medical history | |||||||
| Stroke | 15 | 10 | 2 | 2 | 13 | 22 | <0.001 |
| Transient ischemic attack | 53 | 37 | 10 | 12 | 43 | 72 | <0.001 |
| Stroke or transient ischemic attack | 56 | 39 | 12 | 14 | 46 | 77 | <0.001 |
| Myocardial infarction | 10 | 7 | 7 | 8 | 3 | 5 | 0.44 |
| Angina | 20 | 14 | 9 | 11 | 11 | 18 | 0.19 |
| Myocardial infarction or angina | 26 | 18 | 13 | 16 | 13 | 22 | 0.34 |
| Diabetes | 9 | 6 | 4 | 5 | 5 | 8 | 0.38 |
| Peripheral vascular disease | 32 | 22 | 21 | 25 | 11 | 18 | 0.34 |
| Smoking | |||||||
| Ex-smoker | 74 | 51 | 40 | 48 | 34 | 57 | 0.52 |
| Current smoker | 20 | 14 | 13 | 16 | 7 | 12 | |
| Alcohol use (heavy)c | 11 | 8 | 6 | 7 | 5 | 8 | 0.8 |
| Current medication | |||||||
| Antiplatelet drug | 64 | 44 | 21 | 25 | 43 | 72 | <0.001 |
| Acetylcholinesterase inhibitor | 63 | 44 | 50 | 60 | 13 | 22 | <0.001 |
| Carotid artery stenosis >50% | 19 | 13 | 7 | 8 | 12 | 21 | 0.03 |
| APOE genotype (% with APOE4 allele) | 79 | 55 | 47 | 64 | 32 | 55 | 0.28 |
| Blood pressure | |||||||
| History of hypertension | 56 | 39 | 27 | 32 | 29 | 48 | <0.05 |
| Mean | SD | Mean | SD | Mean | SD | p | |
| Systolic (mm Hg) | 139 | 26 | 138 | 24 | 139 | 29 | 0.81 |
| Diastolic (mm Hg) | 79 | 15 | 80 | 15 | 78 | 15 | 0.44 |
| Lipid levels (mmol/liter)d | |||||||
| Total cholesterol | 5.3 | 1.0 | 5.5 | 1.0 | 5.2 | 1.1 | 0.08 |
| Low-density lipoprotein cholesterol | 3.2 | 0.9 | 3.2 | 1.0 | 3.1 | 0.9 | 0.31 |
| High-density lipoprotein cholesterol | 1.4 | 0.4 | 1.4 | 0.4 | 1.3 | 0.4 | 0.18 |
TABLE 1. Cardiovascular Risk Factors in Alzheimer's Disease and Vascular Dementia Patientsa
| Characteristic | Cerebral Emboli Absent (N=79) | Cerebral Emboli Present (N=63) | Analysis | |||
|---|---|---|---|---|---|---|
| N | % | N | % | p | ||
| Male | 42 | 52 | 33 | 52 | 1.0 | |
| Carotid disease | ||||||
| >50% | 11 | 14 | 8 | 13 | 1.0 | |
| >70% | 0 | 1 | 2 | 0.44 | ||
| Venous-to-arterial circulation shunt | ||||||
| Significant (patent foramen ovale) | 15 | 18 | 22 | 35 | 0.04 | |
| Major (large shunt) | 5 | 6 | 9 | 14 | 0.18 | |
| Myocardial infarction or angina | 12 | 15 | 14 | 22 | 0.35 | |
| Myocardial infarction | 5 | 6 | 5 | 8 | 0.75 | |
| Angina | 10 | 12 | 10 | 16 | 0.54 | |
| Stroke or transient ischemic attack | 32 | 40 | 26 | 41 | 0.97 | |
| Stroke | 7 | 9 | 8 | 13 | 0.61 | |
| Transient ischemic attack | 30 | 37 | 23 | 36 | 1.0 | |
| Peripheral vascular disease | 19 | 24 | 13 | 21 | 0.84 | |
| Diabetes | 7 | 9 | 2 | 3 | 0.32 | |
| Smoker (current) | 11 | 14 | 9 | 14 | 1.0 | |
| Antiplatelet treatment | 29 | 36 | 35 | 56 | 0.03 | |
| Mean | SD | Mean | SD | p | ||
| Age (years) | 75.7 | 7.2 | 75.7 | 7.4 | 1.0 | |
| Mini-Mental State Examination | 20.6 | 6.3 | 21.2 | 6.2 | 0.60 | |
| Alzheimer's Disease Assessment Scale-Cognitive | 25.1 | 13.3 | 23.0 | 13.4 | 0.35 | |
