Association of Estrogen Levels With Neuropsychological Performance in Women With Schizophrenia
Abstract
OBJECTIVE: This study sought to determine the relationship of estrogen levels with psychiatric symptoms and neuropsychological function in female patients with schizophrenia. METHOD: Psychiatric symptoms were assessed and average estrogen and progesterone levels from four consecutive weekly blood samples were measured in 22 female inpatients with schizophrenia who were also administered a neuropsychological battery. RESULTS: There were strong positive correlations between average estrogen level and cognitive function, especially measures of global cognitive function, verbal and spatial declarative memory, and perceptual-motor speed. Correlations of hormone levels with psychiatric symptoms were nonsignificant. CONCLUSIONS: Higher estrogen levels in female patients with schizophrenia are associated with better cognitive ability. These results may have implications for potential treatment of cognitive dysfunction with adjunctive estrogen in female patients with schizophrenia.
Sex differences in terms of age at onset, symptom expression, and course of illness have been consistently demonstrated in patients with schizophrenia (1, 2). Female patients have a later age at onset (3), better treatment response (up to menopause) (4), and more affective and paranoid symptoms and fewer negative symptoms than male patients (5–8). Findings are inconsistent as to whether there are sex differences in neuropsychological function in schizophrenia (9). Some studies have found that male schizophrenic patients perform worse than female patients on measures of cognitive function (9, 10). Others have found that female patients are more impaired than male patients (11, 12) or that there are no differences (13–15). We previously found that male inpatients were more impaired than female inpatients in one cognitive domain (spatial memory), but these differences were eliminated after differences in symptom severity were controlled (16).
Exacerbations of symptoms during premenstrual and postpartum periods (17, 18) and the need for higher medication doses (19) in postmenopausal patients suggest an important role of the sex hormone estrogen in the modulation of psychopathology in schizophrenia. Antidopaminergic properties of estrogen have been shown in animal studies in which estrogen reduced dopamine concentrations (20, 21) and dopamine D2 receptor sensitivity in the brain (22). Estrogen treatment also increases the density of dendritic spines on pyramidal neurons specific to the CA1 region of the rat hippocampus (23).
In normal subjects, there is evidence that estrogen levels relate to cognition throughout the menstrual cycle, with high levels of estrogen at the mid-luteal point associated with better verbal memory and diminished spatial ability (24). In surgically menopausal women, those given estrogen for 2 months after surgery maintained or improved their performance on measures of verbal learning and memory, whereas those given placebo stayed the same or deteriorated on these measures relative to their presurgery baseline (25). Estrogen appeared to have a selective effect on verbal declarative memory, since other measures were unchanged in either group. In normal postmenopausal women, those receiving estrogen performed better on measures of verbal memory (26), proper-name recall (27), and measures of problem solving and psychomotor speed (28) than did those not receiving estrogen. Female patients with Alzheimer’s disease who were treated with estrogen replacement showed improvements on measures of general cognitive ability and confrontation naming (29–31).
To our knowledge, Kulkarni et al. have conducted the only published study of the effects of estrogen augmentation on symptoms in patients with psychotic disorders (32). In this open-label study, 11 women with acute psychotic illnesses were given ethynylestradiol for 8 weeks and were compared with seven women who did not receive estrogen. The adjunctive estrogen group showed a rapid decrease in positive and overall psychiatric symptoms by day 5 compared to the neuroleptic-only group. By day 45, both groups were equally symptomatic. The authors concluded that estrogen augmentation may be effective in the rapid reduction of positive symptoms because of its antidopaminergic properties.
Before carrying out larger studies on the effects of estrogen in psychotic patients, it is important to understand the existing relationship of estrogen to cognitive function and symptoms in patients with schizophrenia. To that end, we examined the relationship of gonadal hormone levels to symptoms and neurocognitive function in 22 women with schizophrenia. We hypothesized that higher levels of estrogen would be associated with better cognitive function (particularly better verbal memory) and less severity of illness in women with schizophrenia. In addition, we conducted exploratory analyses of the relationship of progesterone levels to cognitive ability.
