Improvement in Social Competence With Short-Term Atypical Antipsychotic Treatment: A Randomized, Double-Blind Comparison of Quetiapine Versus Risperidone for Social Competence, Social Cognition, and Neuropsychological Functioning
Abstract
Objective: While neuropsychological test performance is correlated with social outcomes in patients with schizophrenia, there is little evidence to date that changes in neuropsychological performance are associated with changes in these outcomes. As part of an efficacy and tolerability study of atypical antipsychotics, the authors used a performance-based measure of social competence as a short-term outcome measure and examined the correlations between changes in social competence and improvements on neuropsychological tests. Method: Patients with schizophrenia were randomly assigned in a 1:1 ratio to receive treatment with either quetiapine (dose range: 200–800 mg/day) or risperidone (dose range: 2–8 mg/day) for an 8-week period. Results: Of 673 patients initially randomized, 289 had baseline and endpoint neuropsychological and functional competence data. Scores on the performance-based measure of social competence significantly improved with both treatments, as did a number of aspects of neuropsychological performance. Improvements in several aspects of neuropsychological performance were correlated with the extent of improvement in social competence. There were no overall differences between the treatments in their impact on social competence and neuropsychological performance. Conclusions: Short-term treatment with both quetiapine and risperidone resulted in improvements in social competence, with these improvements associated with improvements on some of the neuropsychological measures. In addition to their clinical importance, these results support the use of performance-based competence assessments as outcome measures in clinical trials.
Impairments in adaptive life skills are common and severe in schizophrenia. These impairments are found in social (1) , occupational (2) , self-care (3) , and independent living domains (4) . Much of the disability associated with schizophrenia can be related to these adaptive life skills deficits, and these skills deficits are a major source of the indirect costs of schizophrenia, which are estimated to be substantially greater than the direct treatment costs of the illness (5) . These functional impairments are seen at the time of the first episode of illness (2) and even in the premorbid phases (6) , with the concept of poor premorbid adjustment in schizophrenia being related to the presence of adaptive life skills deficits before the clear onset of the psychotic features of the illness. Further, these deficits do not appear to improve with the remission of psychotic symptoms (7) , implicating other causes for these impairments.
Many cross-sectional studies and some recent longitudinal data have implicated neuropsychological impairments and negative symptoms as the primary determinants of impairments in everyday functioning (8) . When the influence of cognitive and negative symptoms on the concurrent severity of these impairments is examined, it has been noted that the severity of neuropsychological impairments is more strongly correlated with the severity of impaired everyday functioning than are negative symptoms (9 , 10) . The correlations between neuropsychological impairments and impairments in everyday living skills are found across a wide range of neuropsychological ability domains and adaptive life skills (11) . For instance, patients with more cognitive impairments have greater impairments in social functioning (12) , are less likely to have successful social relationships (13) , and are less likely to live independently (14) .
Recent interest has focused on the pharmacological treatment of cognitive impairment in schizophrenia, with interventions including both atypical antipsychotic medications alone and adjunctive medications (15 – 17) . The rationale for these interventions is clear: if cognitive impairments predict deficits in various aspects of everyday living skills, cross-sectionally (8) and longitudinally (18) , then enhancement of cognitive functioning might lead to improvements in everyday functioning. The results of these interventions have been quite mixed, with moderate improvements in multiple cognitive domains reported for atypical antipsychotic medications (15) and limited success for adjunctive treatments (16 , 17) . However, the short-term nature of the typical clinical trial would not be suitable to detect change in functional skills. Most assessments of everyday living skills rely on observation and reporting by either the patient or a family member or caregiver, therefore this measurement domain poses many challenges. Many patients with schizophrenia do not have caregivers who could report on their neuropsychological and living skills, and some evidence suggests inconsistencies with patient self-reports (19) .