| Interview for Deterioration in Daily Living Activities in Dementia | 84.2 | 40.5 | 82.6 | 44.4 | 0.82 | |
| Neuropsychiatric Inventory | 18.7 | 13.3 | 17.2 | 14.9 | 0.52 | |
| Blood pressure | ||||||
| Systolic (mm Hg) | 135.2 | 21.6 | 143.2 | 30.2 | 0.09 | |
| Diastolic (mm Hg) | 80.2 | 15.7 | 77.9 | 14.3 | 0.38 | |
| Lipid levels (mmol/liter)a | ||||||
| Total cholesterol | 5.32 | 1.00 | 5.36 | 1.07 | 0.84 | |
| High-density lipoprotein cholesterol | 1.42 | 0.41 | 1.37 | 0.36 | 0.50 | |
| Low-density lipoprotein cholesterol | 3.13 | 0.89 | 3.23 | 1.01 | 0.51 | |
TABLE 2. Demographic and Clinical Characteristics of Alzheimer's Disease and Dementia Patients With and Without Spontaneous Cerebral Emboli
Spontaneous cerebral emboli were detected in 63 (44%) of the 144 dementia patients, 48 (33%) at the initial assessment and an additional 15 (11%) during follow-up assessments. Cerebral emboli were associated with blood pressure, antiplatelet medication (p=0.03), and a significant venous-to-arterial circulation shunt indicating a patent foramen ovale (p=0.04).
Overall Progression of Dementia
The expected progressive deterioration in cognition and function over 2 years was observed (Figure 2). Overall, there was an increase in scores on the ADAS-Cog, by a mean of 10.2 (95% confidence interval [CI]=7.2–13.3), a decrease in scores on the MMSE, by a mean of 5.5 (95% CI=4.2–6.8), and an increase in scores on the Interview for Deterioration in Daily Living Activities in Dementia, by a mean of 38.3 (95% CI=28.5–48.1) (p<0.001). The rate of progression was similar in Alzheimer's disease and vascular dementia patients; APOE4 genotype positivity had little influence.
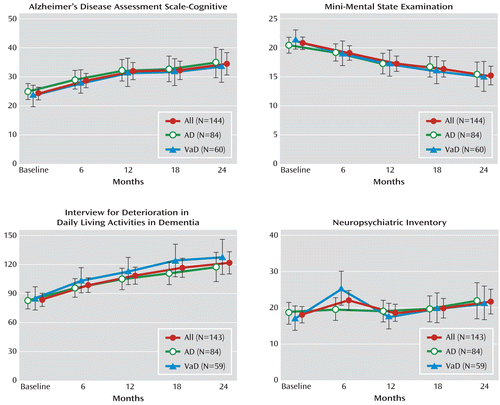
FIGURE 2. Progression of Dementia Over 24 Months in Patients With Alzheimer's Disease or Vascular Dementiaa
a A generalized estimating equation model was used, with exchangeable correlation structure and time as a categorical/continuous factor. Abbreviations: AD=Alzheimer's disease; VaD=vascular dementia.
Cerebral Emboli and Dementia Progression
The changes in outcome measures at 6-month intervals over 2 years between cerebral emboli-positive and emboli-negative patients are shown in Figure 3.
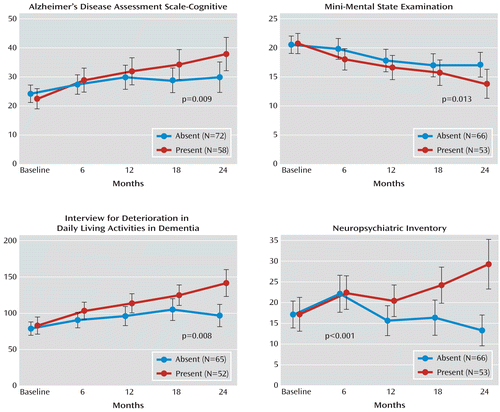
FIGURE 3. Effect of Spontaneous Cerebral Emboli on Dementia Progression in Patients With Alzheimer's Disease or Vascular Dementiaa
a A generalized estimating equation model was used, with exchangeable correlation structure and with time and spontaneous cerebral emboli and their interaction as categorical factors. The regression modeling was adjusted for age, sex, type of dementia, and age at onset as well as APOE4 positivity and additional confounders significant at the 10% level.
