Bone Mineral Density, Markers of Bone Turnover, and Cytokines in Young Women With Borderline Personality Disorder With and Without Comorbid Major Depressive Disorder
Abstract
OBJECTIVE: The pathogenesis of bone loss in major depressive disorder is a matter of debate. Studies of bone loss in nonpsychiatric medical disorders have found an association between the activation of osteoclastic cells and an imbalance of pro- and antiinflammatory cytokines. Since major depressive disorder is also associated with alterations in serum cytokine concentrations, the authors hypothesized that bone loss in patients with major depressive disorder and comorbid borderline personality disorder may be associated with cytokines capable of activating osteoclastic cells. METHOD: Twenty-two patients with borderline personality disorder and comorbid current or lifetime major depressive disorder were compared with 16 patients with borderline personality disorder who did not have major depressive disorder and 20 healthy volunteers. Bone mineral density was assessed by means of dual-energy x-ray absorptiometry. Markers of bone turnover as well as endocrine and immune measures were determined. RESULTS: The bone mineral density of 10 patients with borderline disorder plus current major depressive episode was significantly lower than that of the healthy subjects and the patients with borderline personality disorder without depression. Values of crosslaps, osteocalcin, serum cortisol, tumor necrosis factor-α (TNF-α), and interleukin-6 were significantly higher in the patients with borderline disorder plus current major depressive episode than in the healthy subjects. Crosslaps correlated positively with TNF-α but negatively with bone mineral density at the lumbar spine. Patients with borderline personality disorder who did not have current or lifetime depression displayed no alterations of either bone mineral density or the immunological and hormonal measures examined. CONCLUSIONS: Young women with comorbid borderline personality disorder and major depressive disorder have an elevated risk for osteoporosis. Borderline personality disorder per se is not associated with low bone mineral density. These data suggest that the immune and endocrine disturbances associated with depressive disorders in the context of borderline personality disorder may play a role in the pathophysiological process underlying bone loss in the patients studied.
Following initial reports of pathological fractures in patients suffering from major depressive disorder, several other groups also described low bone mineral density in major depressive disorder and other psychiatric disorders (1–3). Bone mineral density was found to be 6%–15% lower in subjects with major depressive disorder than in matched control subjects, and osteoporosis was observed in up to 42% of the patients (3). Low bone mineral density is associated with a greater risk of bone fractures. The estimated risk increases by a factor of 1.5 to 3 for each reduction of standard deviation in bone mineral density (4). Since osteoporosis is often a silent disease that might go undiagnosed for a long time, identification of risk factors associated with the development of low bone mineral density may be crucial to identifying groups at risk for osteoporosis and preventing further bone mineral reduction.
To date it is unclear whether depression simply magnifies the physiological bone loss that occurs after the age of 35 or whether the disturbance of bone metabolism begins earlier. Examination of cross-sectional studies and the correlations between steroid hormones and bone density in psychiatric patients yielded heterogeneous results (reviewed in reference 3). Biochemical indicators of bone metabolism also yielded heterogeneous results, pointing to either a low or a high bone turnover osteoporosis (5, 6).
A dysregulation of the hypothalamic-pituitary-adrenal (HPA) system, with higher concentrations of serum cortisol in major depressive disorder, has frequently been reported (reviewed in reference 7). Given the reports of osteoporosis in patients who are chronically treated with glucocorticoids or who suffer from Cushing’s syndrome, higher serum cortisol concentrations have been hypothesized to be one mechanism by which depression might induce bone loss (3). Furthermore, a dysregulation of pro- and antiinflammatory cytokines in major depressive disorder has been reported, including increased serum levels of interleukin-1β, interleukin-2 (IL-2), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and the soluble IL-2 and IL-6 receptors (8–14). IL-6 and TNF-α have also been implicated in bone resorption attributable to their activation of osteoclastic cells (15, 16). Therefore, it seems reasonable to hypothesize a role of cytokines in the pathophysiological process underlying the bone loss in major depressive disorder. However, the exact pathogenic mechanisms of osteoporosis in major depressive disorder have not been sufficiently characterized.