It has been suggested that performance-based measures of functional capacity circumvent the lack of informants and potential response bias (20) . A further benefit of performance-based measures of functional capacity is that these measures are proximal in nature (18) in that they do not require that the skills are actually deployed in the real-world environment. Thus they measure capacity (i.e., what the person can do under optimal circumstances) rather than real-world performance (i.e., what the person does do ). Distal measures, such as indicators of real-world social outcomes (number of friends), independent living outcomes (moving to less restrictive levels of care), and employment (seeking or obtaining employment), require both the capacity in the behavioral execution of the skills and environmental factors that encourage (or at least do not interfere with) performance of the skills. Thus, the recent NIMH-MATRICS initiative has recommended the use of a performance-based measure of functional capacity in such clinical trials aimed at cognitive enhancement in patients with schizophrenia (21) . Similarly, these recommendations included assessment of social cognition (i.e., measures affect perception and understanding social rules) as an outcome measure as well, although it was acknowledged that possibly less was known about social cognition than performance-based outcome measures.
The current study was a randomized, double-blind, 8-week clinical trial comparing the efficacy and tolerability of quetiapine and risperidone in treating patients with schizophrenia. Clinical assessments were the main focus of the investigation and have been presented elsewhere (22) . The main clinical assessments were the Positive and Negative Syndrome Scale (PANSS) and Clinical Global Impression (CGI) severity and improvement ratings. Only a selected set of PANSS variables were used for correlation analyses in this article (total score and positive and negative symptom subscale scores).
Neuropsychological performance, social cognition, and a performance-based measure of social competence were the outcome measures of this study. Neuropsychological assessments were selected from aspects of neuropsychological functioning shown to be related to social functioning outcomes, including vigilance, episodic memory, and processing speed. Social competence was measured with the Social Skills Performance Assessment (23) . The Social Skills Performance Assessment is a validated measure of interactive social skills, performed in a role-played manner with an examiner. Social cognition, indexed by the ability to perceive the intensity of affective expression, was measured with the Penn Emotional Acuity Test (24) . Changes in these outcomes were measured from baseline to endpoint and compared across the two atypical antipsychotic treatments. Exploratory data analyses examined the correlation between changes in neuropsychological functioning and changes in scores on the Penn Emotional Acuity Test and Social Skills Performance Assessment in each of the two treatment groups.
Method
Study Design
This was an 8-week, multicenter, double-blind, parallel-designed, randomized, flexible-dose study initiated in hospitalized patients with schizophrenia. Patients were hospitalized for 1 week and then treated as outpatients for the remainder of the study.
Subjects were male and female patients 18–65 years of age with a diagnosis of DSM-IV schizophrenia (295.X, 295.70), a baseline PANSS score of ≥60, a CGI severity rating ≥4, and a score of ≥4 on one of the following PANSS positive symptom subscale items: delusions, conceptual disorganization, hallucinatory behavior, or suspiciousness/persecution.
Patients were required to have stable laboratory and electrocardiogram (ECG) results and to have a negative urine drug screen at study entry. Subjects diagnosed with schizoaffective disorder, major depressive disorder, substance dependence not in remission, mental retardation, and psychotic disorders due to a general medical condition were excluded. Patients who had been hospitalized in the current facilities for more than 1 month before randomization and patients who had a known history of intolerance or lack of response to antipsychotic medications, including clozapine, were also excluded. Sleep medication and benzodiazepines were allowed as needed but were not allowed within 24 hours of clinical or neuropsychological assessments. The appropriate institutional review boards at every research site approved the study, and all patients or their legal guardians provided written informed consent.
Eligible patients were randomly assigned in a 1:1 ratio to receive either quetiapine or risperidone. Quetiapine was initiated at 50 mg/day and titrated to 400 mg/day by day 4. Risperidone was started at 1 mg/day and increased to 4 mg/day by day 4. The investigators could flexibly adjust the dose of both medications thereafter. The dose range was 200–800 mg/day for quetiapine and 2–8 mg/day for risperidone. Quetiapine was administered in two daily doses, as was risperidone.
