Common Pattern of Cortical Pathology in Childhood-Onset and Adult-Onset Schizophrenia as Identified by Proton Magnetic Resonance Spectroscopic Imaging
Abstract
Objective:Multislice proton magnetic resonance spectroscopic imaging (1H-MRSI) permits simultaneous acquisition and mapping of signal intensities of N-acetyl-containing compounds (mainly N-acetylaspartate, NAA), choline-containing compounds (CHO), and creatine plus phosphocreatine (CRE) from multiple whole-brain slices consisting of small single-volume elements. Previous 1H-MRSI studies of adult patients with schizophrenia showed small NAA relative signals in the hippocampal area and in the dorsolateral prefrontal cortex in comparison with healthy subjects. As part of a program to address the pathophysiological continuity between childhood-onset and adult-onset schizophrenia, the authors performed 1H-MRSI of patients with childhood-onset schizophrenia to specifically test whether the hippocampal area and dorsolateral prefrontal cortex show the same abnormalities as seenin adult-onset schizophrenia. Method: A 1.5-T nuclear magnetic resonance machine was used to test 14 patients (mean age, 16.4 years) and 14 comparison subjects. Ratios of areas under the metabolite peaks of the proton spectra were determined (i.e., NAA/CRE, NAA/CHO, CHO/CRE) for multiple cortical and subcortical regions.Results:The patients showed significantly lower NAA/CRE ratios bilaterally in the hippocampal area and the dorsolateral prefrontal cortex than the comparison subjects. There were no significant differences in CHO/CRE or in NAA ratios in any other area sampled. Conclusions:The present study shows that patients with childhood-onset schizophrenia have smaller than normal regional NAA relative signals, suggesting neuronal damage or malfunction in the hippocampal area and dorsolateral prefrontal cortex. These differences were similar in magnitude to those found in patients with adult-onset schizophrenia. The present data extend other evidence of a biological continuum between childhood- and adult-onset schizophrenia. Am J Psychiatry 1998; 155: 1376-1383
Childhood-onset schizophrenia is a rare disease affecting children before puberty; i.e., the first psychotic symptoms appear by age 12 years (1, 2). The pathophysiology of childhood-onset schizophrenia has been investigated in only a few studies, and in general the results support the hypothesis that childhood-onset schizophrenia is in continuity with the adult-onset form of schizophrenia. Clinical studies have shown that the symptoms in childhood-onset schizophrenia are generally similar to and more severe than those seen in adult patients (2). Other studies have suggested similar patterns of autonomic activity and reactivity (3) and smooth-pursuit eye movements (4). Neuropsychological studies (5, 6) have for the most part shown similar deficits in cognitive processing capacity. Moreover, recent studies (7, 8) with magnetic resonance imaging (MRI) have shown abnormalities of the lateral ventricles, the basal ganglia, and the mesial temporal lobe, structures that have been classically involved in adult-onset schizophrenia. All these studies suggest that childhood- and adult-onset schizophrenia may share some pathophysiological features and that the earlier onset in childhood-onset schizophrenia is another indication of the fact that this disorder may be a more severe form of the same disease.
Proton magnetic resonance spectroscopy (1H-MRS) allows in vivo measurement of some aspects of brain biochemistry.1H-MRS detects signals arising from N-acetyl-containing moieties, mainly N-acetylaspartate (NAA). NAA is located within neurons (9), it increases during development (10), and it has been shown to be an intraneuronal marker sensitive to a number of pathological processes affecting the integrity of neurons (11-14). At relatively long echo time, 1H-MRS also detects signals arising from choline-containing compounds (CHO) (15, 16) and creatine plus phosphocreatine (CRE). Several previous single-voxel 1H-MRS studies of patients with schizophrenia have shown low NAA signal intensity or concentration in the mesial temporal lobes (17, 18), in the region of the hippocampus (19, 20), and in the frontal lobe (21), but not without controversy (21-24). 1H-MRS has evolved to 1H-MRS imaging (1H-MRSI), a mapping approach that permits acquisition of signals arising from a large number of small single-volume elements (25). Using this technique, we and others have previously shown low levels of NAA measures in the hippocampal area and the dorsolateral prefrontal cortex of patients with adult-onset schizophrenia (26-29). These findings were independent of drug treatment and were consistent with developmental neuronal pathology (28, 30).