Our main a priori hypothesis was that young women with major depressive disorder already suffer from low bone mineral density. Because of the heterogeneous literature with respect to the type of alterations of bone metabolism in major depressive disorder, we were not able to formulate a directed a priori hypothesis (high versus low bone turnover osteoporosis) with sufficient certainty, but we expected higher bone turnover in the context of elevated concentrations of proinflammatory cytokines.
Approximately 70% of major depressive episodes in young women occur in the context of personality disorders (17, 18). Among women admitted to the hospital with depressive disorders, the leading associated personality disorders are borderline personality disorder (up to 30% comorbidity) and avoidant personality disorder (17). We decided to start the exploration of bone density and bone metabolism in young women with depressive disorders in the context of borderline personality disorder and to compare them with women who had borderline personality disorder but no current or lifetime major depressive disorder as well with healthy women.
Method
This study included 38 female patients who were consecutively admitted to our specialized unit for treatment of borderline personality disorder and who met DSM-IV diagnostic criteria for borderline personality disorder. Diagnoses were determined by using German versions of the Structured Clinical Interview for DSM-IV (SCID) and the SCID for Personality Disorders. Sixteen of the 38 patients with borderline personality disorder had no history of major depressive disorder and no current major depressive episode; 22 of the 38 patients were diagnosed as suffering from comorbid major depressive disorder. Ten of these 22 patients had current major depressive episode, and the remaining 12 had a lifetime history of major depressive disorder but no current major depressive episode.
Exclusion criteria for the patients were current or lifetime anorexia nervosa, schizophrenia, oligophrenia, pregnancy, estrogen deficiency, infectious or (auto-) inflammatory disease, and age of 17 years or younger. Twenty healthy normal-weight women of similar age served as the comparison group. Exclusion of psychiatric disorders in the comparison group was also done by using the SCID.
Sociodemographic data for the participating subjects are presented in Table 1. Eleven of the 38 patients received selective serotonin reuptake inhibitors (three of the 16 patients with borderline personality disorder alone, three of the 12 with borderline personality disorder plus lifetime major depressive disorder, and five of the 10 with borderline personality disorder plus current major depressive episode) for the treatment of bulimia nervosa or the current depressive episode. None of the patients received treatment with mood stabilizers, benzodiazepines, or neuroleptics. The study was approved by the local ethics committee. All patients gave their written informed consent.
Bone mineral density was measured in all patients by means of dual-energy x-ray absorptiometry at the lumbar spine, right femur, left femur, and the forearm of the nondominant hand with a Lunar Prodigy Densitometer equipped with software containing data for an adult reference population (version 2.15.092, Lunar Corp., Madison, Wis.). These normative data were collected from a large sample of healthy subjects enrolled in longitudinal studies of bone density and are specified for age, gender, body mass index, and ethnic group (19). Staff members involved in the determination of bone density were aware of the patient status of the subject but not of comorbid diagnoses. Bone mineral density was not measured in the healthy comparison group, in compliance with government regulations restricting the use of radiation in healthy subjects. Individual bone mineral density values were expressed as z scores for group comparisons within the patient subgroups (borderline personality disorder plus current major depressive episode; borderline personality disorder plus lifetime major depressive disorder; borderline personality disorder alone), and T scores were used to estimate the fracture risk. Osteopenia was defined in accordance with the World Health Organization guidelines (20) as a T score ≤–1.
