Decreased Conflict- and Error-Related Activity in the Anterior Cingulate Cortex in Subjects With Schizophrenia
Abstract
OBJECTIVE: People with schizophrenia have exhibited reduced functional activity in the anterior cingulate cortex during the performance of many types of cognitive tasks and during the commission of errors. According to conflict theory, the anterior cingulate cortex is involved in the monitoring of response conflict, acting as a signal for a need for greater cognitive control. This study examined whether impaired conflict monitoring in people with schizophrenia could underlie reduced anterior cingulate activity during both correct task performance and error-related activity. METHOD: Functional activity in the anterior cingulate of 13 schizophrenia patients and 13 healthy comparison subjects was investigated by using event-related fMRI and a Stroop task that allowed simultaneous examination of activity during both conflict (incongruent trials) and error (commission of error trials). RESULTS: In the presence of comparable reaction time measures for conflict as well as comparable error rates, the schizophrenia subjects showed both decreased conflict- and error-related activity in the same region of the anterior cingulate cortex. Moreover, those with schizophrenia did not exhibit significant post-conflict or post-error behavioral adjustments. CONCLUSIONS: Concurrently reduced conflict- and error-related activity in the anterior cingulate cortex along with reduced trial-to-trial adjustments in performance has not previously been reported in schizophrenia. The current results suggest that impaired conflict monitoring by the anterior cingulate cortex might play an important role in contributing to cognitive control deficits in patients with schizophrenia.
People with schizophrenia exhibit deficits in cognitive control (1). An important brain region involved in cognitive control is the anterior cingulate cortex (2). The goal of the current study was to test whether anterior cingulate cortex dysfunction in people with schizophrenia can be attributed to impairment in a specific cognitive function, the monitoring of response conflict.
Schizophrenia patients have frequently been reported to exhibit reduced anterior cingulate cortex activity at rest (3), during the performance of many different types of cognitive tasks (4–6), and during the commission of errors (7). In event-related potential studies, people with schizophrenia exhibit a reduced error-related negativity (8–11), an event-related potential component associated with the commission of errors with a source consistent with the anterior cingulate cortex (12, 13).
One theory of anterior cingulate cortex function is that it is involved in monitoring response conflict (i.e., the simultaneous activation of two competing responses) (2). For example, in the Stroop task, conflict occurs between color naming and word reading (e.g., the word “RED” printed in green ink). Response conflict occurs in many different tasks (e.g., underdetermined responding) and might explain why anterior cingulate cortex activity has been observed in a wide range of functional brain imaging studies (14).
According to conflict theory, the occurrence of response conflict elicits a signal for greater cognitive control, resulting in recruitment of brain regions to overcome conflict through increased control (2). Moreover, conflict theory posits that anterior cingulate cortex activity during errors is also the result of response conflict (15), since after an error ongoing stimulus evaluation leads to concurrent activation of both the correct and the just executed incorrect response. Hence, conflict theory predicts that the same area of the anterior cingulate cortex should be active during both conflict occurrence and error commissions (errors being a high conflict state), and that both signals should be associated with adjustments in control (2, 15). An alternative view of anterior cingulate cortex function is that it is directly involved in attentional selection. However, previous research suggests that the anterior cingulate cortex is involved in detecting conflict, whereas the prefrontal cortex is involved in selective attention (16, 17).
On the basis of conflict theory, we predicted that impaired anterior cingulate cortex activity in schizophrenia would be related to impaired conflict monitoring. If true, people with schizophrenia should exhibit reduced anterior cingulate cortex activity in the same area of the anterior cingulate cortex during both high-conflict correct trials and during commission of errors. However, concurrently decreased anterior cingulate cortex activity during both conflict and error trials has not been examined in the same study. Moreover, conflict theory predicts reduced behavioral adjustments after the occurrence of both conflict and errors in schizophrenia. Previous research has yet to examine whether people with schizophrenia exhibit reduced post-conflict behavioral adjustments and has been inconclusive regarding whether people with schizophrenia show reduced post-error adjustments, with two studies reporting reduced (7, 8) and two studies reporting intact (10, 11) adjustments.
