A Functional Magnetic Resonance Imaging Study of Social Cognition in Schizophrenia During an Acute Episode and After Recovery
Abstract
Objective: Difficulty with social interactions is a characteristic of schizophrenia. The authors used functional magnetic resonance imaging (fMRI) to investigate brain activation changes during a social cognition paradigm in patients with schizophrenia during and after an acute episode and their association with social and executive function. Method: In a longitudinal study design, 14 patients with schizophrenia experiencing an acute episode had an fMRI scan. They returned for a follow-up scan after clinical improvement. Fourteen healthy comparison subjects were also scanned twice with approximately the same time interval between scans as in the patient group. The authors employed a social cognition fMRI paradigm involving empathic and forgivability judgments. Schizophrenia symptoms, social functioning and illness insight scales, and the Wisconsin Card Sorting Test were used to examine whether improvement on these measures was associated with recovery of brain activation in response to the social cognition paradigm. Results: After recovery from the acute episode, patients exhibited increased activation in the left medial prefrontal cortex, which was, in turn, significantly correlated with improved insight and social functioning. Decreased symptom severity and improved performance on the Wisconsin Card Sorting Test were not significantly associated with increased left medial prefrontal cortex activation. Conclusions: This is the first study to the authors’ knowledge to use a social cognition paradigm to reveal improved left medial prefrontal cortex activation in schizophrenia after recovery from an acute episode. These results suggest that restored left medial prefrontal cortex activation may mediate improvement of insight and social functioning in patients with schizophrenia.
Social cognition is a specialized domain of cognition hypothesized to have developed in order to solve social and adaptive problems. The capacity for understanding another’s mental state may have evolved from the ability to experience our own mental states (1) . In healthy subjects, the neural bases of various aspects of social cognition have been explored, including the “theory of mind,” empathy, moral/ethical judgments, and social cooperation (2 , 3) . In parallel, there is an increasing interest in social cognition in schizophrenia (4) because difficulties with social cognition might directly contribute to impaired social functioning in these patients.
The medial prefrontal cortex may play a predominant role in social cognition. A number of neuroimaging studies have consistently reported medial prefrontal activation during a variety of social cognition tasks (5) . For example, in a previously reported fMRI study of healthy volunteers that employed the same social cognition paradigm as the current study, we observed that prefrontal (including the medial prefrontal cortex) and left temporal regions exhibited greater activation while subjects made empathic and forgivability judgments compared with baseline judgments concerning social situations (6) .
There have been only a few neuroimaging studies examining the neural basis of social cognition in people with schizophrenia. Russell and colleagues (7) reported left middle and inferior frontal cortex underactivation in people with schizophrenia during mental state attribution. Using an intention attribution task, Brunet and colleagues (8) showed right medial prefrontal cortex underactivation in people with schizophrenia. These findings raise the question as to whether deficient activation during social cognition can “recover” with clinical improvement. In support of this suggestion, patients with schizophrenia showed a return of task-related brain activation similar to that of healthy subjects following verbal memory training (9) and treatment with typical (10) and atypical antipsychotic medication (11) . There is also some evidence that social cognition improves in parallel with clinical improvement in schizophrenia (12) .
Hence, we hypothesized that, on a social cognition fMRI paradigm, 1) the medial prefrontal cortex of patients with schizophrenia is underactivated during an acute episode in relation to healthy comparison subjects and their own activation levels after recovery from an acute episode and that 2) the improvement in medial prefrontal activation is associated with enhanced illness insight and social functioning. To test these hypotheses, we scanned 14 patients during an acute episode of their illness and again following clinical improvement. Fourteen healthy comparison subjects were also scanned twice, with approximately the same time interval between scans as in the patient group, to control for the effects of time, scan repetition, and paradigm exposure. In view of the suggested association between Wisconsin Card Sorting performance and social functioning (13) , we also examined the relationship between Wisconsin Card Sorting Test performance and brain activation changes.