Serum was taken after an overnight fast and stored at –40˚C until analysis. Intact parathormone, 1,25-hydroxy-vitamin D, and laboratory markers of bone turnover (intact osteocalcin and C-terminal telopeptides of type-I collagen, referred to as crosslaps) served as laboratory markers of bone turnover and were determined by using commercial immunoradiometric assay (Nichols Institute Diagnostics, Bad Vilbel, Germany) and enzyme-linked immunosorbent assay (ELISA) (Osteometer Biotech A/S, Herlev, Denmark) kits, respectively. Osteocalcin (a biochemical indicator of bone formation) and crosslaps (a biochemical indicator of bone resorption) were shown to specifically and sensitively reflect alterations in bone metabolism in patients with and without bone diseases and to correlate with bone mass (21, 22). Cortisol was determined by the radioimmunoassay technique (DPC, Naunheim, Germany). TNF-α receptors I and II, IL-2, interferon-γ (IFN-γ), insulin-like growth factor I, leptin, and osteoprotegerin were determined by using available ELISA kits according to the manufacturer’s instructions (all from R&D Systems, Wiesbaden, Germany). High-sensitivity ELISA kits were used to determine TNF-α and IL-6 (Quantikinine, R&D Systems).
Data were analyzed by using SPSS (version 10.0, SPSS, Inc., Chicago). Analysis of variance (ANOVA) and the Mann-Whitney U test (for z scores of the patient groups) were used to compare study groups. Further analysis was performed with analysis of covariance (ANCOVA), and Pearson’s coefficients of correlation were calculated. The t test was used to compare the z scores of patients with those of the reference population. A p value below 0.05 (two-tailed) was considered significant. All values are given as means and standard deviations when appropriate.
Results
Bone mineral density expressed as a z score was significantly lower in patients with borderline personality disorder plus current major depressive episode at the lumbar spine (t=–2.9, df=28, p<0.02) and at the forearm of the nondominant hand (t=–2.8, df=28, p<0.03) than in the healthy reference group matched for sex, age, and body mass index. Among the three patient groups with borderline personality disorder, z scores differed significantly (F=4.9, df=2, 34, p<0.02). In patients with borderline personality disorder plus current major depressive episode, z scores were significantly lower at the lumbar spine than they were in patients with borderline personality disorder alone (U=30.5, p<0.02) (Figure 1).
Osteopenia, defined by a T score less than or equal to one standard deviation below that of the reference population at peak bone mass, was observed at the lumbar spine in two (20%) of 10 patients with borderline personality disorder plus current major depressive episode and one (6%) of 16 patients with borderline personality disorder alone (6%). Osteopenia was also observed at the right femur in two (20%) of 10 patients with borderline personality disorder plus current major depressive episode and one (8%) of 12 patients with borderline personality disorder plus lifetime major depressive disorder. Osteopenia was also observed at the left femur in two (20%) of 10 patients with borderline personality disorder plus current major depressive episode, one (8%) of 12 patients with borderline personality disorder plus lifetime major depressive disorder, and two (12%) of 16 patients with borderline personality disorder alone. Osteopenia was also observed at the forearm of the nondominant hand in two (20%) of 10 patients with borderline personality disorder plus current major depressive episode, two (16%) of 12 patients with borderline personality disorder plus lifetime major depressive disorder, and three (19%) of 16 patients with borderline personality disorder alone.
ANOVA revealed significant differences among the three patient groups and the healthy comparison group with regard to age (F=4.2, df=1, 3, p=0.01), fasting cortisol (F=2.8, df=1, 3, p<0.05), osteocalcin (F=3.1, df=1, 3, p<0.04), crosslaps (F=6.4, df=1, 3, p=0.001), TNF-α (F=3.0, df=1, 3, p=0.01), and IL-6 (F=5.3, df=1, 3, p=0.001) (Figure 2). For further analysis, ANCOVA with the covariate age was performed. The effects of the covariate age were not significant, with p ranging from 0.10 to 0.35. We found a significant group effect for fasting cortisol (F=2.6, df=1, 4, p<0.05), osteocalcin (F=3.0, df=1, 4, p<0.03), crosslaps (F=5.1, df=1, 4, p=0.002), TNF-α (F=3.3, df=1, 4, p<0.01), and IL-6 (F=5.3, df=1, 4, p=0.001).