Method
Participants
Thirteen people who met DSM-IV criteria for schizophrenia (five women and eight men; mean age=35.6 years [SD=8.9]; parental education mean=12.6 years [SD=2.7]) and 13 nonpsychiatric comparison subjects matched by age, gender, parental education, and ethnicity (four women and nine men; mean age=36.0 [SD=4.6]; parental education mean=13.1 years [SD=3.0]; all p values for between-group differences >0.55) participated in this study. (Data from this study and for these participants have not been published elsewhere.) Exclusion criteria were substance abuse/dependence history within 6 months, lifetime history of significant head injury or neurological illness, or any contraindication for MRI scanning. Diagnoses were determined with the SCID together with chart review and consultation with each patient’s treatment team. All patients were clinically stable and medicated outpatients. Two patients were being treated with a typical antipsychotic (haloperidol), eight were receiving atypical antipsychotics (olanzapine, N=4; quetiapine, N=1; clozapine, N=3), two were being treated with a typical and atypical combination (risperidone plus haloperidol; quetiapine plus haloperidol), and one was receiving an atypical combination regimen (risperidone and olanzapine). Mean patient symptom levels were as follows: BPRS=28.1 (SD=7.1), Positive and Negative Syndrome Scale=8 (SD=13.4), GAF=57.3 (SD=7.7). Comparison subjects were excluded for lifetime history of an axis I disorder or for a first-degree family history of psychotic disorder. All subjects were right-handed, had normal or corrected to normal vision in the scanner, and normal color vision as determined with Ishihara testing.
Stroop Task
Participants performed a single-trial version of the Stroop color-naming task. Stimuli consisted of one of three words (RED, GREEN, BLUE) printed in one of three colors. Trials were either congruent (e.g., the word “RED” in red ink) or incongruent (e.g., the word “RED” in green ink). Participants were instructed to name the color of the stimulus and to ignore the word. Participants responded with a button press using the middle three fingers of their right hand, with one finger for each color. Trials were 3.5 seconds long (with no intertrial interval) and consisted of a color word for 1 second followed by 2.5 seconds of a fixation cross (“+”). Each block consisted of 80 trials, with approximately 70% congruent trials. Participants were trained to competence to respond with button press responses corresponding to red, green, and blue colors (about 86 trials). They were told to be accurate, with speed also strongly emphasized. We followed the procedures of the initial study that reported post-conflict behavioral adjustments (18) and reemphasized response speed before the next block whenever error rates fell below 10% in a given block. In addition, to equate the two groups on the total number of errors, the number of blocks varied as a function of frequency of errors, with on average 6.15 blocks (SD=0.56) for schizophrenia subjects and 7.46 blocks (SD=1.05) for comparison subjects, with each participant completing at least six blocks. Our approach of individually adjusting speeded response instruction during the course of the experiment resulted in comparable total error rates between groups (schizophrenia subjects: mean=12.6% [SD=8.5]; comparison subjects: mean=9.5% [SD=4.9]). Given the difference in number of trials across participants, we repeated all behavioral and imaging analyses using only the first six blocks (all participants completed at least six blocks), with virtually identical results and all significant results remaining significant.
We were interested in analyzing two related behavioral effects (2): post-conflict adjustments ([iC–cC] + [cI–iI], where iC=congruent trial preceded by an incongruent trial, cI=incongruent trial preceded by a congruent trial, etc.) and post-error adjustments (i.e., comparing trials after errors versus trials after correct responses). For post-error adjustments, given the large reaction time difference between congruent and incongruent trials, we first calculated post-error slowing separately for congruent and incongruent post-error trials and then summed these two scores to create a composite post-error adjustment score. For post-conflict adjustments, we excluded trials with an exact stimulus repetition across consecutive trials, which could artifactually influence post-conflict adjustment scores (19). After removing stimulus repetitions, one patient with a high error rate had only six iI trials (iI trials were the most infrequent of all trial types). This patient was excluded from the analysis of post-conflict adjustments (resulting in 12 patients with post-conflict adjustment scores). Given our hypothesis that adjustments both after conflict and after errors are the result of anterior cingulate cortex activity, we also calculated an overall total adjustment score by summing the amount of post-conflict and post-error adjustment. Given the overall median reaction time difference between schizophrenia and comparison subjects, we used proportion scores (8).