Method
Subjects
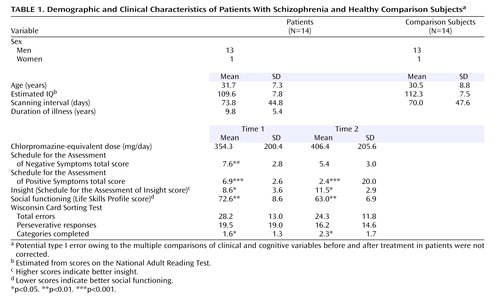
Nineteen patients with a DSM-IV diagnosis of schizophrenia were recruited to participate in this study and had an fMRI scan during an acute episode. Fourteen of the patients had a second scan (mean=73.8, SD=44.8, days after the first scan) whose data were used for analysis. The acute episode was severe enough to warrant inpatient admission. Recovery was after or just before discharge from the inpatient unit, which would indicate a clinical improvement as judged by the team responsible for clinical care. The final patient sample comprised 13 men and one woman, with a mean age of 31.7 years (SD=7.3). Healthy comparison subjects (13 men and one woman; mean age of 30.5 years, SD=8.8) were also scanned twice (mean=70.0, SD=47.6 days apart). All subjects were right-handed. Exclusion criteria for both groups were contraindications to magnetic resonance scanning, neurological disorders (including previous head injury), and learning disabilities. There were no statistically significant between-group differences in age or estimated IQ ( Table 1 ). The study was approved by the local research ethics committee. After complete description of the study to the subjects, written informed consent was obtained.
For all 14 patients, treatment involved conventional inpatient treatment. All patients were receiving antipsychotic medication throughout the study. The mean daily dose in chlorpromazine equivalents (14) was 354.3 mg/day at the first scan and 406.4 mg/day at the second scan. Nine patients were receiving atypical (clozapine [N=4], olanzapine [N=4], or risperidone [N=1]), and five were receiving typical antipsychotics at the first scan. One patient was switched from a depot typical antipsychotic to an oral atypical antipsychotic (clozapine) between the first and second scans. The others remained taking the same antipsychotic medication throughout the study. Within a week of scanning, schizophrenia symptoms were rated with the Schedule for the Assessment of Positive Symptoms (SAPS) (15) and the Schedule for the Assessment of Negative Symptoms (SANS) (16) . Illness insight was measured with the Schedule of Assessment of Insight (17) , which is a semistructured interview that assesses three dimensions of insight (treatment compliance, recognition of illness, and relabeling of psychotic phenomena). Social functioning was assessed by using the Life Skills Profile, a measure of social function and disability in schizophrenia (18) . Patients completed a 64-card version of the Wisconsin Card Sorting Test (19) .
Tasks During fMRI
The experimental task was identical to that of Farrow et al. (6) . Briefly, all subjects underwent two functional scans (empathic and forgivability judgments). Each scan (lasting 306 seconds) incorporated six 51-second epochs in an alternating A/B boxcar design. There were three active conditions and three baseline social reasoning conditions per scan. Each 51-sec epoch consisted of reading a scenario for 16 seconds, followed by making five serial judgments (each lasting 7 seconds) regarding the scenario. Pilot work at the beginning of this study ensured that a time window of 16 seconds for reading and 7 seconds for response was sufficient for patients with schizophrenia. Therefore, we used the identical fMRI parameters used in our previous studies with healthy volunteers (6) and patients with posttraumatic stress disorder (20) . The same tasks, with a counterbalanced order within and across subjects, were used for the first and second fMRI sessions.
The serial judgments used a forced-choice paradigm wherein two possible answers were presented. Both possible answers were matched for sentence length and complexity, and there were no right or wrong answers. Empathy scenarios described another person in need or distress (e.g., “You come home. Your friend has had an unpleasant experience that day. Which is the more likely explanation for your friend’s state of mind?”), and forgiveness scenarios requested a choice as to which of two crimes was more forgivable (e.g., “A young man appearing in court. He is 17 years old. He has been in care for many years and had a troubled childhood. Which of the following crimes would you see as more forgivable?”). Baseline social reasoning scenarios related to social situations (e.g., “You are approaching a large traffic jam on the motorway. It is not rush hour. There have been no roadwork signs. Which is the more likely explanation?”). Data relating to the judgment phase were analyzed.