The purpose of the present study of patients with childhood-onset schizophrenia was to investigate whether the hippocampal area and dorsolateral prefrontal cortex show the same neurochemical pattern seen in adult patients with schizophrenia and, if so, the relative extent of the abnormalities.
METHOD
Subjects
We studied 14 patients with a diagnosis of childhood-onset schizophrenia according to DSM-III-R criteria; their mean age was 16.4 years (SD=1.7), and 11 of the 14 were boys. After the procedure had been fully explained, written informed consent was obtained from the parents and the children before scanning. Recruitment and diagnostic assessment have been presented in detail elsewhere (8). Briefly, the diagnoses were based on interviews of the children and parents that included portions of the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiological Version (31) and of the Diagnostic Interview for Children and Adolescents, DSM-III-R version (32). All patients were taking neuroleptics at the time of the scan. The comparison group consisted of 14 healthy volunteers matched for sex, age (mean age=16.1 years, SD=2.1), and parental socioeconomic status (p>0.1) (33). They had been recruited both from the community and from the normal volunteer office of the National Institutes of Health (NIH). Exclusion criteria included a history of significant head injury, alcohol or drug abuse, and serious medical/neurological illness.
MRSI Procedure
Multiple-slice 1H-MRSI was performed on a conventional GE Signa 1.5-T MRI system (GE Medical Systems, Milwaukee) equipped with self-shielded gradients, according to the method of Duyn et al. (25) as modified by us (26-28). The procedure used in the present study was identical to that described in our earlier reports (26-28). Briefly, the standard quadrature head coil was used in all cases. After a sagittal localizer (fast spin echo, TR=3500 msec, TE=102 msec), a set of T1-weighted 3-mm-thick oblique axial images (spin echo, TR=500 msec, TE=12 msec) was acquired in a plane parallel to the angle of the sylvian fissure. From this set of images (which maximizes the cross-sectional profile of the hippocampus) the volume of brain to be included in the MRSI slices was chosen. Phase-encoding procedures were used to obtain a 32×32 array of spectra from volume elements in each selected slice. Each volume element (“voxel”) had nominal dimensions of 7.5 mm × 7.5 mm × 15.0 mm (0.84 ml). Actual volume, based on full width at half maximum (FWHM) after filtering of k-space, was 1.4 ml (25). The filter was a radial cosine filter starting at half-maximum radius. The 1H-MRSI sequence involves a spin echo slice selection with TR of 2200 msec and TE of 272 msec and includes suppression of water and most of the signal arising from lipids in skull marrow and in surface tissues. To suppress lipid signals from the skull and scalp, the 1H-MRSI sequence included an outer-volume saturation pulse.
After acquiring the spectroscopic data, we obtained T1-weighted images again at the same location to visually inspect for subject movement. Any visually apparent shift in the location of the hippocampal area from one scan to the next was considered evidence for movement and was grounds for exclusion from the study. After the second set of T1-weighted images, a three-dimensional MRI data set was acquired as 124 conventional sagittal slices (spoiled gradient recalled acquisition in the steady state, TR=24 msec, TE=5 msec). It was not possible to acquire the last set of images for two patients and for one normal subject.
The raw 1H-MRSI data were processed on Unix workstations (Sun Microsystems, Mountain View, Calif.) by using software developed in-house. First, the locations of NAA, CHO, and CRE peaks were automatically determined for all voxels. Cosine filters were used for apodization of the free induction decay to minimize bleed before Fourier transformation. Voxels in which these metabolite signals could not be identified (e.g., voxels located outside the head and on or near the skull’s surface) were then manually nulled. The signal strength in a range of 0.2 ppm (0.1 ppm on each side of the center of the peak) around the NAA, CHO, and CRE signal positions was integrated to produce four 32×32 arrays of metabolite signals. Metabolite signals are reported as ratios of the area under each peak: NAA/CRE, NAA/CHO, and CHO/CRE.