Image Acquisition and Analysis
Acquisition
Functional scans were acquired by using a 1.5-T GE Signa whole body scanner with a standard head coil (GE Medical Systems, Milwaukee). Three functional T1-weighted scouts (in the axial, coronal, and sagittal planes) were used to localize the anterior commissure-posterior commissure (AC-PC) line. Twenty-three contiguous axial slices (thickness: 3.8 mm) with 3.75×3.75 mm in-plane resolution were obtained beginning 11.4 mm below the AC-PC line. Scans used a two-shot T2-weighted spiral scanning pulse sequence (TR=1750, TE=35 msec, flip angle=70°, field of view=24 cm), thus allowing full image acquisition every 3.5 seconds, with one image acquired for each Stroop trial. Structural images were obtained before and in the same plane as functional images using a standard T1-weighted pulse sequence.
Preprocessing
Incremental (scan to scan) and total movement were corrected by using Automated Image Registration (20). Participants were excluded from the study if 1) the mean estimated absolute movement from the reference scan was greater than 4 mm or degrees or 2) there was incremental movement greater than 2 mm (after removal for movement of one patient and no comparison subjects, there were 13 participants in each group). There were no significant differences between the two groups on estimated movement parameters. Although nonsignificant, there were two movement parameters, total movement in the x and in the y dimensions, for which schizophrenia subjects tended to have more movement than comparison subjects (p<0.20). We identified the three patients with the largest amount of movement (with movement in the x or y dimension >3 mm). When all three were removed, all p values for between-group differences for every one of the 12 movement parameters was >0.50; yet all significant between-group differences in anterior cingulate cortex brain activation remained significant (p<0.005), and they were also significant if any combination of one or two of these three patients were removed. Structural images were cross-registered to a reference brain by minimizing signal intensity differences with 12-parameter Automated Image Registration, after which images were set to a standard mean intensity and smoothed (8-mm full width at half maximum).
Statistical Analysis
Imaging data were analyzed with a random effects single-subject general linear model that used AFNI (21) and NIS software implemented within a locally developed graphical user interface (fiswidgets desktop) (22). Analyses included four mutually exclusive trial covariates: congruent, incongruent, error (either for congruent or incongruent trials), and no response. Partial correlation maps for individual subjects were generated that reflected the extent to which each voxel’s activity conformed to an a priori canonical double gamma hemodynamic response function (results were virtually identical if we used betas, with all significant results still significant), and we examined between-condition contrasts (i.e., incongruent versus congruent; error versus congruent) testing all voxels (i.e., we did not use a priori regions of interest). Statistical threshold for all analyses unless otherwise noted was p<0.005 and eight contiguous voxels (23). A priori calculations of efficiency (24) showed that the current design has essentially the equivalent efficiency for detecting between-condition differences as either a comparable slow event-related design or a fast event-related design with a variable intertrial inteval (for more properties of this type of imaging design, see reference 17).
Results
Behavioral Results
A two (trial type: congruent versus incongruent) by two (group: patient versus comparison) analysis of variance found a large and significant Stroop behavioral effect (congruent reaction time < incongruent reaction time: F=15.95, df=1, 24, p<0.001). Although not significant, schizophrenia subjects tended to have a larger Stroop effect (congruent mean=609.9 msec [SD=215.1]; incongruent mean=807.8 msec [SD=449.6]) than did the comparison subjects (congruent mean=476.9 msec [SD=92.0]; incongruent mean=567.3 msec, [SD=150.9]) (F=2.21, df=1, 24, p=0.15). There was also a tendency for schizophrenia subjects to have slower reaction times in general than comparison subjects (F=3.69, df=1, 24, p<0.07).
