Placebo-Controlled Trial of Dehydroepiandrosterone (DHEA) for Treatment of Nonmajor Depression in Patients With HIV/AIDS
Abstract
OBJECTIVE: Subsyndromal major depressive disorder is common among HIV-positive adults. This study was designed to assess the efficacy of dehydroepiandrosterone (DHEA) as a potential treatment. METHOD: One hundred forty-five patients with subsyndromal depression or dysthymia were randomly assigned to receive either DHEA or placebo; 90% (69 of 77) of the DHEA patients and 94% (64 of 68) of the placebo patients completed the 8-week trial. The primary measure of efficacy was a Clinical Global Impression improvement rating of 1 or 2 (much or very much improved) plus a final Hamilton Depression Rating Scale score ≤8. Outcome was assessed by using intent-to-treat analysis, followed by completer analysis. Safety was assessed by queries about side effects at every study visit plus measures of CD4 cell count and HIV RNA viral load at baseline and week 8. DHEA dosing was flexible (100–400 mg/day). RESULTS: On the basis of clinicians’ ratings, DHEA was superior in the intent-to-treat analysis, where the response rate was 56% (43 of 77) for the DHEA group versus 31% (21 of 68) for the placebo group. In the completer analysis, the response rate was 62% (43 of 69) for the DHEA group, compared to 33% (21 of 64) for the placebo patients. The number needed to treat was 4 on the basis of intent-to-treat data and 3.4 on the basis of completer data. Few adverse events were reported in either treatment group, and no significant changes in CD4 cell count or HIV RNA viral load were observed in either group. CONCLUSIONS: Nonmajor but persistent depression is common in patients with HIV/AIDS, and DHEA appears to be a useful treatment that is superior to placebo in reducing depressive symptoms. The low attrition rate in this group of physically ill patients, together with requests for extended open-label treatment, reflect high acceptance of this readily available intervention.
In clinical trials, antidepressant medications were shown to be as effective in ameliorating major depression in HIV-positive patients as in physically healthy patients (see reference 1 for a review of this literature). However, there is little empirical evidence about effective treatments for subsyndromal major depression and dysthymia, which are more often seen in the HIV/AIDS population (see reference 2 for a review of this literature). Although antidepressants may be helpful, it is sometimes difficult to persuade more mildly depressed patients to take them, and both they and their physicians may be deterred by concerns about drug interactions with antiretroviral medications.
An alternative strategy in treating depression is the use of anabolic steroids. We and others have found that testosterone can be effective in ameliorating a range of symptoms often seen among HIV-positive patients, including depressed mood (3). However, testosterone is not appropriate for many patients (e.g., women, men with normal testosterone levels), and because it is classified as a Schedule III drug by the U.S. Food and Drug Administration (FDA), some doctors are reluctant to prescribe it. Identification of an alternative anabolic steroid such as dehydroepiandrosterone (DHEA), classified by the FDA as a nutritional supplement and available over the counter, would thus fill a gap in the treatment armamentarium.
DHEA (and its more stable metabolite DHEA sulfate [DHEAS]) is a weakly active adrenal androgen and is an intermediary in gonadal androgen and estrogen synthesis. It is the most abundant steroid hormone produced by the adrenal cortex. Like testosterone, DHEA declines with age, with peak levels at age 20–24 years for men and 15–19 years for women. Serum levels of DHEA and DHEAS show great variation; reference ranges overlap across age groups and genders and are 10%–30% lower in women than in men. Low levels of DHEAS have been observed in patients with chronic diseases such as lupus, rheumatoid arthritis, obesity, type 2 diabetes, hypertension, and cardiovascular disease (4, 5).
Several mechanisms of action of DHEA have been proposed. First, DHEA is a precursor to testosterone and estradiol, each of which independently has been associated with mood effects. Second, DHEA is a neurosteroid that modulates neuronal excitability by means of specific interactions with neurotransmitter receptors that are known to modulate mood (6). Another potential mechanism is that DHEA leads to an increase in bioavailable insulin-like growth factor 1 (IGF-1), which promotes release of growth hormone. Patients who are deficient in growth hormone have been reported to have depressive symptoms (7), suggesting an association between growth hormone and depression. Finally, DHEA may modulate proinflammatory cytokines, such as tumor necrosis factor alpha and interleukin-6, that may be etiologically related to depressed mood (8).
DHEA therapy has been studied in treatment of several medical conditions with variable but inconclusive evidence of efficacy (9, 10). The target of treatment also has varied greatly, including effects on body composition, cognition, fatigue, depression, cocaine dependence, cardiovascular risk factors, immunological and endocrine parameters, bone mass, metabolism, and cancer risk, as well as the negative, depressive, and anxiety symptoms of schizophrenia (11).