A rater blind to diagnosis manually drew regions of interest on the T1-weighted coaxial MR images and then transferred them to the same location on the metabolite maps. Regions of interest were drawn with reference to standard anatomical atlases (34), 35) bilaterally in the hippocampal area, the dorsolateral prefrontal cortex, superior temporal gyrus, orbitofrontal cortex, occipital cortex, anterior and posterior cingulate, centrum semiovale, prefrontal white matter, thalamus, and putamen as previously described (26). The average number of voxels per region of interest was similar to that reported in a previous study (26). Voxels that were contained within the anatomical region of interest but not present on the metabolite maps were removed. Thus, the final calculations were performed only on voxels containing 1H spectra. The program computed the average value of the area under each peak in all voxels within the regions of interest on the metabolite maps. To assess the reliability of these measurements, another rater blind to diagnosis independently drew the same regions of interest in 10 randomly selected cases (intraclass correlation coefficient [ICC] for all regions of interest and for all metabolite ratios, 0.94).
Morphometry
Sagittal volume scans were also resliced coronally (as 1.87-mm-thick sections) by means of public domain NIH “Image” 1.57 software on a Macintosh computer, which was then used to manually outline the hippocampal region and the prefrontal lobe areas by a rater blind to the subjects’ diagnosis (26, 28). The hippocampal area was outlined in the slice containing the mammilary bodies, in the three rostral to them, and in the 10 caudal to them. The prefrontal lobe areas were outlined in each slice rostral to the genu of the corpus callosum. All measurements were executed bilaterally and converted into cubic centimeters. To evaluate the reliability of measurements, a second rater, also blind to the subjects’ diagnosis, measured the hippocampal region and prefrontal lobe areas in the same manner for seven subjects randomly selected (ICC for left and right hippocampal region volumes, 0.81; for left and right prefrontal lobe, 0.96). The volumetric measurements were here performed for correlation with the 1H-MRSI data. The volumetric results from six of these patients have already been presented elsewhere (7).
Clinical Measures
A physician also rated each patient for positive and negative symptoms by means of the Scale for the Assessment of Positive Symptoms (36), Scale for the Assessment of Negative Symptoms (37), and Brief Psychiatric Rating Scale (38).
Statistical Analysis
Differences between the patients and the comparison group were tested separately for each metabolite ratio and for each region of interest by a two-way repeated measures analysis of variance (ANOVA), with hemisphere (left or right) as the within-group factor and diagnosis (patients or comparison group) as the between-groups factor. Post hoc analysis was performed by the Tukey honestly significant difference test. Correlations between metabolite ratios and volumetric and clinical measures—including age, age at onset of symptoms (as defined by clinical history), length of illness, and symptom ratings—were performed by the Spearman test.
RESULTS
Figure 1 shows metabolite maps of NAA, CHO, and CRE signal intensities with the coaxial MRIs for a patient and representative spectra from the hippocampal area and the dorsolateral prefrontal cortex. The color images are scaled to the highest value of each metabolite signal intensity for each 1H-MRSI slice, so that the pattern of regional distribution of metabolite signal intensities within the same slice can be compared between subjects, although color intensity from the same anatomical location cannot be compared between subjects.
In the hippocampal area (figures 2, 3, and 4), ANOVAs showed a significant effect of diagnosis for NAA/CRE (F=3.9, df=1, 26, p<0.05) and no effect for NAA/CHO (F=1.3, df=1, 26, p>0.10) and CHO/CRE (F=0.23, df=1, 26, p>0.50). Post hoc analysis showed that the patients had significantly lower bilateral NAA/CRE ratios (p<0.05). No main effect of hemisphere or interaction of diagnosis and hemisphere was found for any metabolite ratio.
In the dorsolateral prefrontal cortex (figures 2–4), ANOVAs showed a significant effect of diagnosis for NAA/CRE (F=7.7, df=1, 26, p<0.009), an effect for NAA/CHO (F=3.6, df=1, 26, p<0.06), and no effect for CHO/CRE (F=0.7, df=1, 26, p>0.40). Post hoc analysis showed that the patients had significantly lower bilateral NAA/CRE ratios (p<0.04) and lower bilateral NAA/CHO ratios (p<0.06). No main effect of hemisphere or interaction of diagnosis and hemisphere was found for any metabolite ratio. No main effect or interaction was found in any other region of interest for any metabolite ratio. ANOVA revealed sporadic effects of hemisphere and no interaction of diagnosis and hemisphere. None of the sporadic results, which were not based on a priori hypotheses or previous results, survived an appropriate Bonferroni correction for the number of regions, even at the trend level.