As expected, comparison subjects exhibited robust, significant post-conflict adjustment (32 msec) (t=2.70, df=12, p<0.05) and post-error slowing (26 msec) (t=2.56, df=12, p<0.05). In contrast, people with schizophrenia exhibited neither significant post-conflict adjustment (7 msec) (t=0.30, df=12, p>0.70) nor significant post-error slowing (4 msec) (t=0.19, df=11, p>0.80). In addition, there was an overall significantly lower total behavioral adjustment score in patients (t=2.12, df=24, p<0.05) (specific between-group comparison for post-conflict adjustment: t=1.63, df=24, p=0.17; for specific post-error slowing: t=1.63, df=23, p=0.12).
fMRI Results
As can be seen in Figure 1 and Table 1, comparison subjects exhibited robust conflict- and error-related activity in the anterior cingulate cortex (extending into the supplementary motor area), with conflict and error regions of activity largely overlapping. In fact, the conflict region fell entirely within the error region, with the error region being more extensive in every dimension (extent of difference in y dimension=3 mm; extent of inferior difference in z dimension=4 mm). Using the anterior cingulate cortex region of conflict-related activity as a region of interest, we found a significant amount of error-related activity in that same region. Similarly, we found a significant amount of conflict-related activity in the anterior cingulate cortex region of error-related activity.
As can be seen in Figure 2 and Table 2, people with schizophrenia also exhibited significant error-related activity in the anterior cingulate cortex, although most of their error activity occurred superiorly on the medial wall. In contrast, people with schizophrenia did not exhibit significant conflict-related anterior cingulate cortex activity. Even after lowering the threshold to p<0.05, people with schizophrenia did not exhibit significant conflict-related activity in the anterior cingulate cortex. As can be seen in Table 2, people with schizophrenia did exhibit robust and significant conflict-related activity in other regions.
As can be seen in Figure 3 and Table 3, comparison subjects exhibited both greater conflict-related activity (number of voxels=22) and error-related activity (number of voxels=29) than people with schizophrenia in a largely overlapping region of the anterior cingulate cortex. Using the anterior cingulate cortex region in which there was a significant between-group difference in conflict-related activity as a region of interest, we found significantly reduced error-related activity in the schizophrenia group. Similarly, we found significantly decreased conflict-related activity in schizophrenia subjects in the region with a between-group difference in error-related anterior cingulate cortex activity. As can be seen in Figure 4, relative to comparison subjects, people with schizophrenia had a comparable time of activation onset in the anterior cingulate cortex, but their amplitude of response was greatly reduced. Thus, people with schizophrenia exhibited reduced conflict- and error-related activity in the same overlapping region of the anterior cingulate cortex.
Discussion
Previous studies have reported reduced anterior cingulate cortex activity in people with schizophrenia performing a variety of tasks, including during the commission of errors and during correct performance of complex tasks likely to elicit conflict (3–7). This is the first study to concurrently examine and report decreased conflict- and error-related activity in the anterior cingulate cortex in the illness. The decrease in activity was in the same region of the anterior cingulate cortex for both conflict and errors. The area of the anterior cingulate cortex exhibiting decreased activity in people with schizophrenia corresponds closely to a region identified in a review of previous imaging studies as exhibiting maximum anterior cingulate cortex conflict activity during manual response tasks (Talairach coordinates: x=3, y=19, z=35) (14). Moreover, healthy participants in the present study tended to exhibit greater trial-to-trial performance adjustments than the schizophrenia subjects, who exhibited a near absence of post-error and post-conflict adjustments. These results are consistent with the hypothesis that anterior cingulate cortex dysfunction in schizophrenia, at least in part, reflects impairment in conflict monitoring that supports trial-by-trial adjustments in cognitive control. It further suggests that poor anterior cingulate cortex conflict monitoring might play a role in the deficits in cognitive regulation exhibited by people with schizophrenia.
Consistent with the conflict theory of anterior cingulate cortex-based performance monitoring and with another recent study that used the Stroop task (17), there was little difference between the area of the anterior cingulate cortex activated by conflict and that activated by errors in the comparison subjects. This contrasts with some reports that the area of the anterior cingulate cortex activated by errors is more anterior and inferior to the area activated by conflict (25–27) in go/no go tasks. An important difference between conflict and error trials in go/no go tasks is that on correct conflict trials people withhold a response but on error trials individuals execute one. In contrast, during the Stroop, correct conflict and error trials both involve overtly responding, perhaps explaining why conflict and error regions during the Stroop are more similar than on go/no go tasks. Moreover, the presumed error-specific region identified in these studies does exhibit conflict activity when considered in the context of the broader literature. The error-related maxima identified by Braver et al. (x=–1, y=21, z=27) (25) and by Ullsperger and von Cramon (x=7, y=19, z=30) (26) clearly fall within the area activated by conflict tasks (Figure 1, Table 1, Table 2, and Table 3) as identified in a review of anterior cingulate cortex activity (14).