DHEA is of particular interest for HIV-positive patients, because declining levels of DHEA have been associated with progression to AIDS in both cross-sectional comparisons of HIV-positive men and women at different stages of HIV illness (12) and in longitudinal studies, including a previous study by our group (13). In addition, lower levels of DHEAS have been found in HIV-positive male patients with lipodystrophy and increased lipid levels, with a consequent increase in the cortisol-to-DHEA ratio (14). Among men with lipodystrophy followed over time, symptom worsening was associated with a decrease in DHEAS serum levels, and lipodystrophy improvement was associated with an increase in DHEAS (15).
We and other investigators (16–18) have conducted open-label studies and small double-blind, placebo-controlled studies of DHEA for treatment of depression, with promising but sometimes inconclusive results. In our previous study (18), unlike the current study, participants were not required to have an axis I depression diagnosis, the minimum Hamilton Depression Rating Scale (HAM-D) score required for eligibility was 8 (rather than 12, as in the current study), attrition was much higher (29% versus 8% in the current study), and only two women completed the study. The dose of oral DHEA in clinical trials has varied greatly, from 30 mg/day to 2250 mg/day (table 2 in reference 19). Because DHEA is unregulated, preparations have been found to vary enormously in their contents, from total absence of DHEA to 150% of the labeled amount (20). Investigators therefore usually get their supply from compounding pharmacists who supply documentation of contents and purity.
The 8-week trial reported here was designed to assess the efficacy of DHEA for persistent subsyndromal major depression or dysthymia in a placebo-controlled, parallel-group design. We addressed the following questions: 1) Is DHEA superior to placebo in the treatment of subsyndromal major depression or dysthymia in HIV-positive patients? and 2) does DHEA have any effect on the immunological or virological markers of HIV or any other adverse effects?
Method
Subjects
Eligible patients were HIV positive, ranged in age from 18 to 70 years, and had a DSM-IV diagnosis of dysthymia or subsyndromal depression of at least 3 months’ duration. Major exclusion criteria included an unstable medical condition, initiation of steroids within the past 3 months (longer-term use was permitted), current major depressive disorder (or major depressive disorder in partial remission), and current (in the past 6 months) substance use disorder. Study enrollment took place between December 2000 and May 2004.
Measures
Except where otherwise noted, the clinicians’ ratings and the self-ratings were performed at study entry and at weeks 4 and 8.
The Structured Clinical Interview for DSM-IV was used for diagnosis of depressive disorders and to rule out substance use disorders and psychotic symptoms or disorders. The grid format (21) of the Structured Interview Guide for the HAM-D (22) was used to rate severity of depressive symptoms.
The Clinical Global Impression scale (CGI) was used to rate global improvement. Scores of 1 or 2 (very much or much improved, respectively) were used to define “responder,” together with a final HAM-D score of ≤8 for assessment of mood improvement (a priori definition). We modified the conventional cutoff of ≤7 because of the prevalence of somatic symptoms associated with HIV and the medications used to treat HIV. We also examined scores for the HAM-D affective (five items) and somatic (eight items) subscales separately. Secondary measures included the Beck Depression Inventory (BDI) and the Endicott Quality of Life Enjoyment and Satisfaction Questionnaire (23).
Adverse effects were examined at every study visit by using a modified version of the Systematic Assessment for Treatment Emergent Events (24), a clinician-administered list of salient events. Each item, if present, is rated on a 1–5 severity scale. Events were considered to be treatment emergent if the score at subsequent visits was 2 or more points higher than at baseline.
Laboratory measures included blood chemistries, liver function tests, hematocrit, thyroid panel, CD4 cell count, HIV RNA viral load, serum testosterone, and serum DHEAS. In addition to the measures of immunological and virological markers, hematocrit was of interest for safety reasons, because other steroid hormones may cause an increase in the number of red blood cells, with possible attendant risks (25).
Current substance use was assessed at study baseline and at a random occasion during the 8-week trial, by using both an instant urine dipstick and a split sample sent to a research laboratory.
Serum levels of DHEAS were assayed at study baseline and at weeks 4 and 8. Medication nonadherence in participants who were randomly assigned to receive DHEA was assumed if laboratory results showed either 1) failure of the DHEAS level to double between baseline and week 8 or 2) week-8 serum level that was less than 50% of that achieved at week 4.
Procedures
The patients received an initial evaluation, including a review of medical and psychiatric history and of current medications; blood was drawn for laboratory assessment; and a letter was faxed to the primary care provider describing the study and requesting written agreement for participation. Eligible patients were then seen by the study psychiatrist (R.R.), who conducted the informed consent process and a confirmatory psychiatric evaluation. Subsequent visits were scheduled at the end of weeks 1, 2, 4, 6, and 8. Randomization in blocks of four was performed by using a computer-generated list of random numbers without substitution for dropouts, stratified by gender. The study protocol was approved by the Institutional Review Boards at both New York State Psychiatric Institute and Weill Medical College (Cornell University), and all patients gave written consent after the risks and benefits of study participation were explained. FDA authorization was obtained for use of DHEA as an investigational drug.