Functional Assessments
The following neuropsychological and functional assessments were performed at baseline (randomization) and at the end of the study (day 56 or withdrawal).
Social skills
The Social Skills Performance Assessment (23) is a task in which the subject participates in two 3-minute role-playing scenarios of selected social problem situations acted out between an interviewer and participant and audiotaped for subsequent centralized scoring. In scene 1, the subject plays the role of a tenant meeting a new neighbor (interviewer). In scene 2, the subject plays the role of a tenant calling the landlord (interviewer) regarding a leak that has gone unrepaired after a previous complaint. Raters were trained in administration of these tests through the viewing of videotapes prior to investigator meetings, where they were observed in the administration of the instrument. They were not trained in the scoring of the instrument or in the scoring criteria in order to reduce any potential biases. Scoring was performed blind to subject identity, treatment condition, and order of assessment (baseline/endpoint) at the University of California, San Diego (UCSD). Each scene was replayed from the tape recording and scored on a graduated scale from 1 (low) to 5 (high) in eight categories: interest; fluency; clarity; focus; social appropriateness; negotiation ability; persistence; and overall conversation. Overall performance was calculated by summing the averaged ratings from each role play, leading to an overall score that could range from 2 to 10. Interrater reliability of scoring the instrument was based on a 20% overlap in ratings, and the average ICC for scores for each of the eight categories was found to be 0.89. Previous data on the practice effects of the Social Skills Performance Assessment indicated that the test-retest reliability was 0.91 and that there was no significant change in performance at a 4-week retest assessment in either healthy subjects or patients with schizophrenia. The effect size for change at retest in the schizophrenia group was 0.15 (Cohen’s d).
Social cognition/affect perception
The Penn Emotional Acuity Test (24) measures the ability to accurately perceive the intensity of facial expressions. A series of 40 faces that range from very sad to very happy are presented. Subjects grade the level of intensity of the expressions of sadness or happiness on the face on a 7-point scale. The dependent variables are the total number of correct responses made.
Neuropsychological Assessments
A limited neuropsychological assessment was performed, because this was seen to be a secondary outcome in the overall clinical trial. As a result, while all of the data that were collected are presented, this assessment does not cover every potential domain of cognitive functioning.
Attention
Two different versions of the Continuous Performance Test of vigilance were employed. In the identical pairs version (25) , patients were asked to make a finger lift response on a computer key whenever the same 4-digit target stimulus occurred twice in a row. One hundred fifty test trials were presented, with the stimulus duration set at 500 msec with a 950-msec interstimulus interval. In the A-X version (26) , subjects pressed a response key whenever they detected the occurrence of an A immediately followed by an X. These target sequences occurred with 10% frequency and were presented with the same stimulus parameters as the identical pairs test. The dependent variable for both conditions was the proportion of correct detections of target stimuli (i.e., hits).
Visuomotor speed
Part A of the Trail Making Test was given, with the time required to complete the test as the dependent variable.
Memory
Verbal memory was examined with the Rey Auditory Verbal Learning Test, which has been used extensively in neuropsychological studies of patients with schizophrenia. A 15-item list of words is presented to the patient in each of five separate learning trials. Following this, a distractor list (list B) is read to the subjects, followed by short-delay free recall. A delayed free-recall test is performed after a 20-minute period, followed by a choice recognition procedure. Dependent variables selected for analysis were the total number of words recalled in the five learning trials and at delayed recall, as well as delayed recognition discrimination. The same form of the test was used at both assessments because of concerns regarding the equivalence of the available alternative forms and previous findings suggesting that practice effects on verbal learning tests in patients with schizophrenia may be less than the influence of alternate forms (27) .
Executive function
Part B of the Trail Making Test evaluates both visuomotor speed and the ability to alternate between sets. The dependent variable is the time required to complete the task.