On the surface, the present results appear inconsistent with those of Laurens et al. (28) who reported decreased rostral anterior cingulate cortex activity but not caudal anterior cingulate cortex activity during errors in schizophrenia. The area of reduced error-related activity reported by Laurens et al. (x=–8, y=44, z=12) is quite distinct from the anterior cingulate cortex activity in the current study and from the putative error distinct region identified by others (25–27). As suggested by Laurens et al., the region they identified might reflect emotional responses to errors rather than conflict-based performance monitoring, which appears disrupted in the present study. In similar event-related potential research, we have found different event-related potential error components localized to distinct areas of the anterior cingulate cortex, with one component (hypothesized to reflect conflict-related activity) associated with the caudal anterior cingulate cortex and another (hypothesized to reflect an emotional response to committing an error) being associated with the rostral anterior cingulate cortex (29). In the current study, neither schizophrenia nor comparison subjects exhibited significant rostral anterior cingulate cortex activity. Therefore, our results do not address whether patients might have an additional emotion processing deficit in the rostral anterior cingulate cortex, which we consider to be an interesting possibility that merits further research.
A second difference between the Laurens et al. study and the present one is that Laurens et al. did not report group differences in the caudal anterior cingulate cortex region associated with conflict processing. In that study, comparison subjects did not exhibit significant caudal anterior cingulate cortex activity in the conflict contrast, rendering a test of this function in patients impossible. The present study is, to our knowledge, the first to concurrently examine both conflict- and error-related activity in people with schizophrenia, finding both decreased conflict- and error-related activity in the same anterior cingulate cortex region.
An important caveat to the current results is that all patients were chronic outpatients taking (mostly atypical) antipsychotic medication. Dopamine blocking drugs may effect the functioning of the anterior cingulate cortex (30). Thus, it will be important in future studies to examine anterior cingulate cortex functional activity in unmedicated schizophrenia subjects.
According to conflict theory, anterior cingulate cortex conflict monitoring generates a signal that results in greater recruitment of cognitive control (2). Thus, conflict monitoring should result in behavioral adjustments in performance. In the current study, healthy participants exhibited both significant post-conflict adjustments and post-error slowing. In contrast, schizophrenia subjects exhibited a near absence of these adjustments. This is the first study to report a lack of post-conflict adjustment in people with schizophrenia. Previous research has been inconsistent with regard to whether people with schizophrenia exhibit reduced post-error slowing (7, 8, 10, 11). It is possible that not measuring post-error slowing separately for congruent and incongruent trials could account for these inconsistent results. The current results suggest that reduced anterior cingulate cortex conflict monitoring in schizophrenia could result in impaired recruitment of prefrontal cortex-based cognitive control. Thus, the current results suggest that poor anterior cingulate cortex conflict monitoring could have a broad influence on the cognitive difficulties experienced by people with schizophrenia.
Given previous research associating cognitive control deficits with symptoms (31), one issue for future research is to examine associations between anterior cingulate cortex conflict monitoring and symptoms of schizophrenia. For example, psychotic symptoms have been attributed to a deficit in monitoring (32). At the same time, given the role of the anterior cingulate cortex in cognitive control (2), it is possible that anterior cingulate cortex dysfunction could contribute to disorganization. Another important implication of the current results is for the relationship between impaired cognition and functional outcome in schizophrenia (33). Anterior cingulate cortex conflict monitoring has been found to contribute to a number of cognitive domains, such as learning and memory (14). Hence, disturbances in conflict monitoring might contribute to many cognitive deficits found to be predictive of functional outcome in the disorder. We would suggest that the relationship between anterior cingulate cortex function in schizophrenia to medication treatment and functional outcome should be the subject of further investigation.
 |
 |
 |
Received May 18, 2004; revision received Aug. 13, 2004; accepted Aug. 20, 2004. From the Department of Psychological Sciences, University of Missouri, Columbia; the University of Pittsburgh Department of Psychiatry, Pittsburgh; the Center for the Study of Brain, Mind, and Behavior and the Department of Psychology, Princeton University, Princeton, N.J.; the Department of Psychology, University of Minnesota, Minneapolis; the Department of Radiology, University of Pittsburgh Medical Center, Pittsburgh; and the Departments of Psychiatry and Psychology and the University of California Imaging Research Center, UC Davis. Address correspondence and reprint requests to Dr. Carter, UC Davis Imaging Research Center, 4701 X St., Sacramento, CA 95817; [email protected] (e-mail). Supported by NIMH grants MH-059883 and KO2 MH-64190 to Dr. Carter and an Established Investigator Award from the National Alliance for Research on Schizophrenia and Depression to Dr. Cohen.