Post hoc analysis revealed higher concentrations of fasting cortisol in patients with borderline personality disorder plus current major depressive episode than in healthy comparison subjects (p=0.006) and in patients with borderline personality disorder plus current major depressive episode than in those with borderline personality disorder plus lifetime major depressive disorder (p=0.01) (Table 1). Crosslaps were found to be higher in patients with borderline personality disorder plus current major depressive episode than in healthy comparison subjects (p<0.001), in patients with borderline personality disorder plus current major depressive episode than in those with borderline personality disorder alone (p=0.002), and in patients with borderline personality disorder plus lifetime major depressive disorder than in healthy comparison subjects (p<0.05).
TNF-α was higher in patients with borderline personality disorder plus current major depressive episode than in the comparison subjects (p=0.003) and in patients with borderline personality disorder plus current major depressive episode than in those with borderline personality disorder alone (p=0.006). IL-6 was higher in patients with borderline personality disorder plus current major depressive episode than in healthy comparison subjects (p<0.001), in patients with borderline personality disorder plus current major depressive episode than in those with borderline personality disorder plus lifetime major depressive disorder (p<0.02), and in patients with borderline personality disorder plus current major depressive episode than in those with borderline personality disorder alone (p=0.004).
Regression analysis revealed that TNF-α correlated positively with crosslaps (r=0.322, N=38, p<0.05) but negatively with the z score at the lumbar spine (r=–0.362, N=38, p<0.03). Crosslaps correlated negatively with z scores at the lumbar spine (r=–0.333, N=38, p<0.05). z scores at the right femur correlated negatively with concentrations of serum cortisol (r=–0.33, N=38, p<0.05). Body mass index correlated with z scores at the forearm of the nondominant hand (r=0.416, N=38, p<0.02). No correlations were found between age or body mass index with any of the immunological and hormonal measures examined (data not shown). Addition of medication status to the statistical models had no relevant influence on any of the reported results.
Concentrations of other cytokines and hormones analyzed, including osteoprotegerin, were similar among the four groups (Table 1). Serum concentrations of IFN-γ and IL-2 were beneath the detection limit in all four groups. White and red blood cell counts, serum electrolytes, and the concentrations of phosphate, magnesium, insulin, thyroid hormones, and prolactin were also similar among the groups (data not shown).
Substance-related disorders were found in eight, posttraumatic stress disorder in 13, and bulimia nervosa in 20 of the 38 patients. ANCOVA revealed no effect of these present or past comorbid disorders on either bone mineral density or the z scores or on the hormonal and immunological data (data not shown).
Discussion
Our data demonstrate low bone mineral density at the lumbar spine in young female patients of normal weight or overweight who had comorbid borderline personality disorder and current major depressive episode. An important aspect of our study is the relative youth of this group of patients (their mean age was 24 years). Earlier studies examining osteoporosis and major depressive disorder were carried out in older groups of patients whose mean ages ranged from 41 to 75 years (3). Moreover, we found high values for serum crosslaps, which are a marker for osteoclastic activity, and high values for osteocalcin in these patients, indicating high bone turnover.
Patients with borderline personality disorder and a lifetime history of major depressive disorder had nonsignificantly lower bone mineral density than normal, and patients with borderline personality disorder but no history of major depressive disorder had z scores similar to those of the reference group. Our data suggest that the pathophysiological process associated with acute major depressive episode may contribute to the bone loss observed in our patient group. Our data do not support the hypothesis that the pathophysiological process associated with borderline personality disorder per se may lead to a substantial alteration of bone metabolism.
Another finding is that of higher concentrations of TNF-α, IL-6, and fasting cortisol in the patients with borderline personality disorder plus current major depressive episode. Our findings are in accord with those of other researchers who found higher concentrations of proinflammatory cytokines and a dysregulation of the HPA system in patients with current major depressive episode (reviewed in references 7, 10). However, these studies were not stratified for comorbid personality disorders.