Verbal fluency
Two tests were administered: category and letter fluency. Category fluency involves naming animals, fruits, and vegetables for 1 minute per category, while phonological fluency examined naming of words starting with the letters F, A, and S for 1 minute per letter. Total scores for category and letter fluency were the dependent variables.
Data Analysis
Statistical tests on all data were performed at the 5% two-tailed significance level. Multivariate tests for differences between the treatment groups for changes on the set of cognitive battery measures were conducted using multivariate analysis of covariance (MANCOVA) with medication group as a fixed effect and investigator (site) and baseline scores as covariates. Results at baseline and week 8 (or early termination) are reported. These were the only time points at which the tests were administered. Further, paired t tests were applied to determine if the change scores from baseline were significant within each treatment group. Exploratory correlational analyses examined the relationships between changes in neuropsychological performance, social cognition, and social competence. The overall relationship between changes in Social Skills Performance Assessment and neuropsychological and social cognition variables was examined with a simultaneous entry regression analysis. Correlation analyses examined the relationship between changes on the PANSS subscales and changes in the other variables.
Results
Patient Disposition
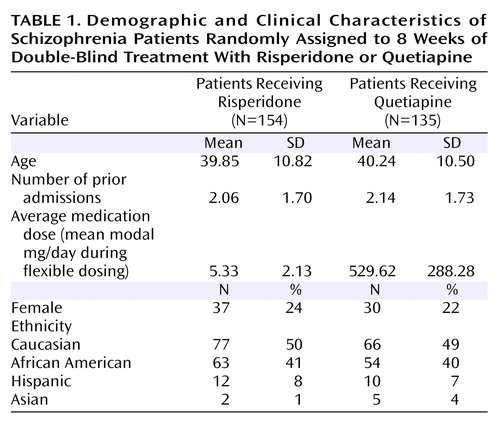
In total, 673 patients were randomly assigned to a treatment condition (quetiapine: N=338; risperidone: N=335). Of these, 326 (96.4%) patients in the quetiapine group and 319 (95.2%) patients in the risperidone group had received previous antipsychotic treatment with olanzapine (36.1% [N=122] and 40.4% [N=135], respectively), risperidone (29.3% [N=99] and 27.5% [N=92]), haloperidol (16.3% [N=55] and 18.9% [N=63]), quetiapine (13.3% [N=45] and 9.9% [N=33]), ziprasidone (8.0% [N=27]) and 4.2% [N=14]), chlorpromazine (2.4% [N=8] and 2.4% [N=8]), or loxapine (0.9% [N=3] and 1.2% [N=4]). In addition, two patients (0.6%) in the quetiapine group had received clozapine, and one patient (0.3%) in the risperidone group had received molindone. Neuropsychological, social cognition, and social competence assessment data were obtained for 538 of these patients; of these, however, 216 patients had data at baseline only while seven patients had postbaseline data only. Of the 315 patients (149 for quetiapine and 166 for risperidone) with both a baseline and final assessment, 26 patients (14 for quetiapine and 12 for risperidone) had nonvalid final assessments and were excluded. Therefore, 289 patients (135 for quetiapine and 154 for risperidone) had valid neuropsychological, social competence, or social cognition assessment data at baseline and endpoint (week 8). We performed t tests for each of the assessments, comparing the neuropsychological, social competence, and social cognition baseline scores of patients with some endpoint data (N=289) and without endpoint data (N=216), and none of these t tests were significant.