Method
Subjects were 22 female inpatients at Napa State Hospital who met criteria for DSM-III-R chronic schizophrenia. Patients or their legal guardians gave written informed consent for a study of sex differences in neuropsychological function and brain structure. These results have been published elsewhere (16, 33). Approximately 100 charts were screened over a 2-year period and reviewed for exclusionary criteria (acute medical illness, HIV-positive status, seizure disorder, psychosurgery, prior loss of consciousness greater than 30 minutes, intravenous amphetamine abuse, inhalant use, substance abuse within last 30 days). Consenting patients were interviewed by a psychiatrist or psychologist with the Structured Clinical Interview for DSM-III-R (34). Consensus diagnoses were reached by a psychiatrist and two psychologists after review of the diagnostic interview. Demographic and clinical characteristics are shown in Table 1.
Patients were administered a comprehensive neuropsychological test battery by a trained psychologist. This battery has been described elsewhere (36, 37). Because neuropsychological tests yield a large number of dependent measures, internally consistent summary scales were constructed (coefficient alphas ranged from 0.67 to 0.87). These scales were created by converting raw scores to z scores (mean=0, SD=1) so that different measures could be combined. A global cognitive scale was created by using the average z score of the six cognitive domain summary scales. The use of summary scales enhances measurement reliability and reduces type I error by substantially reducing the number of statistical tests needed.
Psychiatric symptoms were assessed by two raters during the week of neuropsychological testing with the 18-item Brief Psychiatric Rating Scale (38). The negative symptom scale score was the average of the blunted affect, emotional withdrawal, and motor retardation items. The positive symptom scale score was the average of the hallucinatory behavior, unusual thought content, and conceptual disorganization items. The anxious-depression scale score was the average of the anxiety, guilt feelings, and depressed mood items (39, 40). The interrater reliability at our center (determined by using intraclass correlations) for the positive, negative, and anxious-depression subscale scores was high (0.89, 0.81, and 0.82, respectively).
Estrogen and Progesterone Measurement
Blood samples were obtained weekly for 4 consecutive weeks starting during the first week of neuropsychological testing. Testing and symptom ratings were conducted without knowledge of serum estrogen and progesterone levels.
Menstrual history, determined from the medical record and patient interview, included dates of last menstrual period and age of first menstruation. Because patient self-report was frequently unreliable, it was difficult to determine where patients were in their cycles. Consequently, we used an average of the four weekly blood draws to get an assessment of overall estrogen and progesterone levels. In addition, neuropsychological testing frequently occurred over a several-day period because of scheduling issues. Thus, it was difficult to select the blood level that was closest in time to the testing. To offset our inability to precisely determine the day of menstrual cycle at the time of the blood draw, we also assessed the association of cognitive function with the highest estrogen level as well as with a measure of hormone level variability (standard deviation) across the 4 weeks.
The highest estrogen level obtained over the four weekly blood draws is shown for each subject in Figure 1. The first half (approximately 14 days) of the menstrual cycle is the follicular phase. This phase begins with menstruation and ends with the pre-ovulatory peak (approximately day 13), when estrogen levels are highest, and with ovulation (day 14). The next 14 days are the luteal phase, during which progesterone levels slowly rise to peak at the midway point (mid-luteal) followed by a second, slightly lower peak of estrogen relative to the pre-ovulatory peak (41). The preovulatory and postovulatory lower limits of values for normal women (90 pmol/liter and 550 pmol/liter, respectively) were taken from laboratory values of normal women reported in the study of Riecher-Rossler et al. (42). Similar to data from 32 female patients with schizophrenia in the Riecher-Rossler et al. study (42), our patients had abnormally low estrogen levels compared to normative data. Only one patient (number 14) showed a value over 550 pmol/liter, which would indicate normal follicular maturation with ovulation.
Of the 22 subjects, three were taking oral contraceptives, seven were receiving estrogen-replacement therapy, and 12 were receiving neither. For subjects receiving neither oral contraceptives nor estrogen-replacement therapy, estrogen and progesterone levels were evaluated by an expert in reproductive endocrinology to determine whether they had an ovulatory cycle. These expert clinical ratings were based on the patterns of estrogen and progesterone levels over the four consecutive weekly blood samples. An ovulatory cycle was defined as relatively high estrogen followed by high progesterone with low levels during the other weeks. These classifications were made blind to neuropsychological performances or symptom ratings. Seven of the 12 subjects were considered to have an ovulatory cycle; five were considered nonovulatory.