Dose schedule
Micronized DHEA in 100-mg tablets and matching placebo, provided by Belmar Pharmacy (Lakewood, Colo.), were dispensed at each visit. The initial dose was 100 mg/day, on the basis of our prior experience. The dose was increased to 200 mg/day after 1 week and to 300 mg/day after 2 weeks in the absence of clinical improvement and dose-limiting side effects. After 4 weeks, an additional dose increase to 400 mg/day was permitted in the absence of clinical improvement and dose-limiting side effects.
Week 8
At this visit, the screening psychologist joined the study psychiatrist and patient to conduct final assessments and to reach consensus agreement about outcome on the CGI. On the basis of the CGI rating, patients were categorized as responders or nonresponders. Patients with partial response were included in the nonresponder category.
Statistical Analyses
Patients seen at least once after baseline were included in the intent-to-treat analysis. Primary endpoints were analyzed by using the chi-square test for response rates, paired t tests, and analysis of covariance with adjustment for baseline scores on all rating scales other than the CGI. According to convention, we report log10 viral copies because of the wide possible range and report 1.69 log10 viral copies for all values reported as “undetectable.” All tests used an alpha level of 0.05, two-tailed.
Results
Sample Composition
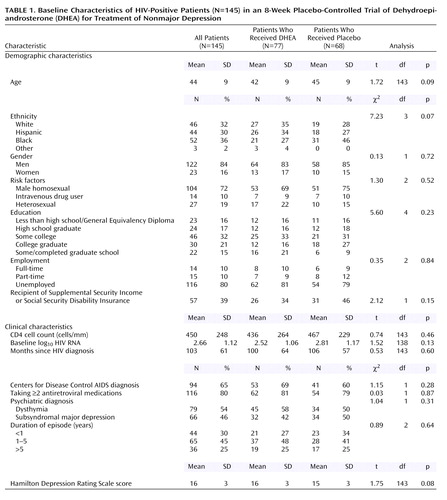
A total of 273 men and women were screened; 145 eligible patients entered the study (77 were randomly assigned to receive DHEA, and 68 were randomly assigned to receive placebo), and 92% (N=133) completed the 8-week double-blind trial. Of the 128 screened patients who did not enter the study, 23% (N=29) were not clinically depressed, 8% (N=10) were too depressed, 25% (N=32) were active substance users, 3% (N=4) had unstable health, 13% (N=17) had concurrent psychiatric diagnoses, 14% (N=18) did not meet the other inclusion criteria, and 14% (N=18) declined.
Twelve men (four in the placebo group) did not complete the trial. Reasons were unrelated to side effects, except in three cases (two patients in the DHEA group and one in the placebo group). All withdrew within the first 4 weeks, usually after the first study visit. Ethnicity was unrelated to attrition. Attrition rates were 11% for white men, 9% for Hispanic men, and 6% for black men. The total study group of 145 patients and the completer group of 133 patients differed on four measured variables: dropouts were younger (mean age=36 years, SD=8, compared with mean=44 years, SD=9, for completers) (t=3.03, df=143, p=0.003), were less likely to be taking antiretrovirals (50% versus 83% for completers) (χ2=7.36, df=1, p=0.007), were more likely to have an elevated log10 viral load (mean=3.38, SD=1.2, compared with mean=2.60, SD=1.09, for completers) (t=–2.26, df=138, p<0.03), and were more likely to be employed full- or part-time (33% or 8%, respectively, versus 8% or 11%, respectively, for completers) (χ2=8.42, df=2, p<0.02).
Patients who were randomly assigned to receive DHEA did not differ from those who were randomly assigned to receive placebo on any of the measured demographic or medical variables, as shown in Table 1. The average age of the overall group was 44 years, 84% were men, 36% were black, 32% were white, 30% were Hispanic, and 2% were of other ethnicity. Men having sex with men was the most common mode of HIV transmission. As a group, the patients were well educated, and most were currently unemployed. Twenty-four of the gay men (23%) were Central American or South American and came to the United States for access to HIV treatments unavailable at home or because of discrimination that precluded employment there. The average duration since notification of HIV status was about 8.6 years (SD=5.1). Two-thirds had an AIDS diagnosis. The mean baseline CD4 cell count was 450 cells/mm (SD=248), although the historical nadir was considerably lower (mean=205 cells/mm, SD=208). Of the 80% (N=116) of patients who were taking antiretroviral medications, 42% (N=49) had an undetectable viral load. The range of baseline DHEAS serum levels was very wide both for men (23–475 μg/dl) and women (7–212 μg/dl). However, baseline DHEAS serum levels were uncorrelated with CD4 cell counts (Pearson’s r=0.09, N=145, p=0.28). Twenty percent of the men in each group (N=15 in the DHEA group; N=14 in the placebo group) were taking a stable regimen of testosterone supplementation at study entry.