The correlation of TNF-α with serum crosslaps and low bone mineral density (expressed as z scores) points to a possible role in the pathophysiological process underlying the bone loss in this group of depressive patients. Bone tissue is continuously rebuilt in a coordinated process of osteoclastic resorption and osteoblastic bone mass increase (23). Enhanced bone resorption has been associated with an inappropriate osteoclast activation (24, 25). Several factors, including high concentrations of glucocorticoids and proinflammatory cytokines such as TNF-α and IL-6, have been shown to activate osteoclastic cells in vivo and in vitro. It therefore seems reasonable to hypothesize that the higher levels of serum cortisol and proinflammatory cytokines in patients with borderline personality disorder plus current major depressive episode may play a role in the observed decrease in bone mineral density.
Since the concentrations of osteoprotegerin were similar among the groups and the osteoblastic marker osteocalcin was higher in patients with borderline personality disorder plus current major depressive episode, our findings suggest that the bone loss observed in patients with borderline personality disorder plus current major depressive episode may mainly be attributed to osteoclastic activation rather than to an inhibition of osteoblastic cells. A negative correlation between serum TNF-α and bone mineral density has also been reported in patients suffering from postmenopausal osteoporosis and in healthy adolescent girls (26, 27).
Other disease- and medication-related processes, such as impaired fluid and electrolyte balance, dietary and vitamin D deficiency, decreased exercise, and decreased exposure to sunshine may play a role in bone loss (5, 6). Cross-sectional assessment, however, shows that none of the available studies reported them as mediating factors for low bone mineral density in depression. Our data show similar concentrations of parathormone, estradiol, and 1,25-hydroxy-vitamin D in all groups examined. These findings do not support the hypothesis that a deficiency of vitamin D or estradiol or alterations of the parathormone metabolism may be underlying causes of bone loss in our patients.
There are several limitations to our study. First, the small number of patients in each group limits the interpretation of the negative results and increases the risk of spurious results in the comparison of multiple measures. Second, there was no comparison group with major depressive episode and another comorbid personality disorder. This makes it difficult to differentiate whether the reported alteration of bone density is an effect of depression alone or the result of an interaction between depression and borderline personality disorder or its respective risk factors. A third limitation is the restricted ability of the study to control for potential confounding factors like medication or exercise. Studies of alterations in cytokine concentrations in depressed patients during antidepressant treatment (13, 28) showed a decrease of proinflammatory cytokines (TNF-α and IL-6) after treatment with an SSRI or tricyclic agents. In another cross-sectional study (29), no influence of previous antidepressant treatment was observed on the concentrations of TNF-α and IL-6 at admission to a hospital. Furthermore, in a longitudinal study (30), the SSRI paroxetine was shown to leave concentrations of several cytokines (including TNF-α, IL-6) unchanged.
TNF-α and IL-6 have been reported to increase during exercise (31). Patients with major depressive disorder tend to reduce their physical activity. We are not aware of any study attributing the changes in proinflammatory cytokines in depression to changes in exercise. Taken together, these findings and observations suggest that the concentrations of TNF-α and IL-6 may have been underestimated because of confounding factors in our patient groups. However, we cannot completely exclude that previous medication or exercise may have contributed to the observed findings.
In summary, young women with comorbid borderline personality disorder and current major depressive episode constitute a group at risk of developing osteoporosis. Possible pathophysiological processes include hypercortisolism and an up-regulation of the proinflammatory cytokines IL-6 and TNF-α.
 |
Received July 22, 2003; revision received Feb. 17, 2004; accepted March 1, 2004. From the Department of Psychiatry and Psychotherapy, Institute of Radiology, Institute of Clinical Chemistry, University of Lübeck, Germany. Address correspondence and reprint requests to Dr. Kahl, Klinik für Psychiatrie und Psychotherapie, Universitätsklinikum Schleswig-Holstein, Campus Lübeck, Ratzeburger Allee 160, 23538 Lübeck, Germany; [email protected] (e-mail). Supported by grant MUL2301 from the University of Lübeck.