Data Analysis
Spearman rho correlations were used because some variables were not normally distributed. These correlations are also uninfluenced by outliers. In addition to analyses for the entire group, correlations between average estrogen level and neuropsychological performance were also conducted within two subgroups of patients, those receiving either oral contraceptives or estrogen replacement therapy (N=10) and those not receiving either medication (N=12).
Confirmatory hypothesis testing was performed only on the relationships of average estrogen levels with the neuropsychological summary scales and with symptom subscales. Correlations with individual neuropsychological tests are presented for descriptive purposes. Analysis of correlations with progesterone was considered exploratory.
Results
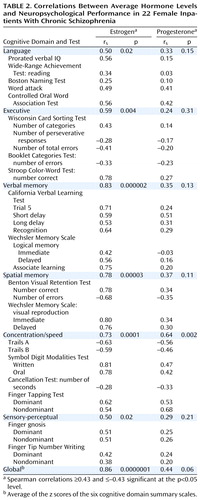
Correlations between average estrogen and progesterone levels and neuropsychological performance are shown in Table 2; correlations for the six cognitive domain summary scales are shaded. As can be seen, average estrogen level was strongly related to global cognitive function (Figure 2). Correlations for each individual scale included in the global scale were also highly significant. Only the concentration/speed scale was associated with higher progesterone levels. Neither estrogen nor progesterone levels were significantly correlated with psychiatric symptoms (Table 3).
Within each of the two patient subgroups (those receiving either oral contraceptives or estrogen replacement therapy and those receiving neither), correlations between average estrogen level and global cognitive function remained essentially the same (rs=0.84 and 0.83, respectively). Correlations of highest level of estrogen during the 4-week period with the six cognitive domain summary scales ranged from 0.44 to 0.76. Correlations between the six cognitive domain summary scales and the measurement of hormone level variability (standard deviation) across the four estrogen measures ranged from 0.15 to 0.62.
Neither patient age (rs=–0.16, p<0.47) nor neuroleptic dose (rs=–0.32, p<0.15) was significantly related to average estrogen level. Thus, medication does not appear to have been a major factor in the diminished hormone levels in these patients.
Discussion
To our knowledge, this is the first study to investigate the association of hormone levels with cognitive function in women with schizophrenia. Declarative memory appears to be most strongly related to estrogen levels, but our results strongly suggest that higher average estrogen levels are associated with better neuropsychological performance in many areas of cognition. Even a conservative Bonferroni correction with alpha set at 0.007 would have resulted in significant correlations between average estrogen level and four of the six cognitive domain summary scales (executive, verbal memory, spatial memory, and concentration/speed) as well as the global cognitive scale.
In normal subjects, there appears to be a more specific relationship of estrogen to verbal memory and verbal fluency (24, 43); however, to our knowledge, there have been no studies of the relationship of estrogen with cognition that have used large batteries of neuropsychological tests in normal women. Additionally, it should be noted that in normal postmenopausal women and in those with Alzheimer’s disease, adjunctive estrogen also appears to improve cognitive processes such as general problem-solving ability, perceptual-motor speed, and naming ability (27–29). Thus, our finding that estrogen levels are associated not only with memory functions but many cognitive functions appears to be consistent with the literature.
Average progesterone level was also positively associated with better functioning on measures of perceptual-motor speed. In investigations of within-subject variation across the menstrual cycle, studies generally have not differentiated between estrogen and progesterone levels (24, 44) in their relationship to cognition. However, both levels of these hormones are high during midcycle, when speeded dexterity and verbal articulatory tasks are performed best. Because both hormones tend to follow a concurrent pattern, it is difficult to differentiate which hormone is most influential on cognitive processes unless direct serum measures are used, as in the current study. As can be seen in Table 2, correlations of estrogen with cognition were generally much stronger than the correlations with progesterone. As noted, the analyses of progesterone with cognition were exploratory and must therefore be considered more tentative than those of estrogen. In contrast to the relationships of hormones to cognitive function, our results suggest that estrogen levels are not strongly associated with psychiatric symptoms in these patients.