Performance
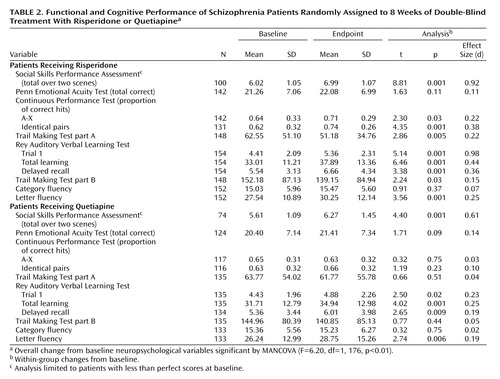
Table 1 presents the descriptive characteristics of the treatment groups, and Table 2 presents the performance data on the social competence, social cognition, and neuropsychological tests as a function of treatment group. The first MANCOVA compared performance across all of the neuropsychological tests but not the social cognition and social skills measures. Note that missing neuropsychological assessment data were deleted on a pairwise basis, leading to a reduction in the overall number of subjects. The MANCOVA found that there were no significant differences from baseline to endpoint across treatments on the neuropsychological change scores (Wilks’s lambda=0.96; F=1.05, df=1, 190, p=0.40). For performance on the Penn Emotional Acuity Test, the MANCOVA found that there were no significant differences associated with treatment (F=0.01, df=1, 226, p=0.95). When treatment-associated differences in performance on the Social Skills Performance Assessment were examined, there was no statistically significant difference associated with treatment (F=4.26, df=1, 171, p=0.06), although the difference tended to favor risperidone treatment. Post hoc tests based on the MANOVA results were computed to compare cognitive changes between the groups. These tests revealed that there were two cognitive domains where risperidone treatment was superior to quetiapine: Continuous Performance Test identical pairs hits and Trail Making Test part A time (both p<0.05).
Changes From Baseline
Paired tests within treatment group were used to identify changes from baseline performance on all of the study-dependent variables. For treatment with quetiapine, statistically significant improvements were detected in phonological fluency, as well as trial 1 performance, total learning, and delayed recall on the Rey Auditory Verbal Learning Test, and on the Social Skills Performance Assessment. For risperidone treatment, significant improvements were seen for phonological fluency, Trail Making Tests parts A and B, trial 1 performance, total learning, and delayed recall on the Rey Auditory Verbal Learning Test, and correct detections on both versions of the Continuous Performance Test as well as the Social Skills Performance Assessment. Neither medication was associated with significant differences in performance on the Penn Emotional Acuity Test or on category fluency.
Pearson product-moment correlations were calculated between changes on the Social Skills Performance Assessment and all of the neuropsychological variables. These correlations were calculated for each medication group separately and are presented in Table 3 . For both treatments, some aspects of neuropsychological change were associated with improvements in social competence measured by the Social Skills Performance Assessment. For quetiapine treatment, significant correlations were seen between Social Skills Performance Assessment scores and improvements in score on the Trail Making Test part B and Rey Auditory Verbal Learning Test total learning and delayed recall. For risperidone treatment, improvements on phonological fluency, A-X Continuous Performance Test, Trail Making Test part B, and Rey Auditory Verbal Learning Test total learning were correlated with improvements in performance on the Social Skills Performance Assessment. Note that the magnitude of the correlations was nearly identical for each variable for each treatment and the differences in patterns of statistical significance were due to differences in the treatment group size. Simultaneous entry regression analyses were used to determine the overall relationship between changes in Social Skills Performance Assessment and neuropsychological and social cognition variables, with these analyses calculated separately for each treatment group. For quetiapine treatment, the overall regression was significant (F=4.15, df=8, 65, p<0.05). The total variance accounted for in the analysis was 20%, with both Trail Making Test part B and word list total learning contributing significant independent variance ( Figure 1 ). For risperidone treatment, the overall regression was also significant (F=2.42, df=8, 89, p<0.05). Total variance accounted for in the analysis was 19%, with improvements in Trail Making Test part B performance and total learning on the Rey Auditory Verbal Learning Test also both accounting for significant variance in improvements in Social Skills Performance Assessment performance.

Intercorrelations of the cognitive change scores were also computed within each treatment group. For quetiapine treatment, the correlations ranged from r=0.02 (changes in A-X Continuous Performance Test hits and Rey Auditory Verbal Learning Test total learning) to r=0.53 (change in trial 1 learning and total learning on the Rey Auditory Verbal Learning Test). In the risperidone group, these correlations ranged from r=0.00 (changes in A-X Continuous Performance Test hits and Rey Auditory Verbal Learning Test total learning) to r=0.65 (change in trial 1 learning and total learning on the Rey Auditory Verbal Learning Test).