Most patients reported symptoms of chronic or recurrent depression associated with stressors such as coming out (gay men), periods of homelessness or loss of employment when sick, learning about one’s HIV-seropositive status, immigration and worries about obtaining political asylum, or substance abuse. For 70% of patients, the duration of current episode was ≥1 year (Table 1). Sixty percent (87 of 145) reported periods of past depression before learning about their HIV-seropositive status. Of these 87 patients, 55 (38% of the participants) had a history of probable major depression. Seventy percent (N=102) had received treatment for depression at some point before study entry (26% [N=38] had received psychotherapy, 21% [N=30] had received medication, and 23% [N=34] had received both psychotherapy and medication).
Of the 145 patients, 54% (N=79) had dysthymia and 46% (N=66) had subsyndromal major depression. Patients with subsyndromal major depression met three or four of the nine criteria for major depressive disorder, including depressed mood and/or loss of interest. The most common symptoms, apart from depressed mood (95%, N=138), were loss of interest (83%, N=120), insomnia (61%, N=88), and fatigue (56%, N=81). Other cognitive symptoms (guilt, difficulties with concentration, suicidal ideation) were uncommon; each symptom was found in <15% (N=22 or fewer) of patients.
Efficacy of DHEA for Depression: Week-8 Treatment Outcome
Intent-to-treat analysis
The response rates were 56% (43 of 77) for DHEA patients and 31% (21 of 68) for placebo patients (χ2=9.13, df=1, p=0.003). Endpoint HAM-D scores, adjusted for baseline scores, were numerically but not statistically significantly lower in the DHEA group, compared to the placebo group (mean=8.1, SD=5.3, and mean=9.4, SD=5.0) (F=2.36, df=1, 143, p=0.13). Women responded at a similar rate as men to DHEA (seven of 13 [54%] for women, compared to 36 of 64 [56%] for men), but women had a higher response rate to placebo (five of 10 [50%] for women, compared to 16 of 58 [28%] for men), although the difference was not significant and the wide confidence limits found for this small group of women rendered the finding suggestive at best.
By using a ≥50% decline in HAM-D scores as the measure of treatment outcome in the intent-to-treat analysis, the response rate was 57% (44 of 77) in the DHEA group and 35% (24 of 68) in the placebo group (χ2=6.92, df=1, p=0.009).
Completer analysis
In the completer group of 133 patients, the response rates were 62% in the DHEA group (43 of 69) and 33% (21 of 64) in the placebo group (χ2=11.58, df=1, p<0.001), as shown in Table 2. With adjustment for baseline HAM-D scores, week-8 HAM-D scores were significantly lower in the DHEA group (mean=7.1, SD=4.5) than in the placebo group (mean=8.9, SD=4.6) (F=5.00, df=1, 131, p<0.03). Neither baseline nor week-8 HAM-D scores differed between patients with a diagnosis of dysthymia and those with subsyndromal major depression (data not shown). Although scores for the HAM-D affective and somatic items were examined separately, the change in the affective subscale score was significant (F=4.51, df=1, 131, p<0.04), and the change in the somatic subscale score approached statistical significance (F=3.58, df=1, 131, p=0.06). The onset of persistent improvement (defined as a CGI score of 1 or 2 that was maintained through week 8) was rather evenly distributed across weeks 4, 6, and 8 and did not differ between the responders to DHEA and those who responded to placebo (data not shown).
By using the criterion of a ≥50% drop in HAM-D scores as an outcome measure, the response rates were 64% (N=44) of completers randomly assigned to receive DHEA and 38% (N=24) of those randomly assigned to receive placebo (χ2=9.17, df=1, p=0.002).
For the secondary measures, the mean change in BDI scores at week 8 did not differ between treatment groups (DHEA group: mean=13.6, SD=8.3; placebo group: mean=12.3, SD=8.5) (F=0.95, df=1, 131, p=0.33, analysis of covariance, with adjustment for baseline score). We also examined the data for the BDI affective/cognitive and somatic item sets separately and found comparable results (data not shown). However, within both treatment groups, those classified as responders on the basis of CGI and HAM-D scores had significantly lower BDI scores than the nonresponders (data not shown).
The Endicott measure of quality of life similarly did not differentiate between groups overall (F=0.007, df=1, 131, p=0.93), but within each treatment group, those classified as responders on the basis of their CGI scores showed significantly greater improvement in their ratings of life satisfaction than did those classified as nonresponders.
Effect of DHEAS serum level on treatment outcome
For men, the mean doses of DHEA were 386 mg/day for responders and 390 mg/day for nonresponders (t=0.30, df=54, p=0.77). For women, the mean doses were 243 mg/day for responders and 300 mg/day for nonresponders (t=–1.27, df=11, p=0.25). For men who received DHEA, the mean achieved DHEAS serum levels at week 8 were 1898 μg/dl (SD=1345) for responders and 1582 μg/dl (SD=1307) for nonresponders. For women who received DHEA, the DHEAS serum levels at week 8 were 1238 μg/dl (SD=1086) for responders and 1171 μg/dl (SD=508) for nonresponders. Differences in serum levels at week 8 between responders and nonresponders in the DHEA group were not statistically significant for men (t=1.08, df=54, p=0.29) or women (t=0.14, df=11, p=0.89) or for the combined group of men and women (t=1.09, df=66, p=0.27).