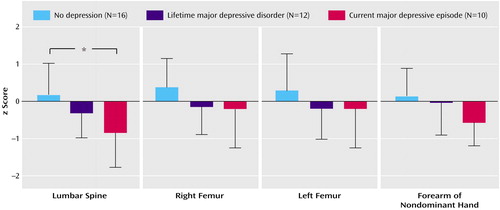
Figure 1. Comparison of z Scores at the Lumbar Spine, Right Femur, Left Femur, and Forearm of the Nondominant Hand in 38 Patients With Borderline Personality Disorder With or Without Comorbid Major Depressive Disorder
*p<0.05.
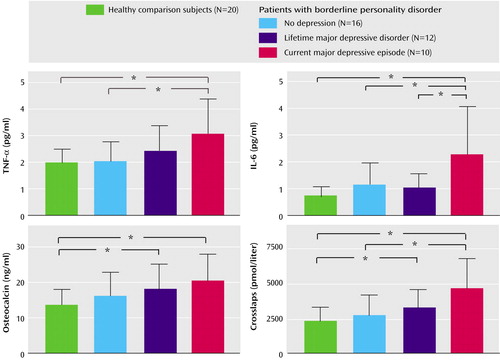
Figure 2. Tumor Necrosis Factor-α (TNF-α), Interleukin-6 (IL-6), and Markers of Bone Turnover in 38 Patients With Borderline Personality Disorder With or Without Comorbid Major Depressive Disorder and 20 Normal Comparison Subjects
*p<0.05.
1. Van Vort WB, Rubenstein M, Rose RP: Osteoporosis with pathological hip fractures in major depression. J Geriatr Psychiatry Neurol 1990; 3:10–12Crossref, Medline, Google Scholar
2. Halbreich U, Palter S: Accelerated osteoporosis in psychiatric patients: possible pathophysiologic processes. Schizophr Bull 1996; 22:447–454Crossref, Medline, Google Scholar
3. Cizza G, Ravn P, Chrousos GP, Gold PW: Depression: a major, unrecognized risk factor for osteoporosis. Trends Endocrinol Metab 2001; 12:198–203Crossref, Medline, Google Scholar
4. Whooley MA, Kip KE, Cauley JA, Ensrud KE, Nevitt MC, Browner WS (Osteoporotic Fractures Research Group): Depression, falls and risk of fracture in older women. Arch Intern Med 1999; 159:484–490Crossref, Medline, Google Scholar
5. Michelson D, Stratakis C, Hill L, Reynolds J, Galliven E, Chrousos G, Gold P: Bone mineral density in women with depression. N Engl J Med 1996; 335:1176–1181Crossref, Medline, Google Scholar
6. Herrán A, Amado JA, García-Unzueta MT, Vázquez-Barquero JL, Perera L, González-Macías J: Increased bone remodelling in first-episode major depressive disorder. Psychosom Med 2000; 62:779–782Crossref, Medline, Google Scholar
7. Heuser I: The hypothalamic-pituitary-adrenal axis in depression. Pharmacopsychiatry 1998; 31:10–13Crossref, Medline, Google Scholar
8. Maes M: Major depression and activation of the inflammatory response syndrome. Adv Exp Med Biol 1999; 461:25–46Crossref, Medline, Google Scholar
9. Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H: Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 1997; 9:853–858Crossref, Medline, Google Scholar
10. Van West D, Maes M: Activation of the inflammatory response system: a new look at the etiopathogenesis of major depression. Neuro Endocrinol Lett 1999; 20:11–17Medline, Google Scholar
11. Frommberger UH, Bauer J, Haselbauer P, Fraulin A, Riemann D, Berger M: Interleukin-6 (IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci 1997; 247:228–233Crossref, Medline, Google Scholar
12. Kiecolt-Glaser JK, Glaser R: Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res 2002; 53:873–876Crossref, Medline, Google Scholar
13. Lanqillon S, Krieg J-C, Bening-Abu-Shach U, Vedder H: Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 2000; 22:370–379Crossref, Medline, Google Scholar
14. Dantzer R, Wollman E, Vitkovic L, Yirmiya R: Cytokines and depression: fortuitous or causative association? Mol Psychiatry 1999; 4:328–332Crossref, Medline, Google Scholar
15. Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL: TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 2000; 106:1481–1488Crossref, Medline, Google Scholar