A computer controlled experimental events and recorded data using Psyscope (21) . The computer output was displayed by a projector on a screen visible to the subject in the scanner. Subjects used their right index and middle finger to press buttons on an intrascanner response box, which was optically connected to a Psyscope button box by means of an interface (New Micros, Inc., Dallas).
fMRI Data Acquisition and Analysis
fMRI was performed by using a 1.5 T system (Eclipse, Philips Medical Systems, Cleveland) at the University of Sheffield. Functional images were acquired at 104 time points by using gradient-recalled, echo-planar imaging (TE=40 msec, TR=3000 msec, field of vision=240 mm, in-plane matrix=128×128×20×6 mm slices, acquisition time=5 minutes and 6 seconds), covering the entire cerebrum and most of the cerebellum.
Data were analyzed with statistical parametric mapping (SPM99, http://www.fil.ion.ucl.ac.uk/spm) and were motion-corrected, spatially normalized, and smoothed by using a Gaussian kernel (full-width half-maximum of 8 mm). Blood-oxygen-level-dependent (BOLD) response was modeled by a boxcar waveform convolved with a canonical hemodynamic response function and its temporal derivative. Random effects models were used to quantify group activations associated with empathic and forgivability judgments at both scanning times and to examine within- and between-group differences. A second-level conjunction analysis was performed in order to identify brain areas exhibiting conjointly increased activation in response to both empathic and forgivability judgments in the patient group following clinical improvement. The mean BOLD signal changes in conjointly activated areas were then correlated with clinical and cognitive variables. Statistical parametric maps (t) were generated with a height threshold of p<0.005, uncorrected for multiple comparisons. Anatomical localizations in the maps (t) were transformed into the stereotactic space of Talairach and Tournoux (22) .
Results
Clinical and Behavioral Data
Patients’ symptom severity was significantly reduced (SANS total score: t=3.4, df=12, p<0.01; SAPS total score: t=6.5, df=12, p<0.001) following treatment. Their insight (t=2.9, df=12, p<0.05) and social functioning (t=4.1, df=12, p<0.01) scores significantly improved. There was a modest increase in executive functioning (Wisconsin Card Sorting categories completed) in the patient group ( Table 1 ).
During scanning, the mean TR for patients (mean=3.6, SD=0.2 seconds) was not significantly slower than that of comparison subjects (mean=3.3, SD=0.2 seconds). Both comparison subjects and patients were faster at responding to judgments involving social reasoning than those involving empathic (F=48.2, df=1, 26, p<0.001) or forgivability judgments (F=103.1, df=1, 26, p<0.001).
Imaging Data
Brain Activation Changes Following Recovery From an Acute Episode of Schizophrenia and Their Clinical Correlates
The improvement of brain activation in patients (second versus first scans) was contrasted with time-related changes in healthy comparison subjects (second versus first scans) to observe the effect of recovery from an acute episode in patients with schizophrenia. As shown in data supplement 1 (available online as a data supplement at http://ajp.psychiatryonline.org) and Figure 1 , there were significant increases in activation in the left medial prefrontal cortex (Brodmann’s area 10/9) along with the right fusiform gyrus (Brodmann’s area 19), the posterior middle temporal (Brodmann’s area 37) and lingual gyri (Brodmann’s area 18), and the left inferior parietal lobule (Brodmann’s area 40) during empathic judgments in patients. Forgivability judgments also led to increased activation of the left medial prefrontal cortex (Brodmann’s area 10/9), as well as the cuneus and precuneus (Brodmann’s area 18/7), the posterior middle temporal (Brodmann’s area 37), and the paracentral (Brodmann’s area 31) areas of the left hemisphere.