The baseline level of DHEAS and change in DHEAS level at week 8 were assessed in relation to treatment outcome. The men in the DHEA group who were responders had nonsignificantly higher baseline serum levels of DHEAS, compared with nonresponders (mean=253 μg/dl, SD=162, and mean=183 μg/dl, SD=125, respectively) (t=1.68, df=54, p=0.10). The mean change in DHEAS between baseline and week 8 similarly did not differentiate between male responders and nonresponders (change mean=1645 μg/dl, SD=1300, for responders, versus mean=1312 μg/dl, SD=1321, for nonresponders) (t=0.91, df=54, p=0.37). Looking at the data another way, the change in DHEAS level between baseline and week 8 was uncorrelated with the change in HAM-D score; again there was no significant relationship to clinical response (r=–0.11, N=56, p=0.43). Data for men and women combined were comparable, with no relationship between change in DHEAS level and clinical outcome (r=–0.11, N=68, p=0.38).
Fourteen patients (seven men [6%] and seven women [30%]) had baseline serum DHEAS levels below the age-adjusted reference range. Of these patients, nine were randomly assigned to DHEA treatment and had an overall response rate of 44% (four of the nine). Although the numbers are very small, there is no indication that baseline deficiency enhanced the likelihood of response to DHEA treatment.
DHEAS serum levels at weeks 4 and 8 were examined for patients who received DHEA as a proxy measure of medication adherence. We identified 16 patients (including three women) randomly assigned to receive DHEA whose DHEAS serum levels at week 8 did not at least double from baseline (e.g., a patient with a DHEAS serum level of 38 μg/dl at baseline and 43 μg/dl at week 8) (N=6) or whose DHEAS serum level dropped by more than 50% between weeks 4 and 8 (e.g., from 1541 μg/dl at week 4 to 353 μg/dl at week 8) (N=10). Among the patients who received DHEA and whose DHEAS serum levels increased at least 100%, the response rate was 70% (37 of 53), compared to 38% (six of 16) for the 16 patients whose serum levels of DHEAS did not at least double or dropped by more than 50% (χ2=5.46, df=1, p<0.02, or p=0.04, Fisher’s exact test).
Effect of steroid supplementation on treatment outcome
There was no difference in response rate for the 25 men who had been taking a stable regimen of steroids (most commonly gel-preparation testosterone) for at least 3 months. Of those who had been taking testosterone and who were randomly assigned to receive DHEA, 55% (six of 11) were responders, compared with 67% (30 of 45) of those who had not been taking testosterone (p=0.45, Fisher’s exact test). In the placebo group, the men who had been taking steroids had a response rate was 9% (one of 11), compared to 35% (15 of 43) for men who were not taking supplemental steroids (p=0.10, Fisher’s exact test).
Double-blind guesses
To determine whether the patients’ psychiatrists or the patients could penetrate the double blind and thus be likely to be biased in assessing outcome, each was asked to “guess” the treatment before the blind was broken at week 8. Overall, the doctors guessed correctly 60% of the time (61% accuracy in identifying patients who received DHEA and 59% accuracy in identifying patients who received placebo). They were more accurate in identifying DHEA responders (83%) than in identifying placebo responders (21%), suggesting that they guessed “active drug” in the presence of clinical response. The patients’ guesses were correct 55% of the time overall; 64% of those who received DHEA and 45% of those who received placebo were correct in their guesses. Neither the doctors’ nor the patients’ guesses were significantly better than chance.
Safety of DHEA for Treatment of Depression
Treatment effects on steroid, medical, immunological, and virological markers
Total serum testosterone levels did not change in the DHEA group for men (baseline: mean=601 ng/dl, SD=237; week 8: mean=638 ng/dl, SD=323) (paired t=1.25, df=55, p=0.22). However, women who received DHEA had a substantial increase in serum testosterone levels (baseline: mean=44 ng/dl, SD=20; week 8: mean 119 ng/dl, SD=123) (paired t=–2.50, df=12, p<0.03). No significant changes in testosterone level were observed for either men or women in the placebo group.
No significant changes in hematocrit were found; neither the DHEA group nor the placebo group showed an increase over baseline levels. Similarly, in the DHEA group there were no statistically or clinically significant changes in CD4 cell count (baseline: mean=449 cells/mm, SD=266; week 8: mean=436 cells/mm, SD=281) (t=0.73, df=68, p=0.47) or log10 HIV RNA viral load (baseline: mean=2.47, SD=1.0; week 8: mean=2.49, SD=1.1) (t=–0.31, df=66, p=0.76).