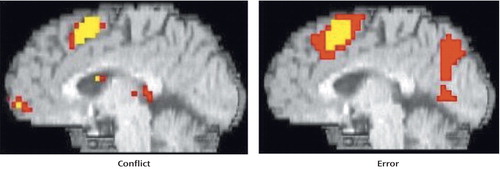
Figure 1. Conflict- and Error-Related Anterior Cingulate Cortex Activity in Comparison Subjects During Stroop Task Performancea
aNote that the conflict region and error region entirely overlapped, with the error region being more extensive in every dimension.
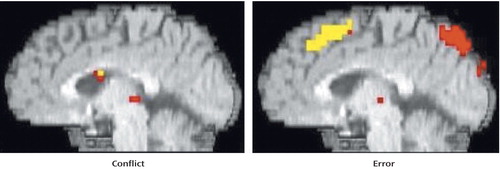
Figure 2. Conflict- and Error-Related Anterior Cingulate Cortex Activity in People With Schizophrenia During Stroop Task Performance
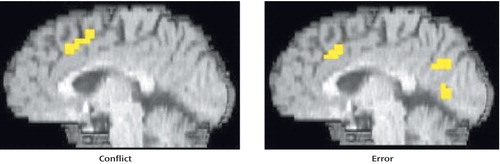
Figure 3. Regions of Greater Conflict- and Error-Related Anterior Cingulate Cortex Activity in Comparison Subjects Relative to People With Schizophrenia During Stroop Task Performance
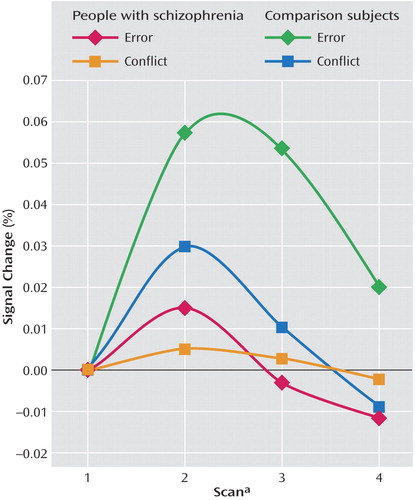
Figure 4. Conflict- and Error-Related Anterior Cingulate Cortex Response Amplitude in People With Schizophrenia and Comparison Subjects During Stroop Task Performancea
aFull scan every 3.5 seconds.
1. Cohen JD, Servan-Schreiber D: Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol Rev 1992; 99:45–77Crossref, Medline, Google Scholar
2. Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD: Conflict monitoring and cognitive control. Psychol Rev 2001; 108:624–652Crossref, Medline, Google Scholar
3. Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, Carpenter WT: Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry 1992; 49:522–530Crossref, Medline, Google Scholar
4. Rubia K, Russell T, Bullmore ET, Soni W, Brammer MJ, Simmons A, Taylor E, Andrew C, Giampietro V, Sharma T: An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophr Res 2001; 52:47–55Crossref, Medline, Google Scholar
5. Hofer A, Weiss EM, Golaszewski SM, Siedentopf CM, Brinkhoff C, Kremser C, Felber S, Fleischhacker WW: An fMRI study of episodic encoding and recognition of words in patients with schizophrenia in remission. Am J Psychiatry 2003; 160:911–918Link, Google Scholar
6. Meyer-Lindenberg A, Poline J-B, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF: Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry 2001; 158:1809–1817Link, Google Scholar
7. Carter CS, MacDonald AW III, Ross LL, Stenger VA: Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry 2001; 158:1423–1428Link, Google Scholar
8. Alain C, McNeely HE, He Y, Christensen BK, West R: Neurophysiological evidence of error-monitoring deficits in patients with schizophrenia. Cereb Cortex 2002; 12:840–846Crossref, Medline, Google Scholar
9. Bates AT, Kiehl KA, Laurens KR, Liddle PF: Error-related negativity and correct response negativity in schizophrenia. Clin Neurophysiol 2002; 113:1454–1463Crossref, Medline, Google Scholar
10. Kopp B, Rist F: An event-related brain potential substrate of disturbed response monitoring in paranoid schizophrenic patients. J Abnorm Psychol 1999; 108:337–346Crossref, Medline, Google Scholar
11. Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM: Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. J Abnorm Psychol 2002; 111:22–41Crossref, Medline, Google Scholar
12. Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E: A neural system for error detection and compensation. Psychol Sci 1993; 4:385–390Crossref, Google Scholar
13. Falkenstein M, Hoormann J, Christ S, Hohnsbein J: ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol 2000; 51:87–107Crossref, Medline, Google Scholar
14. Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A: Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex 2001; 11:837–848Crossref, Medline, Google Scholar
15. Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD: Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 1998; 280:747–749Crossref, Medline, Google Scholar
16. MacDonald AW III, Cohen JD, Stenger VA, Carter CS: Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 2000; 288:1835–1838Crossref, Medline, Google Scholar
17. Kerns JG, Cohen JD, MacDonald AW III, Cho RY, Stenger VA, Carter CS: Anterior cingulate conflict monitoring and adjustments in control. Science 2004; 303:1023–1026Crossref, Medline, Google Scholar
18. Gratton G, Coles MG, Donchin E: Optimizing the use of information: strategic control of activation of responses. J Exp Psychol Gen 1992; 121:480–506Crossref, Medline, Google Scholar
19. Mayr U, Awh E, Laurey P: Conflict adaptation effects in the absence of executive control. Nat Neurosci 2003; 6:450–452Crossref, Medline, Google Scholar
20. Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC: Automated Image Registration, I: general methods and intrasubject, intramodality validation. J Comput Assist Tomogr 1988; 22:139–152Crossref, Google Scholar
21. Cox RW: AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:1672–1673Crossref, Google Scholar
22. Fissell K, Tseytlin E, Cunningham D, Karthickeyan I, Carter CS, Schneider W, Cohen JD: Fiswidgets: a graphical computing environment for neuroimaging analysis. Neuroinformatics 2002; 1:111–125Crossref, Google Scholar
23. Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC: Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995; 33:636–647Crossref, Medline, Google Scholar
24. Dale AM: Optimal experimental design for event-related fMRI. Hum Brain Mapp 1999; 8:109–114Crossref, Medline, Google Scholar
25. Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A: Anterior cingulate cortex and response conflict: effects of frequency, inhibition, and errors. Cereb Cortex 2001; 11:825–836Crossref, Medline, Google Scholar
26. Ullsperger M, von Cramon DY: Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage 2001; 14:1387–1401Crossref, Medline, Google Scholar
27. Mathalon DH, Whitfield SL, Ford JM: Anatomy of an error: ERP and fMRI. Biol Psychol 2003; 64:119–141Crossref, Medline, Google Scholar
28. Laurens KR, Ngan ETC, Bates AT, Kiehl KA, Liddle PF: Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain 2003; 126:610–622Crossref, Medline, Google Scholar
29. van Veen V, Carter CS: The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci 2002; 14:593–602Crossref, Medline, Google Scholar
30. Suhara T, Okubo Y, Yasuno F, Sudo Y, Inoue M, Ichimiya T, Nakashima Y, Nakayama K, Tanada S, Suzuki K, Halldin C, Farde L: Decreased dopamine D2 receptor binding in the anterior cingulate cortex in schizophrenia. Arch Gen Psychiatry 2002; 59:25–30Crossref, Medline, Google Scholar
31. Kerns JG, Berenbaum H: The relationship between formal thought disorder and executive functioning component processes. J Abnorm Psychol 2003; 112:339–352Crossref, Medline, Google Scholar
32. Frith CD: The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychol Med 1987; 17:631–648Crossref, Medline, Google Scholar
33. Green MF, Kern RS, Braff DL, Mintz J: Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull 2000; 26:119–136Crossref, Medline, Google Scholar