In comparing our patients’ highest estrogen level across the four blood samples to the data of Riecher-Rossler et al. (42), we found that our patients had low levels of estrogen compared to ranges seen in healthy comparison women. While neuroleptic medications may increase prolactin levels (45) and presumably reduce estrogen levels, we found no statistically significant relationship of antipsychotic medication dose with serum estradiol level. Riecher-Rossler et al. (42) also found no relationship of medication dose and estrogen level in their cohort of schizophrenia patients. However, one might not expect a strict linear relationship between dose and estrogen given that different antipsychotic medications, regardless of dose, have different effects on prolactin and hormone levels. Future studies should examine the relationship of prolactin levels to estrogen levels as one way of assessing possible medication effects. Also, nonovulatory women would be expected to have lower-than-normal estrogen levels, but low values were present even in the ovulatory subgroup. Thus, it appears that lower-than-normal levels in these women with schizophrenia may be a feature of the illness itself rather than a secondary consequence of nonovulatory status.
Some limitations of the study results should be considered. Because we could not determine exactly where patients were in their cycles at the time of testing and because patients would likely be in different cycle phases, we chose to measure average estrogen and progesterone levels. It could be argued that relationships might be obscured by not looking at the four time points individually. On the other hand, the average of four measurements should give a more reliable and robust measure because of the greater number of measurements. Indeed, the strength of the associations that we found between average estrogen levels and cognition in female patients with schizophrenia argues strongly against any relationship having been obscured.
In addition, we computed correlations between the patients’ highest estrogen level across all four blood samples and measures of neuropsychological function. These positive correlations were also significant (rs=0.76, p<0.00004 for the global scale). However, the correlation of 0.86 for the average of the four estrogen measures suggests that the average measure is somewhat more robust than the individual highest level. We also calculated standard deviations of the four estrogen levels for each subject to get a measure of variability of estrogen levels over the month. In this case, correlations were moderately strong, indicating that greater variability of estrogen during the cycle was associated with better cognitive function (rs=0.55, p<0.008). Greater variability could be associated with ovulatory versus nonovulatory status. Within the two groups of subjects not receiving adjunctive hormones, seven were ovulatory and five were nonovulatory. Although these groups are too small for statistical testing, relationships between average estrogen level and global neuropsychological function were again virtually identical (rs=0.61 for ovulatory; rs=0.60 for nonovulatory). Thus, it appears that between-subject differences in overall estrogen level account for more variance than within-subject variation of estrogen.
These findings strongly suggest that average estrogen level over a 4-week period is a meaningful measure and that overall estrogen levels may be more important for cognition than week-to-week fluctuations. These findings have positive implications for the treatment of schizophrenia patients, since they suggest that raising overall estrogen levels may have a beneficial impact throughout the cycle.
Ten of the 22 patients were receiving oral contraceptives or estrogen-replacement therapy, both of which would affect estrogen levels in women. However, correlations of average estrogen level with neuropsychological performance were virtually identical within both subgroups, suggesting that higher levels of either endogenous or exogenous estrogen were beneficial in their relationship to cognitive functioning.
Our female patients with schizophrenia were a severely ill group of early-onset patients who are not likely to be representative of women with schizophrenia as a whole. Thus, our findings may not be generalizable to less severely ill patients. Future studies should examine less severely ill patients and include a normal comparison group to determine the relationship of estrogen levels and their periodic fluctuation with neuropsychological measures. Second, the relationship of hormone levels (estrogen and testosterone) with cognition in male patients with schizophrenia should be explored. Third, the effects of adjunctive estrogen in female patients with schizophrenia on symptoms and cognition should be investigated in double-blind, placebo-controlled studies. To date, there have been very few treatment studies in schizophrenia patients that have focused on the amelioration of cognitive dysfunction, yet cognitive dysfunction has been strongly related to clinical outcome (46). Further exploration is needed to address the question of the extent to which estrogen is related to global cognitive function or more specifically to memory processes. These data add to a growing body of research suggesting that estrogen has an important role in mental processes in normal women (26) as well as in women with Alzheimer’s disease (47) and other neuropsychiatric diseases such as schizophrenia (2).