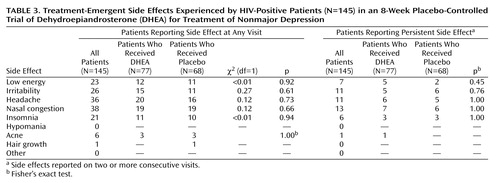
Treatment-emergent side effects
As shown in Table 3, treatment-emergent side effects were relatively uncommon and did not differ between treatment groups. Table 3 shows the number of patients who reported side effects at any visit, as well as the number of patients who reported side effects that persisted across two or more study visits.
Discussion
DHEA appears to be a useful treatment for nonmajor depression among men and women with HIV/AIDS. On the basis of clinicians’ ratings, the true drug effect in the study group was about 30% (DHEA response rate minus placebo response rate). The number needed to treat was 4 in the intent-to-treat analysis and 3.4 in the completer analysis; these results are considered to be in the “effective” range (26). DHEA is well tolerated, with few side effects. The high level of interest in this “alternative” medication may account for the remarkably low attrition we observed, compared to that in depression treatment studies of physically healthy patients. (In a meta-analysis of antidepressant trials [27], attrition of as much as 33% was found regardless of antidepressant drug class.) Our group had substantially higher rates of attrition in placebo-controlled trials with conventional antidepressants for HIV-positive patients. Other factors may include more rigorous initial screening, including urine toxicology screens, and the study patients’ extensive contact with the research and treatment staff.
In response to many requests, we added a 4-month extension phase for patients who responded to DHEA (either responders in the double-blind phase or placebo nonresponders who started DHEA at week 8) and who completed 4 months of treatment (N=44). At their final month-8 visit, their mean HAM-D mean score was 2.4 (SD=3.1), and their mean BDI score was 5.5 (SD=6.2). At this visit, the only side effects reported by more than one patient were low energy (N=2), headache (N=3), nasal congestion (N=5), and insomnia (N=2), all of which were “mild” or “moderate.” Overall, the results of this intermediate-term 8-month follow-up suggest that mood response is maintained with minimal and possibly nonspecific side effects, although long-term effects remain unknown.
The study patients had been depressed for a long time. The modal duration of depression was between 1 and 5 years, but even among the patients who reported a duration of depression of less than 2 years, almost all had extended periods of prior depression that, for the most part, antedated knowledge of their HIV status. Chronic subsyndromal major depressive disorder and dysthymia are far more common in this population than is major depression, but the nonmajor forms are equally likely to interfere with social activities, relationships and even employment (28). It should be kept in mind that apart from depressed mood and loss of interest, the predominant symptoms of depression in these HIV-positive patients, of whom 80% were taking rather toxic antiretroviral medications, were somatic.
In the literature, subsyndromal depression is increasingly recognized as a clinically significant entity (29, 30) that is commonly observed in both general and clinical populations. Although there has been disagreement about the comparative efficacy of antidepressants versus placebo (31), a number of research groups, including our own, have demonstrated that physically healthy patients with chronic mild depression show superior response to antidepressants, compared to placebo (32, 33). Our findings with HIV-positive patients are in concordance with these findings, despite the difference in the study participants’ physical health status.
We found a noticeable difference between the clinicians’ ratings and the patients’ self-reports: clinicians’ ratings reflected significant differences between treatment groups, but the self-reports did not. However, by using the clinicians’ classification of responders and nonresponders and comparing these subgroups within treatment arms, we found significant differences, with responders showing more improvement than nonresponders on self-ratings of depression and quality of life. Although the overall response rate of 31% among placebo patients in our study was substantially lower than that reported in some placebo-controlled clinical trials for mildly depressed patients (34), as a group the placebo patients did improve on all measured parameters.
The doses of DHEA used and the DHEAS serum levels achieved in this study clearly reflect a pharmacological rather than replacement model of treatment. We found no relationship between baseline DHEAS serum levels and probability of response, nor did the 10% of patients with baseline DHEAS serum levels below the reference range show a better outcome than the rest of the patients. These results are not consistent with the findings of Wolkowitz et al. (35), who found that among six depressed patients treated openly with DHEA in doses from 30–90 mg/day for 4 weeks, mood improvements were significantly related to increases in DHEAS. Perhaps our substantially higher doses and consequently higher achieved levels of DHEAS obscured whatever differences might have been seen. Alternatively, our findings may be more robust, given the larger number of patients included.