Correlations With Clinical Changes
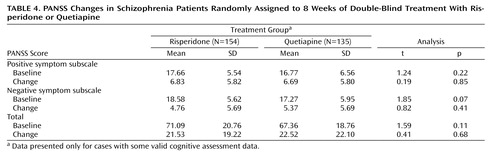
Patients receiving treatment with either quetiapine or risperidone experienced a decrease in total, positive, and negative PANSS scores from baseline ( Table 4 ). There were no differences in either baseline scores or magnitude of change scores between the two treatment groups. In order to examine the possible associations between clinical changes and changes in social competence, social cognition, and neuropsychological variables, Pearson correlations were calculated between all variables and changes in PANSS positive, negative, and total scores. For quetiapine treatment, two out of 33 correlations were significant, with changes in PANSS total scores predicting improvements in delayed recall (r=–0.26, p<0.05), and changes in PANSS positive scores predicting improvements in total learning (r=–0.25, p<0.05). No correlations between changes in negative symptoms and neuropsychological or Social Skills Performance Assessment changes reached significance. For risperidone treatment, again two correlations were significant, with improvements in PANSS positive symptom scores being associated with improvements in Rey Auditory Verbal Learning Test trial 1 performance (r=–0.30, p<0.05) and improvements in total learning (r=–0.33, p<0.05).
Discussion
In this first study of treatment with atypical antipsychotic medications and changes in performance-based measures of social competence, two important findings emerged. The first is that social competence measures are positively affected by treatment with the atypical antipsychotic medications quetiapine and risperidone. The second is that these treatment-related changes in social skills performance are correlated with concurrent improvements in neuropsychological performance. A total of 20% of the variance in improvements in neuropsychological performance and social competence was found to overlap, suggesting that the beneficial effects of these medications on cognitive performance are also related to these changes in functional skills.
Previous studies of the relationships between cognitive and functional skills have most often been cross-sectional and observational in their research design. Therefore, the results of this treatment study also make an important point regarding the validity of inferences regarding the influences of the cognitive abnormalities seen in schizophrenia on functional status. The findings here also present positive evidence for the sensitivity of measures of functional capacity for detection of treatment-related changes. Since most previous studies of atypical antipsychotics and cognition lacked an assessment of functional outcome, it was only assumed that the cognitive changes had functional relevance.
Only one previous study examined treatment-related changes on a cognitive measure, the Wisconsin Card Sorting Test, and its relationship to performance-based measures of social competence. There was no evidence of improvement on either measure in that study (28) . Further, a previous open-label study of quetiapine found improvements in cognitive functioning and quality of life, but no substantial relationship between these variables (29) . A previous study that examined the test-retest stability of cognitive measures similar to those employed in this study (27) found that no measures manifested a statistically significant practice effect and that the effect sizes for change were considerably smaller than the improvements seen in this study. Similar results came from a systematic retest study of the Social Skills Performance Assessment (30) , where the retest effect size in patients with schizophrenia was found to be 0.14 (Cohen’s d), which is notably smaller than the treatment-related changes seen in this study.
In addition to the large patient cohort and broader neuropsychological assessment, we argue that the results of this study are important because of the use of a performance-based measure of social competence that does not require environmental opportunities or special support for deployment of social skills. Social competence was measured with a proximal measure of performance that was not dependent on environmental factors that might be required to facilitate performance. The effect size for improvement was substantial. These data indicate that outcome measures sensitive to functional skills deficits may be useful, even in short-term trials, if they validly measure skills competence. Conversely, outcome measures that require real-world performance of functional skills may place too great a burden on pharmacological interventions, which are unlikely to be able to influence environmental factors such as performance opportunities, interfering factors such as anxiety, or social constraints (e.g., eligibility for disability payments).