16. Hofbauer LC, Khosla S, Lacey DL, Dunstan CR, Boyle WJ, Riggs BL: The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res 2000; 15:2–12Crossref, Medline, Google Scholar
17. Corruble E, Ginestet D, Guelfi JD: Comorbidity of personality disorders and unipolar major depression: a review. J Affect Disord 1996; 37:157–170Crossref, Medline, Google Scholar
18. Rossi A, Marinangeli MG, Butti G, Scinto A, Di Cicco L, Kalyvoka A, Petruzzi C: Personality disorders in bipolar and depressive disorders. J Affect Disord 2001; 65:3–8Crossref, Medline, Google Scholar
19. Genant HK: Universal standardization for dual x-ray absorptiometry: patient and phantom cross-calibration results. J Bone Miner Res 1995; 10:997–998Crossref, Medline, Google Scholar
20. WHO Study Group: Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organ Tech Rep Ser 1994; 843:1–129Medline, Google Scholar
21. Ravn P, Fledelius C, Rosenquist C, Overgaard K, Christiansen C: High bone turnover is associated with low bone mass in both pre- and postmenopausal women. Bone 1996; 19:291–298Crossref, Medline, Google Scholar
22. Peichl P, Griesmacherb A, Marteau R, Hejc S, Kumpan W, Muller MM, Broll H: Serum crosslaps in comparison to serum osteocalcin and urinary bone resorption markers. Clin Biochem 2001; 34:131–139Crossref, Medline, Google Scholar
23. Hofbauer LC, Neubauer A, Heufelder AE: Receptor activator of nuclear factor-kappaB ligand and osteoprotegerin: potential implications for the pathogenesis and treatment of malignant bone diseases. Cancer 2001; 92:460–470Crossref, Medline, Google Scholar
24. Orr FW, Lee J, Duivenvoorden WCM, Singh G: Pathophysiologic interactions in skeletal metastasis. Cancer 2000; 88(12 suppl):2912–2918Google Scholar
25. Guise TA: Molecular mechanisms of osteolytic bone metastases. Cancer 2000; 88(suppl 1):2892–2898Google Scholar
26. Zheng SX, Vrindts Y, Lopez M, De Groote D, Zangerle PF, Collette J, Franchimont N, Geenen V, Albert A, Reginster JY: Increase in cytokine production (IL-1 beta, IL-6, TNF-alpha but not IFN-gamma, GM-CSF or LIF) by stimulated whole blood cells in postmenopausal osteoporosis. Maturitas 1997; 26:63–71Crossref, Medline, Google Scholar
27. Wennberg P, Nordstrom P, Lorentzon R, Lerner UH, Lorentzon M: TNF-alpha gene polymorphism and plasma TNF-alpha levels are related to lumbar spine bone area in healthy female Caucasian adolescents. Eur J Endocrinol 2002; 146:629–634Crossref, Medline, Google Scholar
28. Tuglu C, Kara SH, Caliyurt O, Vardar E, Abay E: Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology (Berl) 2003; 170:429–433Crossref, Medline, Google Scholar
29. Haack M, Hinze-Selch D, Fenzel T, Kraus T, Kühn M, Schuld A, Pollmacher T: Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: effects of confounding factors and diagnosis. J Psychiatr Res 1999; 33:407–418Crossref, Medline, Google Scholar
30. Hinze-Selch D, Schuld A, Kraus T, Kuhn M, Uhr M, Haack M, Pollmacher T: Effects of antidepressants on weight and on the plasma levels of leptin, TNF-alpha and soluble TNF receptors: a longitudinal study in patients treated with amitriptyline or paroxetine. Neuropsychopharmacology 2000; 23:13–19Crossref, Medline, Google Scholar
31. Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK: Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol 1999; 15:287–291Crossref, Google Scholar