 |
 |
 |
Presented in part at the sixth International Congress on Schizophrenia Research, Colorado Springs, Colo., April 12–16, 1997. Received Feb. 24, 2000; revision received Dec. 27, 2000; accepted Jan. 5, 2001. From the University of California, Davis–Napa Psychiatric Research Center, Napa State Hospital. Address reprint requests to Dr. Hoff, UC Davis–Napa Psychiatric Research Center, Napa State Hospital, 2100 Napa-Vallejo Highway, Napa, CA 94558; [email protected] (e-mail). Supported by grants from the National Alliance for Research on Schizophrenia and Depression, the California Department of Mental Health, and NIMH (MH-30854).
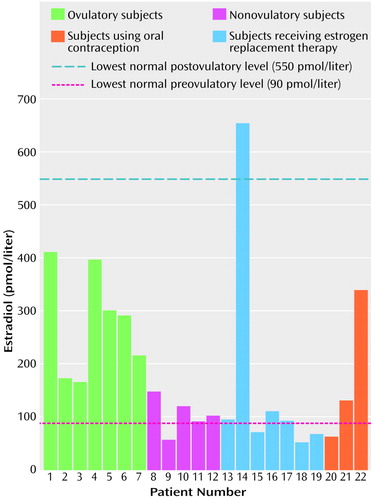
Figure 1. Highest Estrogen Levela of 22 Female Inpatients With Chronic Schizophrenia
aDetermined across four weekly blood samples.
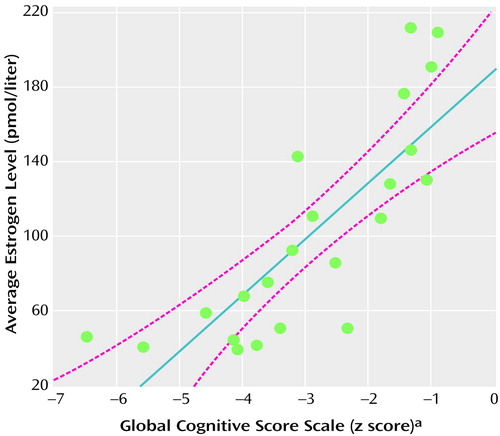
Figure 2. Association of Average Estrogen Level With Global Cognitive Scale Score in 22 Female Inpatients With Chronic Schizophrenia
aAverage of the z scores of the six cognitive domain summary scales.
1. Goldstein JM, Santangelo SL, Simpson JC, Tsuang MT: The role of gender in identifying subtypes of schizophrenia: a latent class analytic approach. Schizophr Bull 1990; 16:263-275Crossref, Medline, Google Scholar
2. Seeman MV: The role of estrogen in schizophrenia. J Psychiatry Neurosci 1996; 21:123-127Medline, Google Scholar
3. Angermeyer MC, Goldstein JM: Gender differences in schizophrenia: rehospitalization and community survival. Psychol Med 1989; 19:365-382Crossref, Medline, Google Scholar
4. Jonsson H, Nyman AK: Predicting long-term outcome in schizophrenia. Acta Psychiatr Scand 1991; 83:342-346Crossref, Medline, Google Scholar
5. Seeman MV: Gender differences in schizophrenia. Can J Psychiatry 1982; 27:107-112Crossref, Medline, Google Scholar
6. Lewine R: Schizophrenia: an amotivational syndrome in men. Can J Psychiatry 1985; 30:316-318Crossref, Medline, Google Scholar
7. Shtasel DL, Gur RE, Gallacher F, Heimberg C, Gur RC: Gender differences in the clinical expression of schizophrenia. Schizophr Res 1992; 7:225-231Crossref, Medline, Google Scholar
8. Goldstein JM: Gender differences in the course of schizophrenia. Am J Psychiatry 1988; 145:684-689Link, Google Scholar
9. Seidman LJ, Goldstein JM, Goodman JM, Koren D, Turner W, Tsuang MT: Sex differences in olfactory identification and Wisconsin Card Sorting performance in schizophrenia: relation to attention and verbal ability. Biol Psychiatry 1997; 42:104-115Crossref, Medline, Google Scholar
10. Goldstein JM, Seidman LJ, Goodman JM, Koren D, Lee H, Weintraub S, Tsuang MT: Are there sex differences in neuropsychological functions among patients with schizophrenia? Am J Psychiatry 1998; 155:1358-1364Google Scholar
11. Perlick D, Mattis S, Stastny P, Teresi J: Gender differences in cognition in schizophrenia. Schizophr Res 1992; 8:69-73Crossref, Medline, Google Scholar
12. Lewine RR, Walker EF, Shurett R, Caudle J, Haden C: Sex differences in neuropsychological functioning among schizophrenic patients. Am J Psychiatry 1996; 153:1178-1184Google Scholar
13. Goldberg TE, Gold JM, Torrey EF, Weinberger DR: Lack of sex differences in the neuropsychological performance of patients with schizophrenia. Am J Psychiatry 1995; 152:883-888Link, Google Scholar