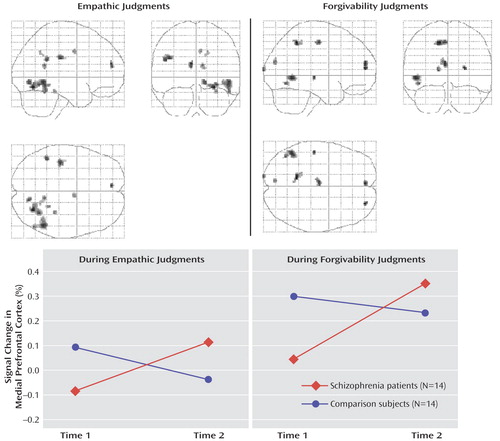
a The upper panel shows brain areas associated with the effect of recovery in patients with schizophrenia during empathic and forgivability judgments (patients’ second versus first scans contrasted with the comparison subjects’ second versus first scans). The lower panel presents raw BOLD signal intensity changes in the left medial prefrontal cortex (peak activation coordinates: x=–8, y=51, z=16 for empathic and x= –4, y=55, z=14 for forgivability judgments) in patients with schizophrenia and healthy comparison subjects.
Because the left medial prefrontal area was consistently associated with the effect of recovery in schizophrenia for both empathic and forgivability judgments, mean BOLD signal changes in the peak voxels of the left medial prefrontal cortex were then extracted in all scans for both tasks in order to examine brain response pattern across scans. As shown in Figure 1 , patients showed greater activation in response to both empathic and forgivability judgments over time, whereas comparison subjects showed an activation change in the opposite direction (F=4.58, df=1, 26, p<0.05).
A second-level conjunction analysis was then performed within the patient group alone to identify brain areas conjointly increased in activation for both empathic and forgivability judgments following recovery and to correlate signal changes in these regions with clinical variables. The left medial prefrontal cortex (Brodmann’s area 10/9: x= -6, y=51, z=16; z=3.40, p<0.0001; 32 voxels), shown in Figure 2 , exhibited the strongest conjoint activation following recovery during both empathic and forgivability judgments. Other significantly activated areas included the right lingual gyrus (Brodmann’s area 18; x=14, y=–76, z=–3; z=3.02, p=0.001; 17 voxels), the cuneus (Brodmann’s area 18; x=–8, y= – 80, z=26; z=3.02, p=0.001; 13 voxels), and the left ventral anterior cingulate gyrus (subgenual anterior cingulate cortex, Brodmann’s area 25; x=–16, y=15, z= –11; z=2.93, p=0.002; 16 voxels).

a Left medial prefrontal cortex (peak activation coordinates: x=–6, y=51, z=16; 32 voxels) activation common to empathic and forgivability judgments in patients with schizophrenia following recovery from an acute episode. The graphs show that the difference between mean blood-oxygen-level-dependent signal changes in the peak activation before and after recovery in patients was significantly correlated with improvement in insight (r=0.81, p<0.001) and was related at a less than significant level to improvement in social functioning scores (r=0.51, p=0.06).
Figure 2 shows that increased activation of the left medial prefrontal cortex in patients was significantly correlated with improvement in insight scores (r=0.81, p<0.001) and was related at a less than significant level to improvement in social functioning scores (r=0.51, p=0.06). Decreased symptom severity (either negative or positive) or improved performance on the Wisconsin Card Sorting Test (total errors, perseverative responses, or categories completed) were not significantly associated with increased left medial prefrontal cortex activation. Mean BOLD signal changes from the other significantly activated areas (the right lingual gyrus, cuneus, and ventral anterior cingulate gyrus) were extracted to examine whether activation changes in these regions also had the same relationship. Increased activation in the right lingual gyrus was moderately correlated with increased insight scores (r=0.56, p<0.05); enhanced left subgenual cingulate gyrus activation was correlated with decreased negative symptom severity (r=0.59, p<0.04).