Because we did not include patients with major depression or those with substance use disorders, our findings cannot be generalized to these subgroups. Furthermore, although we made every effort to enroll women, the number of women in the final study group was too small to provide more than suggestions for future research, including questions about placebo response rate. Other limitations include the absence of long-term follow-up regarding maintenance of response or possible long-term endocrine or other effects of DHEA, as well as the restriction of our study to HIV-positive individuals. Given the high acceptance rate and low side effect profile for DHEA in this group of patients with HIV/AIDS, nearly all of whom were taking multiple concurrent medications, it may be appropriate to evaluate the efficacy of DHEA in other groups of physically ill patients in which mild depression is common. We do not recommend widespread use of DHEA in the absence of confirmatory efficacy research and more data about longer-term use. For patients who are unwilling to take antidepressants, who express a strong preference for an “alternative” treatment, and who have nonmajor depression, DHEA may be a reasonable choice. We suggest documenting informed consent for an “unapproved” treatment and monitoring response and potential adverse events at regular intervals.
 |
 |
 |
Presented in part at the 156th annual meeting of the American Psychiatric Association, San Francisco, May 17–22, 2003. Received Nov. 16, 2004; revision received Jan. 26, 2005; accepted March 7, 2005. From New York State Psychiatric Institute, Columbia University College of Physicians and Surgeons; and Weill College of Medicine, Cornell University, New York. Address correspondence and reprint requests to Dr. Rabkin, New York State Psychiatric Institute, Unit 51, 1051 Riverside Dr., New York, NY 10032; [email protected] (e-mail). Supported by NIMH grant R01 MH-60563. The authors thank Judy Chiu and Kirstie Crespo for assistance in conducting the study and Shu Hsing Lin, Ph.D., for assistance with data analysis.
1. Ferrando SJ, Wapenyi K: Psychopharmacological treatment of patients with HIV and AIDS. Psychiatr Q 2002; 73:33–49Crossref, Medline, Google Scholar
2. Griffin K, Rabkin JG: Psychological distress in people with HIV/AIDS: prevalence rates and methodological issues. AIDS Behav 1997; 1:29–42Crossref, Google Scholar
3. Rabkin JG, Wagner GJ, Rabkin R: A double-blind, placebo-controlled trial of testosterone therapy for HIV-positive men with hypogonadal symptoms. Arch Gen Psychiatry 2000; 57:141–147Crossref, Medline, Google Scholar
4. Tomera JF: Dehydroepiandrosterone and aging. Drugs Today 1996; 32:453–461Google Scholar
5. Johnson M, Bebb R, Sirrs S: Uses of DHEA in aging and other disease states. Ageing Res Rev 2002; 1:29–41Crossref, Medline, Google Scholar
6. Brebner K, Hayley S, Zacharko R, Merali Z, Anisman H: Synergistic effects of interleukin-1beta, interleukin-6 and tumor necrosis factor-alpha: central monoamine, corticosterone, and behavioral variations. Neuropsychopharmacology 2000; 22:566–580Crossref, Medline, Google Scholar
7. Soares CD, Musolino N, Cunha A, Neto M, Caires M, Rosenthal M, Camargo C, Bronstein M: Impact of recombinant human growth hormone treatment on psychiatric, neuropsychological and clinical profiles of GH deficient adults: a placebo-controlled trial. Arq Neuropsiquiatr 1999; 57:182–189Crossref, Medline, Google Scholar
8. Lanquillon S, Drieg J-C, Bening-Abu-Shach U, Vedder H: Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 2000; 22:370–379Crossref, Medline, Google Scholar
9. Wolkowitz OM, Kramer J, Reus V, Costa M, Yaffe K, Walton P, Raskind M, Peskind E, Newhouse P, Sack D, DeSouza E, Sadowsky C, Roberts E: DHEA treatment of Alzheimer’s disease: a randomized double-blind, placebo-controlled study. Neurology 2003; 60:1071–1076Crossref, Medline, Google Scholar
10. Hunt PJ, Gurnell EM, Huppert FA, Richards C, Prevost AT, Wass JA, Herbert J, Chatterjee VK: Improvement in mood and fatigue after dehydroepiandrosterone replacement in Addison’s disease in a randomized, double blind trial. J Clin Endocrinol Metab 2000; 85:4650–4656Medline, Google Scholar
11. Strous RD, Maayan R, Lapidus R, Stryjer R, Lustig M, Kotler M, Weizman A: Dehydroepiandrosterone augmentation in the management of negative, depressive, and anxiety symptoms in schizophrenia. Arch Gen Psychiatry 2003; 60:133–141Crossref, Medline, Google Scholar
12. Laudat A, Blum L, Guechot J, Picard O, Cabane J, Imbert J, Giboudeau J: Changes in systemic gonadal and adrenal steroids in asymptomatic HIV-infected men: relationship with the CD4 cell counts. Eur J Endocrinol 1995; 133:418–424Crossref, Medline, Google Scholar