Cognitive decline.
In patients with cerebral emboli, ADAS-Cog scores revealed significantly faster cognitive deterioration, with a mean increase of 15.4 (95% CI=10.5–20.2) (p=0.009) in these patients, compared with only 6.0 (95% CI=2.3–9.8) in those without cerebral emboli. MMSE scores also revealed more rapid deterioration in emboli positive patients (p=0.01), with a mean decline of 6.9 (95% CI=4.7–8.9) in these patients, compared with only 3.4 (95% CI=1.8–5.1) in emboli-negative patients. We carried out further generalized estimating equation modeling for the proportion of patients who had a >40% increase in ADAS-Cog scores. For cerebral emboli-positive patients, the percentage of patients with >40% cognitive deterioration at 6, 12, 18, and 24 months were 31%, 45%, 53%, and 57%, respectively, compared with 21%, 30%, 23%, and 30%, respectively, in cerebral emboli-negative patients (p=0.003 [data adjusted for all confounders are shown in Figure 3]).
Functional decline.
In patients who were cerebral emboli-positive, scores on the Interview for Deterioration in Daily Living Activities in Dementia showed more rapid cognitive deterioration (p=0.008), with a mean increase of 59.0 (95% CI=40.1–78.0) in these patients, compared with only 17.9 (95% CI=6.1–29.8) in emboli-negative patients.
Behavioral and psychological symptoms.
Patients who had cerebral emboli also showed significantly greater deterioration as indicated by Neuropsychiatric Inventory scores, with a mean increase in score of 12.0 (95% CI=5.7–18.4) in these patients, compared with a mean decrease in score of –3.8 (95%=–7.3 to –0.4) in those with no cerebral emboli (p<0.001).
Is There a Dose Effect?
For those patients with emboli detected at any time during the study, the mean number of emboli (the total number of emboli divided by the number of transcranial Doppler sessions) was 0.27 (range=0.09–11.3). The ranking based on the number of emboli detected per Doppler session was significantly correlated with cognitive deterioration over 2 years as indicated by scores on the ADAS-Cog (rs=0.28, p=0.008), Interview for Deterioration in Daily Living Activities in Dementia (rs=0.36, p<0.001), and Neuropsychiatric Inventory (rs=0.42, p<0.001). However, when this was repeated including only the smaller number of emboli-positive patients, the correlations with scores on these measures failed to approach statistical significance.
Patent Foramen Ovale and Progression of Dementia
Patent foramen ovale was detected in 37 (26%) dementia patients. The effect of patent foramen ovale on the progression of dementia was investigated using generalized estimating equation analysis, and no significant association with scores on the ADAS-Cog, MMSE, or Neuropsychiatric Inventory was found. However, functional decline was greater in patients with patent foramen ovale, with a mean increase in score of 46.7 on the Interview for Deterioration in Daily Living Activities in Dementia for these patients, compared with 35.7 for those with no patent foramen ovale (p=0.002).
Discussion
The presence of spontaneous cerebral emboli in patients with dementia was associated with a more rapid cognitive and functional decline and an overall increase in behavioral and psychological symptoms over 2 years. This confirms our initial results for assessments over a 6-month period (23). The occurrence of cerebral emboli and their effect on the rate of progression of dementia were similar in Alzheimer's disease and vascular dementia patients.
The cognitive decline observed in our dementia patients was similar to that observed in clinical trials in Alzheimer's disease (24, 25). These studies also report a considerable interindividual variability in the progression of dementia. Our study suggests that cerebral emboli may explain at least some of this variability. The difference in the cognitive decline over 2 years between dementia patients with and without spontaneous cerebral emboli is similar to the annual decline in Alzheimer's disease (23–25), which suggests a potential for clinically meaningful effect if emboli were causative and could be inhibited.