Within- and Between-Group Differences in Empathic Judgments
As shown in data supplement 2, during the first scan, the comparison subjects showed increased BOLD response for empathic judgments, compared with baseline social reasoning judgments, in the bilateral medial prefrontal cortex (Brodmann’s area 10) and the inferior frontal gyrus (Brodmann’s area 45). Posteriorly, bilateral middle temporal gyri (Brodmann’s area 21), the left inferior parietal lobule (angular gyrus, Brodmann’s area 39), the right posterior fusiform gyrus (Brodmann’s area 19), the thalamus, the precuneus, and the cuneus were activated. These results replicate our previous findings (6) in a newly acquired healthy volunteer sample. Patients with schizophrenia, on the other hand, exhibited relatively small areas of activation in the left medial prefrontal cortex (Brodmann’s area 10), the right middle frontal gyrus (Brodmann’s area 46), the left superior temporal gyrus (Brodmann’s area 22), the left precuneus (Brodmann’s area 7), and the left lingual gyrus (Brodmann’s area 17). Although the pattern of activation at the first scan in healthy comparison subjects was largely preserved at the second scan (data supplement 2), the patients exhibited a difference in activation patterns between the first and second scans. For instance, the patients showed relatively small but significant activations (not seen at time 1) in areas, including the right anterior fusiform gyrus (Brodmann’s area 37) and the caudate. Between-group comparisons at first scan, as shown in data supplement 2, showed that patients had less activation than those seen in comparison subjects in the left superior/medial prefrontal cortex (Brodmann’s area 8), the right inferior temporal gyrus (Brodmann’s area 19), the right posterior fusiform gyrus (Brodmann’s area 19), the precuneus (Brodmann’s area 7), the left middle/inferior temporal gyrus (Brodmann’s area 20), and the thalamus. Between-group differences during the second scan revealed less activation in patients than in comparison subjects in the bilateral middle frontal gyrus (Brodmann’s area 10), thalamus, and precuneus (Brodmann’s area 7).
Within- and Between-Group Differences in Forgivability Judgments
Comparison subjects at the first scan exhibited increased activation, during forgivability judgments compared with baseline social reasoning judgments, in the bilateral medial prefrontal cortex (Brodmann’s area 10), the bilateral superior frontal gyri (Brodmann’s area 8/9), and the right middle frontal gyrus (Brodmann’s area 8). The posterior cingulate (Brodmann’s area 23), the cuneus (Brodmann’s area 17/19), the thalamus, and a wide region, including the occipital lingual gyrus and posterior fusiform gyrus, were also activated. Patients with schizophrenia also activated the left medial prefrontal cortex (Brodmann’s area 10). As in empathic judgments, the healthy comparison subjects’ activation pattern at the first scan was replicated during the second scan. During the second scan, patients exhibited increased activation in the medial prefrontal cortex (Brodmann’s area 8/10). Between-group analysis revealed that at the first scan, the patients had decreased activation in the left superior frontal (Brodmann’s area 9) and occipital lingual gyri (Brodmann’s area 19), the right posterior fusiform gyrus (Brodmann’s area 19), and the globus pallidus. Patients during the second scan showed diminished activation in a large area around the thalamus, prefrontal areas, and the posterior cingulate (data supplement 3).
Discussion
This study employed a repeated-measures design to investigate the neural basis of social cognition during and after an acute episode in patients with schizophrenia. Symptom ratings, social functioning, illness insight scales, and the Wisconsin Card Sorting Test were used to examine whether improvement on these measures was predicted by recovery of brain activation in response to the social cognition paradigm. Our study showed that patients with schizophrenia displayed less activation than healthy subjects in the left medial prefrontal cortex, among other regions. However, clinical improvement after treatment was accompanied by enhanced activation in this region. The increase in left medial prefrontal cortex activity was specifically associated with improved insight and social functioning (just less than significant) scores in these patients. This increased activation may have provided a partial explanation for the neural basis of improved social cognition after recovery observed in other studies (12) . We also found that reduced severity of negative symptoms was associated with enhanced activation in the left ventral anterior cingulate cortex.
The finding that the medial prefrontal cortex was activated in our social cognition paradigm in comparison subjects is in line with many other neuroimaging studies of the neural basis of understanding the mental states of others (5) . The medial prefrontal cortex is activated when attention is directed to the self (self-awareness) (23) . The common engagement of this area for representing the mental states of others and the self may provide the neural basis for intersubjectivity (24) , the interplay between two different subjective minds. Considering this common engagement, it is important to highlight that the increased activation in the left medial prefrontal cortex was associated with improved “illness insight” in people with schizophrenia. The specific association between improved illness insight and medial prefrontal activation in our repeated scans is strengthened because patients with schizophrenia in general have greater variability in brain activation across time in relation to healthy comparison subjects (25) .