14. Andia AM, Zisook S, Heaton RK, Hesselink J, Jernigan T, Kuck J, Moranville J, Braff DL: Gender differences in schizophrenia. J Nerv Ment Dis 1995; 183:522-528Crossref, Medline, Google Scholar
15. Albus M, Hubmann W, Mohr F, Scherer J, Sobizack N, Franz U, Hecht S, Borrmann M, Wahlheim C: Are there gender differences in neuropsychological performance in patients with first-episode schizophrenia? Schizophr Res 1997; 28:39-50Crossref, Medline, Google Scholar
16. Hoff AL, Wieneke M, Faustman WO, Horon R, Sakuma M, Blankfeld H, Espinoza S, DeLisi LE: Sex differences in neuropsychological functioning of first episode and chronically ill schizophrenic patients. Am J Psychiatry 1998; 155:1437-1439Google Scholar
17. Gerada C, Reveley A: Schizophreniform psychosis associated with the menstrual cycle. J Preventative Psychiatry 1988; 1:5-15Google Scholar
18. Kendell RE, Chalmers JC, Platz C: Epidemiology and puerperal psychoses. Br J Psychiatry 1987; 150:662-673Crossref, Medline, Google Scholar
19. Lloyd D, Simpson JC, Tsuang MT: Are there sex differences in the long-term outcome of schizophrenia? J Nerv Ment Dis 1985; 173:643-649Crossref, Medline, Google Scholar
20. Di Paolo T, Bedard F, Bedard PJ: Influence of gonadal steroids on human and monkey cerebrospinal fluid homovanillic acid concentrations. Clin Neuropharmacol 1989; 12:60-66Crossref, Medline, Google Scholar
21. Dupont A, Di Paolo T, Gagne B, Barden N: Effects of chronic estrogen treatment on dopamine concentrations and turnover in discrete brain nuclei of ovariectomized rats. Neurosci Lett 1981; 22:69-74Crossref, Medline, Google Scholar
22. Hafner H, Behrens S, De Vry J, Gattaz WF: An animal model for the effects of estradiol on dopamine-mediated behavior: implications for sex differences in schizophrenia. Psychiatry Res 1991; 38:125-134Crossref, Medline, Google Scholar
23. McEwen BS, Woolley CS: Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp Gerontol 1994; 29:431-436Crossref, Medline, Google Scholar
24. Hampson E: Variations in sex-related cognitive abilities across the menstrual cycle. Brain Cogn 1990; 14:26-43Crossref, Medline, Google Scholar
25. Phillips SM, Sherwin BB: Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology 1992; 17:485-495Crossref, Medline, Google Scholar
26. Kampen DL, Sherwin BB: Estrogen use and verbal memory in healthy postmenopausal women. Obstet Gynecol 1994; 83:979-983Crossref, Medline, Google Scholar
27. Robinson D, Friedman L, Marcus R, Tinklenberg J, Yesavage J: Estrogen replacement therapy and memory in older women. J Am Geriatr Soc 1994; 42:919-922Crossref, Medline, Google Scholar
28. Schmidt R, Fazekas F, Reinhart B, Kapeller P, Fazekas G, Offenbacher H, Eber B, Schumacher M, Freidl W: Estrogen replacement therapy in older women: a neuropsychological and brain MRI study. J Am Geriatr Soc 1996; 44:1307-1313Google Scholar
29. Henderson VW, Paganini-Hill A, Emanuel CK, Dunn ME, Buckwalter G: Estrogen replacement therapy in older women: comparisons between Alzheimer’s disease cases and nondemented control subjects. Arch Neurol 1994; 51:896-900Crossref, Medline, Google Scholar