13. Ferrando SJ, Rabkin J, Poretsky L: Dehydroepiandrosterone (DHEA) and testosterone: relationship to HIV illness stage and progression over one year. J Acquir Immune Defic Syndr Hum Retrovirol 1999; 22:146–154Crossref, Google Scholar
14. Christeff N, Melchior J-C, de Truchis P, Perronne C, Nunez E, Gougeon ML: Lipodystrophy defined by a clinical score in HIV-infected men on highly active antiretroviral therapy: correlation between dyslipidaemia and steroid hormone alterations. AIDS 1999; 13:2251–2260Crossref, Medline, Google Scholar
15. Christeff N, de Truchis P, Melchior J-C, Perronne C, Gougeon ML: Longitudinal evolution of HIV-1-associated lipodystrophy is correlated to serum cortisol: DHEA ratio and IFN-alpha. Eur J Clin Invest 2002; 32:775–784Crossref, Medline, Google Scholar
16. Wolkowitz OM, Reus VI, Keebler A, Nelson N, Friedland M, Brizendine L, Roberts E: Double-blind treatment of major depression with dehydroepiandrosterone. Am J Psychiatry 1999; 156:646–649Abstract, Google Scholar
17. Bloch M, Schmidt P, Danaceau M, Adams L, Rubinow D: Dehydroepiandrosterone treatment of midlife dysthymia. Biol Psychiatry 1999; 45:1533–1541Crossref, Medline, Google Scholar
18. Rabkin JG, Ferrando SJ, Wagner GJ, Rabkin R: DHEA treatment for HIV+ patients: effects on mood, androgenic and anabolic parameters. Psychoneuroendocrinology 2000; 25:53–68Crossref, Medline, Google Scholar
19. Rabkin JG, Ferrando SJ: DHEA and HIV illness. AIDS Read 1997; 7(1):28–36Google Scholar
20. Parasrampuria J, Schwartz K: Quality control of dehydroepiandrosterone dietary supplement products (letter). JAMA 1998; 280:1565Crossref, Medline, Google Scholar
21. Williams JB: A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry 1988; 45:742–747Crossref, Medline, Google Scholar
22. Kalali A, Williams J, Kobak KA, Lipsitz J, Engelhardt N, Evans K, Olin J, Pearson J, Rothman M, Bech P: The new GRID HAM-D: pilot testing and international field trials. Int J Neuropsychopharm 2002; 5(suppl 1):S147Google Scholar
23. Endicott J, Nee J, Harrison W, Blumenthal R: Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull 1993; 29:321–326Medline, Google Scholar
24. Rabkin JG, Markowitz J, Ocepek-Welikson K: General vs systematic inquiry about emergent clinical events with SAFTEE: implications for clinical research. J Clin Psychopharmacol 1992; 12:3–10Crossref, Medline, Google Scholar
25. Bagatell C, Bremner W: Androgens in men: uses and abuses. N Engl J Med 1996; 334:707–714Crossref, Medline, Google Scholar
26. McQuay HJ, Moore RA: Using numerical results from systematic reviews in clinical practice. Ann Intern Med 1997; 126:712–720Crossref, Medline, Google Scholar
27. Anderson IM, Tomenson BM: Treatment discontinuation with selective serotonin reuptake inhibitors compared with tricyclic antidepressants: a meta-analysis. BMJ 1995; 310:1433–1438Crossref, Medline, Google Scholar
28. Rabkin JG, McElhiney M, Ferrando S, van Gorp W, Lin SH: Predictors of employment of men with HIV/AIDS: a longitudinal study. Psychosom Med 2004; 66:72–78Crossref, Medline, Google Scholar
29. Judd L, Akiskal H, Paulus M: The role and clinical significance of subsyndromal depressive symptoms (SSD) in unipolar major depression. J Affect Disord 1997; 45:5–18Crossref, Medline, Google Scholar
30. Rapaport MH, Judd LL, Schettler PJ, Yonkers KA, Thase ME, Kupfer DJ, Frank E, Plewes JM, Tollefson GD, Rush AJ: A descriptive analysis of minor depression. Am J Psychiatry 2002; 159:637–643Link, Google Scholar
31. Venning G: Antidepressant drugs have previously been shown ineffective in mild depression (letter). BMJ 2000; 3209:311Google Scholar
32. Stewart J, McGrath P, Quitkin F, Rabkin J, Harrison W, Wager S, Nunes E, Ocepek-Welikson K, Tricamo E: Chronic depression: response to placebo, imipramine and phenelzine. J Clin Psychopharmacol 1993; 13:391–396Medline, Google Scholar
33. Thase M, Fava M, Halbreich U, Kocsis J, Koran L, Davidson J, Rosenbaum J, Harrison W: A placebo-controlled, randomized clinical trial comparing sertraline and imipramine for the treatment of dysthymia. Arch Gen Psychiatry 1996; 53:777–784Crossref, Medline, Google Scholar
34. Thase M: How should efficacy be evaluated in randomized clinical trials of treatments for depression? J Clin Psychiatry 1999; 60 (suppl 4):23-31Google Scholar
35. Wolkowitz O, Reus V: Dehydroepiandrosterone in psychoneuroendocrinology, in Psychoneuroendocrinology. Edited by Wolkowitz O, Rothschild A. Arlington, Va, American Psychiatric Publishing, 2003, pp 205-242Google Scholar