We were unable to confirm a dose effect for cerebral emboli. Possibly, the duration of transcranial Doppler monitoring over the study period (mean=5.5 hours over 24 months) was insufficient to detect such an effect. In 48/63 (76%) patients, we detected emboli in only one monitoring session, which is consistent with a previous study of patients with severe carotid artery disease who were monitored for 5 hours (26). Nevertheless, the presence or absence of spontaneous cerebral emboli significantly predicted the progression of clinical dementia.
Atherosclerosis of the aorta, carotid arteries, and cerebral arteries is perhaps the most likely source of cerebral emboli. Emboli may also arise in the heart or paradoxically from the venous blood circulation in patients with a patent foramen ovale (27, 28). Atherosclerosis of the carotid arteries is associated with an increased risk (29) and faster progression (30) of dementia. However, severe carotid disease was infrequent in our dementia patients, and cerebral emboli were not associated with this disease. Atherosclerosis of the circle of Willis has also been associated with Alzheimer's pathology in some (31) but not all (32) studies. However, it is not possible to detect emboli in the circle of Willis.
In our study, cerebral emboli were associated with significant venous-to-arterial circulation shunt, indicating a patent foramen ovale. Paradoxical embolization may therefore be a cause for some, but by no means all, cerebral emboli in dementia patients. Patent foramen ovale was also associated with a more rapid decline in activities of daily living in dementia patients, which is consistent with findings in our previous study, in which patent foramen ovale was associated with the severity of white matter lesions on MRI scans in Alzheimer's disease patients (33), suggesting a thromboembolic etiology for at least some of these white matter lesions (34).
It is possible that the inflammation associated with major surgery and cardiopulmonary bypass may lead to intraoperative emboli formation and cognitive deficits (35). This mechanism may be of special significance in older people, since microglia in the aged or diseased brain switch their phenotype to produce neurotoxic molecules in response to systemic inflammation or other disturbances of brain homeostasis (36, 37). Cerebral emboli-induced repetitive low-grade ischemia in the cerebral microcirculation may potentially be a trigger for microglial activation and inflammatory brain injury leading to dementia.
Limitations
Although our recruitment target was not achieved, the detection rate for spontaneous cerebral emboli was higher and the deterioration in cognitive functioning in dementia was more rapid than we predicted. We adjusted for the use of cholinesterase inhibitors, antiplatelet medication, and statin therapy but not other medications, such as benzodiazepines, hypnotics, anticholinergics, or antipsychotics. We minimized potential bias in detecting cerebral emboli by recording transcranial Doppler sessions for subsequent analysis conducted by two trained vascular technologists who had no knowledge of the underlying diagnosis or clinical outcomes. The number of transcranial Doppler sessions may have been insufficient to measure a dose effect, and some dementia patients with cerebral emboli may have been wrongly allocated emboli-negative status in this study.
Conclusions
The presence of spontaneous cerebral emboli was associated with a more rapid progression of dementia over 2 years in both Alzheimer's disease and vascular dementia patients. Cerebral emboli are potentially preventable and could be a novel target for the prevention and treatment of dementia. Population studies to explore whether cerebral emboli predict the onset of dementia in elderly people at risk of developing dementia are required.
1.
2. : Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol 2006; 5:735–741Crossref, Medline, Google Scholar
3. : Alzheimer's disease brain pathology: vascular origins? results from the HAAS Autopsy Study. Neurobiol Aging 2008; 29:1587–1590Crossref, Medline, Google Scholar
4. : The role of cerebral ischemia in Alzheimer's disease. Neurobiol Aging 2000; 21:321–330Crossref, Medline, Google Scholar
5. : Doppler microembolic signals predict ischemic recurrences in symptomatic carotid stenosis. Acta Neurol Scand 2000; 101:327–331Crossref, Medline, Google Scholar
6. : Asymptomatic embolization detected by Doppler ultrasound predicts stroke risk in symptomatic carotid artery stenosis. Stroke 2005; 36:971–975Crossref, Medline, Google Scholar
7. : Association between intraoperative cerebral microembolic signals and postoperative neuropsychological deficit: comparison between patients with cardiac valve replacement and patients with coronary artery bypass grafting. J Neurol Neurosurg Psychiatry 1998; 65:573–576Crossref, Medline, Google Scholar
8. : Cerebral injury during cardiopulmonary bypass: emboli impair memory. J Thorac Cardiovasc Surg 2001; 121:1150–1160Crossref, Medline, Google Scholar
9. : Cerebral emboli as a potential cause of Alzheimer's disease and vascular dementia: case-control study. BMJ 2006; 332:1119–1124Crossref, Medline, Google Scholar
10. : Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34:939–944Crossref, Medline, Google Scholar
11. , : Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993; 43:250–260Crossref, Medline, Google Scholar
12. : Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
13. : Ultrasound assessment of internal carotid artery stenosis. Clin Radiol 1997; 52:654–658Crossref, Medline, Google Scholar
14. : Optimizing the technique of contrast transcranial Doppler ultrasound in the detection of right-to-left shunts. Stroke 2002; 33:2211–2216Crossref, Medline, Google Scholar
15. : Is transcranial Doppler for the detection of venous-to-arterial circulation shunts reproducible? Cerebrovasc Dis 2007; 23:424–429Crossref, Medline, Google Scholar
16. : Transcranial Doppler detection of venous-to-arterial circulation shunts: criteria for patent foramen ovale. J Clin Ultrasound 2009; 37:276–280Crossref, Medline, Google Scholar
17.
18. : A new rating scale for Alzheimer's disease. Am J Psychiatry 1984; 141:1356–1364Link, Google Scholar
19. : Assessing the severity of dementia: patient and caregiver. Arch Neurol 1991; 48:274–277Crossref, Medline, Google Scholar
20. : The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Crossref, Medline, Google Scholar
21. : The progression of cognitive impairment in dementia with Lewy bodies, vascular dementia and Alzheimer's disease. Int J Geriatr Psychiatry 2001; 16:499–503Crossref, Medline, Google Scholar
22. : A 1-year controlled trial of acetyl-l-carnitine in early-onset Alzheimer's disease. Neurology 2000; 55:805–810Crossref, Medline, Google Scholar
23. : Asymptomatic spontaneous cerebral emboli predict cognitive and functional decline in dementia. Biol Psychiatry 2007; 62:339–344Crossref, Medline, Google Scholar
24. : Combination therapy of donepezil and vitamin E in Alzheimer disease. Alzheimer Dis Assoc Disord 2003; 17:113–116Crossref, Medline, Google Scholar
25. : Potential long-term effects of rivastigmine on disease progression may be linked to drug effects on vascular changes in Alzheimer brains. Int J Clin Pract 2003; 57:756–760Medline, Google Scholar
26. : Are spontaneous cerebral microemboli consistent in carotid disease? Stroke 2002; 33:685–688Crossref, Medline, Google Scholar
27. : Cerebral microemboli and cognitive impairment. J Neurol Sci 2002;
28. : Clinical impact of patent foramen ovale diagnosis with transcranial Doppler. Eur J Ultrasound 2002; 16:11–20Crossref, Medline, Google Scholar
29. : Atherosclerosis and risk for dementia. Ann Neurol 2007; 61:403–410Crossref, Medline, Google Scholar
30. : The role of carotid atherosclerosis in Alzheimer's disease progression. J Alzheimers Dis 2011; 25:719–726Crossref, Medline, Google Scholar
31. : Intracranial atherosclerosis as a contributing factor to Alzheimer's disease dementia. Alzheimers Dement 2011; 7:436–444Crossref, Medline, Google Scholar
32. : Large vessel cerebral atherosclerosis is not in direct association with neuropathological lesions of Alzheimer's disease. Eur Neurol 2009; 62:93–98Crossref, Medline, Google Scholar
33. : Paradoxical embolisation and cerebral white matter lesions in dementia. Br J Radiol 2008; 81:30–34Crossref, Medline, Google Scholar
34. : Cerebral white matter hyperintense lesions are associated with unstable carotid plaques. Eur J Vasc Endovasc Surg 2006; 31:8–13Crossref, Medline, Google Scholar
35. : Relationship between white cell count, neuropsychologic outcome, and microemboli in 161 patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg 2006; 131:1358–1363Crossref, Medline, Google Scholar
36. : Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 2007; 7:161–167Crossref, Medline, Google Scholar
37. : Microglia: a sensor for pathological events in the CNS. Trends Neurosci 1996; 19:312–318Crossref, Medline, Google Scholar