In this study, in both comparison and schizophrenia subjects, activation generally decreased over time. In the left medial prefrontal cortex, however, we found that activation change over time in patients was in the opposite direction of that of comparison subjects. Patients showed increased activation at the second scan, whereas the comparison group showed decreased activation at the second scan ( Figure 1 ). Thus, at the second scan, there were no significant group differences in activation in the left medial prefrontal cortex. Other studies have reported similar task-related brain activation “recovery” in patients with schizophrenia. Wexler and colleagues (9) showed that increased activation in the left inferior frontal cortex was significantly correlated with patients’ behavioral improvement in verbal memory performance following verbal memory training for 10 weeks. On the other hand, studies of healthy volunteers have reported that task-relevant activation may be reduced when they are presented with familiar stimuli or when a task has been previously rehearsed (26) . Increased efficiency or reduced anxiety to task in healthy subjects may explain the reduced activation over time.
To date, we are aware of two neuroimaging studies of social cognition in patients with schizophrenia. Russell and colleagues (7) reported that people with schizophrenia made more errors in the mental state attribution of photographed eyes, and had less activation in the left prefrontal cortex in relation to comparison subjects. A positron emission tomography study by Brunet and colleagues (8) used a sequencing task that involved having to infer a character’s intention and choosing a card to complete sequences. In this task, in contrast to the finding of Russell et al., people with schizophrenia showed decreased activation in the right medial prefrontal cortex. We speculate that language material used in our study (written scenarios and response options) and Russell and colleagues’ (matching words with photographs) might engage the left prefrontal cortex more than the right. The nonverbal material used in the study by Brunet and colleagues might explain why they showed right prefrontal activation. Our study showed that areas exhibiting enhanced activation in patients after recovery (left medial prefrontal cortex, Brodmann’s area 9/10) were not identical to those that were deficient in activation at the first scan (Brodmann’s area 8/9). It is possible that these various prefrontal areas are part of one functionally defined area of the cortex because these areas are frequently reported in social cognition studies (5) . Alternatively, patients may have recruited different prefrontal areas to perform the task when they were acutely ill.
The other brain area that schizophrenia patients consistently failed to activate for both empathic and forgivability judgments at the first scan was the right posterior fusiform gyrus. Schultz and colleagues (27) showed that the right posterior fusiform gyrus, which has been associated with face perception, is activated in response to a task involving perception of moving geometric shapes interacting with each other in a social manner. Another study by Geday and colleagues (28) demonstrated that the right posterior fusiform gyrus was activated in response to emotional pictures of people in social situations as well as facial emotion expressions. These studies suggest that the posterior fusiform gyrus is heavily involved in processing emotionally important cues and attributes of other people. Because patients with schizophrenia consistently showed underactivation in this area during facial emotion recognition (29) , future studies might examine the link between facial emotion recognition and social cognition in schizophrenia.
Healthy comparison subjects showed increased activation in the thalamus during empathic and forgivability judgments compared with baseline social reasoning judgments, whereas patients failed to activate this region. A number of studies have shown functional and structural thalamic abnormalities in schizophrenia (30) . Whether the reduced thalamic activation in patients in this study is directly related to social cognition dysfunction or is associated with a lack of attention and sensory information processing remains to be determined. Nonetheless, studies showing thalamus-anterior cingulate network activation in mothers listening to infant cries (31) and involvement of this network in rodent maternal behavior (32) suggest that this network activity might represent attention to emotional and social stimuli, consisting of a neural basis for social cognition.
Following recovery from the acute episode, the patient group showed increased activation in the left subgenual anterior cingulate cortex, which was associated with reduced negative symptoms. This area was not part of our hypothesis. Subgenual anterior cingulate cortex activity has been related to emotional responsivity and is reported to be decreased in people with depression (33) . The current study, however, did not assess depressive symptoms or neuroleptic-induced movement disorders that partly overlap with negative symptoms. Statistical significance for this activity was weak, and the functional significance of increased subgenual anterior cingulate cortex activation in patients with schizophrenia could be addressed by future studies.