The major limitations of this study are the short duration of treatment and the high percentage of subjects who did not receive the assessments, which might limit the generalizability of the results. However, the lack of baseline differences between cases with and without follow-up data is reassuring and may imply that the results apply more broadly to patients with schizophrenia treated with quetiapine and risperidone. Additional studies are required to assess the effects of these treatments with long-term use. Differences in the rate of dropouts and missing data also have the potential to influence the results. Further, while the cognitive domains assessed are relatively comprehensive even with the limited battery, social cognition is complex and is not thoroughly assessed with the Penn Emotional Acuity Test alone. It should not be concluded that social cognition in general is not positively influenced by cognitive enhancing treatments on the basis of the results of this study.
Conclusions
Short-term treatment with the atypical antipsychotic medications quetiapine and risperidone was associated with improvement on a validated measure of social competence, and these changes were correlated with cognitive improvements. While these data do not address long-term social adjustment, they do indicate that measures of functional capacity can improve in the short term. Future research on this topic must carefully separate changes in competence from changes in real-world performance, which may be influenced by variables that are not necessarily illness-related.
1. Wiersma D, Wanderling J, Dragomirecka E, Ganev K, Harrison G, An Der Heiden W, Nienhuis FJ, Walsh D: Social disability in schizophrenia: its development and prediction over 15 years in incidence cohorts in six European centres. Psychol Med 2000; 30:1155–1167Google Scholar
2. Ho B-C, Andreasen N, Flaum M: Dependence on public financial support early in the course of schizophrenia. Psychiatr Serv 1997; 48:948–950Google Scholar
3. Murray CJL, Lopez AD: Global mortality, disability, and the contributions of risk factors: Global Burden of Disease Study. Lancet 1997; 349:1436–1442Google Scholar
4. Auslander LA, Lindamer LL, Delapena J, Harless K, Polichar D, Patterson TL, Zisook S, Jeste DV: A comparison of the community-dwelling older schizophrenia patients by residential status. Acta Psychiatr Scand 2001; 103:380–386Google Scholar
5. Rice DP: Economic burden of mental disorders in the United States. Economics of Neuroscience 1999; 1:40–44Google Scholar
6. Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, Poulton R: Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry 2002; 59:449–456Google Scholar
7. Harvey PD, Docherty NM, Serper MR, Rasmussen M: Cognitive deficits and thought disorder, II: an 8-month followup study. Schizophr Bull 1990; 16:147–156Google Scholar
8. Green MF: What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153:321–330Google Scholar
9. Velligan DI, Mahurin RK, Diamond PL, Hazleton BC, Eckert SL, Miller AL: The functional significance of symptomatology and cognitive function in schizophrenia. Schizophr Res 1997; 25:21–31Google Scholar
10. Harvey PD, Howanitz E, Parrella M, White L, Davidson M, Mohs RC, Hoblyn J, Davis KL: Symptoms, cognitive functioning, and adaptive skills in geriatric patients with lifelong schizophrenia: a comparison across treatment sites. Am J Psychiatry 1998; 155:1080–1086Google Scholar
11. Evans JD, Heaton RK, Paulsen JS, Palmer BW, Patterson T, Jeste DV: The relationship of neuropsychological abilities to specific domains of functional capacity in older schizophrenic patients. Biol Psychiatry 2003; 53:422–430Google Scholar
12. Smith TE, Hull JW, Romanelli S, Fertuck E, Weiss KA: Symptoms and neurocognition as rate limiters in skills training for psychotic patients. Am J Psychiatry 1999; 156:1817–1818Google Scholar
13. Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD: Determinants of real world functional performance in schizophrenia: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry 2006; 163:418–425Google Scholar
14. Twamley EW, Doshi RR, Nayak GV, Palmer BW, Golshan S, Heaton RK, Patterson TL, Jeste DV: Generalized cognitive impairments, ability to perform everyday tasks, and level of independence in community living situations of older patients with psychosis. Am J Psychiatry 2002; 159:2013–2020Google Scholar
15. Harvey PD, Keefe RS: Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am J Psychiatry 2001; 158:176–184Google Scholar
16. Friedman JI, Adler DN, Howanitz E, Harvey PD, Brenner G, Temporini H, White L, Parrella M, Davis KL: A double-blind placebo controlled trial of donepezil adjunctive treatment to risperidone for the cognitive impairment of schizophrenia. Biol Psychiatry 2002; 51:349–357Google Scholar
17. Friedman JI, Adler DN, Temporini HD, Kemether E, Harvey PD, White L, Parrella M, Davis KL: Guanfacine treatment of cognitive impairment in schizophrenia. Neuropsychopharmacology 2001; 25:402–409Google Scholar
18. Green MF, Kern RS, Heaton RK: Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res 2004; 72:41–51Google Scholar
19. McKibbin CL, Patterson TL, Jeste DV: Assessing disability in older patients with schizophrenia: results from the WHODAS-II. J Nerv Ment Dis 2004; 192:405–413Google Scholar
20. McKibbin CL, Brekke JS, Sires D, Jeste DV, Patterson TL: Direct assessment of functional abilities: relevance to persons with schizophrenia. Schizophr Res 2004; 72:53–67Google Scholar
21. Buchanan RW, Davis M, Goff D, Green MF, Keefe RS, Leon AC, Nuechterlein KH, Laughren T, Levin R, Stover E, Fenton W, Marder SR: A summary of the FDA-NIMH MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr Bull 2005; 31:5–19Google Scholar
22. Zhong K, Harvey P, Brecher M, Sweitzer D: A randomized, double-blind study of quetiapine and risperidone in the treatment of schizophrenia. Neuropsychopharmacology 2004; 29(suppl 1):S232Google Scholar
23. Patterson TL, Moscona S, McKibbin CL, Davidson K, Jeste DV: Social skills performance assessment among older patients with schizophrenia. Schizophr Res 2001; 48:351–360Google Scholar
24. Erwin RJ, Gur RC, Gur RE, Skolnick B, Mawhinney-Hee M, Smailis J: Facial emotion discrimination, I: task construction and behavioural findings in normal participants. Psychiatr Res 1992; 42:231–240Google Scholar
25. Cornblatt BA, Lenzenweger MF, Erlenmeyer-Kimling L: The Continuous Performance Test, Identical Pairs Version, II: contrasting attentional profiles in schizophrenic and depressed patients. Psychiatr Res 1989; 29:65–85Google Scholar
26. Nuechterlein KH, Edell WS, Norris M, Dawson ME: Attentional vulnerability indicators, thought disorder, and negative symptoms. Schizophr Bull 1986; 12:408–426Google Scholar
27. Harvey PD, Heaton RK, Palmer BA, Mohamed S, Kennedy J, Brickman A: Stability of cognitive impairment in elderly schizophrenic patients receiving conventional antipsychotic treatment. Am J Psychiatry 2005; 162:110–117Google Scholar
28. Bellack AS, Schooler NR, Marder SR, Kane JM, Brown CH, Yang Y: Do clozapine and risperidone affect social competence and problem solving? Am J Psychiatry 2004; 161:364–367Google Scholar
29. Velligan DI, Newcomer J, Pultz J, Csernansky J, Hoff AL, Mahurin R, Miller AL: Does cognitive function improve with quetiapine in comparison to haloperidol? Schizophr Res 2002; 53:239–248Google Scholar
30. Patterson TL, McKibbin CL, Mausbach BT, Goldman S, Bucardo J, Jeste DV. Functional Adaptation Skills Training (FAST): A randomized trial of a psychosocial intervention for middle-aged and older patients with chronic psychotic disorders. Schizophrenia Research (in press)Google Scholar