30. Ohkura T, Isse K, Alazawa K, Hamamoto M, Yaoi Y, Hagino N: Low-dose estrogen replacement therapy for Alzheimer’s disease in women. Menopause 1994; 1:125-130Crossref, Google Scholar
31. Henderson VW, Watt L, Buckwalter JG: Cognitive skills associated with estrogen replacement in women with Alzheimer’s disease. Psychoneuroendocrinology 1996; 21:421-430Crossref, Medline, Google Scholar
32. Kulkarni J, de Castella A, Smith D, Taffe J, Keks N, Copolov D: A clinical trial of the effects of estrogen in acutely psychotic women. Schizophr Res 1996; 20:247-252Crossref, Medline, Google Scholar
33. Lauriello J, Hoff A, Wieneke MH, Blankfeld H, Faustman WO, Rosenbloom M, DeMent S, Sullivan EV, Lim KO, Pfefferbaum A: Similar extent of brain dysmorphology in severely ill women and men with schizophrenia. Am J Psychiatry 1997; 154:819-825Link, Google Scholar
34. Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-III-R—Patient Version (SCID-P). New York, New York State Psychiatric Institute, Biometrics Research, 1987Google Scholar
35. Kaplan HI, Sadock BI (eds): Comprehensive Textbook of Psychiatry, 6th ed. Baltimore, Williams & Wilkins, 1995Google Scholar
36. Hoff AL, Riordan H, O’Donnell DW, Morris L, DeLisi LE: Neuropsychological functioning of first-episode schizophreniform patients. Am J Psychiatry 1992; 149:898-903Link, Google Scholar
37. Hoff AL, Riordan H, O’Donnell D, Stritzke P, Neale C, Boccio A, Anand AK, DeLisi LE: Anomalous lateral sulcus asymmetry and cognitive function in first-episode schizophrenia. Schizophr Bull 1992; 18:257-270Crossref, Medline, Google Scholar
38. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799-812Crossref, Google Scholar
39. Thiemann S, Csernansky JG, Berger PA: Rating scales in research: the case of negative symptoms. Psychiatry Res 1987; 20:47-55Crossref, Medline, Google Scholar
40. Faustman WO: Brief Psychiatric Rating Scale, in The Use of Psychological Testing for Treatment Planning and Outcome Assessment. Edited by Maruish ME. Mahwah, NJ, Lawrence Erlbaum Associates, 1999, pp 791-830Google Scholar
41. Goldfien A, Monroe SC: Ovaries, in Basic and Clinical Endocrinology. Edited by Greenspan FS, Strewler GJ. Stanford, Conn, Appleton & Lange, 1997, pp 434-486Google Scholar
42. Riecher-Rossler A, Hafner H, Stumbaum M, Maurer K, Schmidt R: Can estradiol modulate schizophrenia symptomatology? Schizophr Bull 1994; 20:203-214Crossref, Medline, Google Scholar
43. Sherwin BB: Estrogenic effects on memory in women. Ann NY Acad Sci 1994; 743:213-230Crossref, Medline, Google Scholar
44. Hampson E, Kimura D: Reciprocal effects of hormonal fluctuations on human motor and perceptual-spatial skills. Behav Neurosci 1988; 102:456-459Crossref, Medline, Google Scholar
45. Kleinberg DL, Davis JM, De Coster T, Van Vaelen B, Mrecher M: Prolactin levels and adverse events in patients treated with risperidone. J Clin Psychopharmacol 1999; 19:57-61Crossref, Medline, Google Scholar
46. Green MF: What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153:321-330Link, Google Scholar
47. Paganini-Hill A, Henderson VW: Estrogen deficiency and risk of Alzheimer’s disease in women. Am J Epidemiol 1994; 140:256-261Crossref, Medline, Google Scholar