There are some issues to consider in interpreting the results of this study. In our study, the subjects were predominantly men. There is some evidence suggesting that women experience fewer adverse psychosocial consequences of schizophrenia (34) . Second, we used a less conservative activation threshold in this study, which would increase the number of areas detected. We were, however, particularly interested in consistent findings across different comparisons. Finally, the need for the development of social cognition tasks for schizophrenia research should also be noted. The development of our fMRI tasks was based upon literature showing that an individual’s empathic responses arise as a direct result of observing someone in distress (35) . Future studies might want to develop tasks to assess different aspects of social cognition in schizophrenia because social cognition is a wide domain of cognition and consists of multiple emotional and cognitive processes.
In summary, our study supports the hypothesis that improved insight following recovery from an acute episode of schizophrenia is reflected in enhanced medial prefrontal activation during social cognition tasks. The results of our study also suggest that medial prefrontal cortex underactivation in patients relative to healthy comparison subjects may reflect deficits in the representation of self and others and that these deficits may partially be ameliorated when patients can recruit the medial prefrontal cortex for social cognition. Our study highlights the biological responsivity of the prefrontal cortex to therapeutic intervention (10 , 36) and forms the basis upon which to investigate further neural mechanisms for psychosocial function in schizophrenia.
1. Humphrey N: The Inner Eye: Social Intelligence in Evolution. Oxford, UK, Oxford University Press, 2002Google Scholar
2. Blakemore S-J, Winston J, Frith U: Social cognitive neuroscience: where are we heading? Trends Cogn Sci 2004; 8:216–222Google Scholar
3. Preston SD, de Waal FBM: Empathy: its ultimate and proximate bases. Behav Brain Sci 2002; 21:1–71Google Scholar
4. Pinkham AE, Penn DL, Perkins DO, Lieberman J: Implications for the neural basis of social cognition for the study of schizophrenia. Am J Psychiatry 2003; 160:815–824Google Scholar
5. Lee K-H, Farrow TFD, Spence SA, Woodruff PWR: Social cognition, brain networks and schizophrenia. Psychol Med 2004; 34:391–400Google Scholar
6. Farrow TF, Zheng Y, Wilkinson ID, Spence SA, Deakin JF, Tarrier N, Griffiths PD, Woodruff PW: Investigating the functional anatomy of empathy and forgiveness. Neuroreport 2001; 12:2433–2438Google Scholar
7. Russell TA, Rubia K, Bullmore ET, Soni W, Suckling J, Brammer MJ, Simmons A, Williams SC, Sharma T: Exploring the social brain in schizophrenia: left prefrontal underactivation during mental state attribution. Am J Psychiatry 2000; 157:2040–2042Google Scholar
8. Brunet E, Sarfati Y, Hardy-Bayle MC, Decety J: Abnormalities of brain function during a nonverbal theory of mind task in schizophrenia. Neuropsychologia 2003; 41:1574–1582Google Scholar
9. Wexler BE, Anderson M, Fulbright RK, Gore JC: Preliminary evidence of improved verbal working memory performance and normalization of task-related frontal lobe activation in schizophrenia following cognitive exercises. Am J Psychiatry 2000; 157:1694–1697Google Scholar
10. Spence SA, Hirsch SR, Brooks DJ, Grasby PM: Prefrontal cortex activity in people with schizophrenia and control subjects. evidence from positron emission tomography for remission of “hypofrontality” with recovery from acute schizophrenia. Br J Psychiatry 1998; 172:316–323Google Scholar
11. Lund A, Kroken R, Thomsen T, Hugdahl K, Smievoll AI, Barndon R, Iversen J, Landro NI, Sundet K, Rund BR, Ersland L, Lundervold A, Asbjornsen A: “Normalization” of brain activation in schizophrenia: an fMRI study. Schizophr Res 2002; 58:333–335Google Scholar
12. Drury VM, Robinson EJ, Birchwood M: “Theory of mind” skills during an acute episode of psychosis and following recovery. Psychol Med 1998; 28:1101–1112Google Scholar
13. Reed RA, Harrow M, Herbener ES, Martin EM: Executive function in schizophrenia: is it linked to psychosis and poor life functioning? J Nerv Ment Dis 2002; 190:725–732Google Scholar
14. Atkins M, Burgess A, Bottomley C, Riccio M: Chlorpromazine equivalents: a consensus of opinion for both clinical and research implications. Psychiatr Bull 1997; 21:224–226Google Scholar
15. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
16. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
17. David AS: Insight and psychosis. Br J Psychiatry 1990; 156:798–808Google Scholar
18. Rosen A, Hadzi-Pavlovic D, Parker G: The life skills profile: a measure assessing function and disability in schizophrenia. Schizophr Bull 1989; 15:325–337Google Scholar
19. Kongs SK, Thompson LL, Iverson GI, Heaton RK: Wisconsin Card Sorting Test—64 Card Version (WCST-64). Lutz, Fla, PAR Assessment Resources, 2000Google Scholar
20. Farrow TFD, Hunter MD, Wilkinson ID, Gouneea C, Fawbert D, Smith R, Mason S, Lee K-H, Spence SA, Woodruff PWR: Therapy-induced change in functional brain response to empathic and forgivability judgments in posttraumatic stress disorder. Psychiatry Res (Neuroimaging) (in press)Google Scholar
21. Cohen JD, MacWhinney B, Flatt M, Provost J: Psyscope: a new graphic interactive environment for designing psychology experiments. Behav Res Methods Instrum Comput 1993; 25:257–271Google Scholar
22. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical, 1988Google Scholar
23. Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP: Neural correlates of self-reflection. Brain 2002; 125:1808–1814Google Scholar
24. Frith C: Attention to action and awareness of other minds. Conscious Cogn 2002; 11:481–487Google Scholar
25. Manoach DS, Halpern EF, Kramer TS, Chang Y, Goff DC, Rauch SL, Kennedy DN, Gollub RL: Test-retest reliability of a functional MRI working memory paradigm in normal and schizophrenic subjects. Am J Psychiatry 2001; 158:955–958Google Scholar
26. van Turennout M, Bielamowicz L, Martin A: Modulation of neural activity during object naming: effects of time and practice. Cereb Cortex 2003; 13:381–391Google Scholar
27. Schultz RT, Grelotti DJ, Klin A, Kleinman J, Van der Gaag C, Marois R, Skudlarski P: The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philos Trans R Soc Lond B Biol Sci 2003; 358:415–427Google Scholar
28. Geday J, Gjedde A, Boldsen AS, Kupers R: Emotional valence modulates activity in the posterior fusiform gyrus and inferior medial prefrontal cortex in social perception. Neuroimage 2003; 18:675–684Google Scholar
29. Quintana J, Wong T, Ortiz-Portillo E, Marder SR, Mazziotta JC: Right lateral fusiform gyrus dysfunction during facial information processing in schizophrenia. Biol Psychiatry 2003; 53:1099–1112Google Scholar
30. Braus DF, Weber-Fahr W, Tost H, Ruf M, Henn FA: Sensory information processing in neuroleptic-naive first-episode schizophrenic patients: a functional magnetic resonance imaging study. Arch Gen Psychiatry 2002; 59:696–701Google Scholar
31. Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS: A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry 2002; 51:431–445Google Scholar
32. Harris JC: Social neuroscience, empathy, brain integration, and neurodevelopmental disorders. Physiol Behav 2003; 79:525–531Google Scholar
33. Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P: Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatry 2002; 159:1830–1840Google Scholar
34. Leung A, Chue P: Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl 2000; 401:3–38Google Scholar
35. Stotland E: Exploratory investigations of empathy, in Advances in Experimental Social Psychology, vol 3. Edited by Berkowitz L. New York, Academic Press, 1969Google Scholar
36. Spence SA, Green RD, Wilkinson ID, Hunter MD: Modafinil modulates anterior cingulate function in chronic schizophrenia. Br J Psychiatry 2005; 187:55–61Google Scholar