The effect sizes of the NAA/CRE differences were 0.6 for the hippocampal area and 1.3 for the dorsolateral prefrontal cortex. These are comparable to what we previously found (26) in patients with adult-onset schizophrenia (0.5 and 1.3, respectively).
To evaluate whether the lower ratios seen in the patients are due to differences in the numerator or denominator terms, the mean integrated areas of NAA, CHO, and CRE resonances were normalized to the corresponding mean integrated areas in the centrum semiovale (i.e., hippocampal NAA divided by centrum semiovale NAA, hippocampal CHO divided by centrum semiovale CHO, and so forth). We used the centrum semiovale as a reference because in a previous study (27) its metabolite ratios showed a low coefficient of variation among several other regions of interest and because it is probably not involved in the primary pathophysiological process of schizophrenia. While low normalized NAA ratios were found bilaterally in the hippocampal area (ANOVA: F=3.5, df=1, 26, p<0.06) and in the dorsolateral prefrontal cortex (ANOVA: F=2.8, df=1, 26, p<0.10), no differences were found for CHO or CRE, further suggesting that the ratio differences between the patients and normal subjects are due to differences in NAA.
No significant correlation was found between the volume of the hippocampal area and the prefrontal lobe and the ratio measures in the hippocampal area and dorsolateral prefrontal cortex (all r values were between –0.14 and 0.32). Spearman tests did not show any significant correlation between NAA ratios in the hippocampal area and dorsolateral prefrontal cortex and the different items of the various rating scales used. A few non-hypothesis-driven correlations emerged, but they were not significant after Bonferroni correction for the number of comparisons. No correlation was found between any clinical variable (age, age at onset of the disease, current neuroleptic dose, years of treatment with neuroleptic drugs, and length of illness) and MRSI measures in the hippocampal area and the dorsolateral prefrontal cortex (all r values were between –0.33 and 0.40).
DISCUSSION
The present study shows that patients with childhood-onset schizophrenia show lower NAA measures in the hippocampal area and the dorsolateral prefrontal cortex suggestive of neuronal involvement exclusively in these two areas. No correlation was found between NAA measures in the hippocampal area and dorsolateral prefrontal cortex and the volume of these structures as measured with MRI, suggesting that they are not related to volumetric losses; this finding is also similar to what we have observed in patients with adult onsets. Moreover, length of illness did not predict NAA levels, suggesting that the NAA differences are not related to progressive pathology. These results are consistent both in the extent and the location of the damage with earlier findings from patients with adult-onset schizophrenia that showed low NAA values in mesial temporal-limbic and prefrontal cortices (17–(20), (26-29), although some controversy about these findings persists (21-24). Overall, the present study adds more emphasis to the thesis of a common pathophysiological process specifically affecting mesial temporal-limbic and prefrontal circuitries in patients with childhood-onset and adult-onset schizophrenia.
The involvement of the hippocampal area and the dorsolateral prefrontal cortex in the pathophysiology of schizophrenia has long been posited (39). Postmortem studies (40-44) have shown anatomical abnormalities consistent with developmental pathology, particularly in the hippocampal region and the prefrontal cortex. Structural studies with MRI have produced results consistent with those from postmortem analyses, showing most often bilaterally lower volumes of the hippocampal region and of the frontal lobe (45-50), although negative reports have also appeared (51). Functional neuroimaging studies have also implicated the dorsolateral prefrontal cortex and its connections with the hippocampal area in the failure of patients with schizophrenia to produce as robust an activation during working memory tasks as seen in healthy subjects (52). Moreover, previous postmortem, 1H-MRS, and 1H-MRSI studies have shown low NAA values in the mesial temporal lobes (17, 18), the hippocampus/amygdala area (19, 26–28, 53, and the prefrontal cortex (21, 26–29, 53) in patients with schizophrenia. While no postmortem study of childhood-onset schizophrenia is available yet, structural imaging studies have shown cerebral abnormalities that are consistent with those seen in adult-onset schizophrenia. The few MRI studies that have assessed the morphology of brain regions specifically in patients with childhood-onset schizophrenia have shown among other findings a lack of the normal right-greater-than-left asymmetry of the hippocampus and a nonsignificantly lower volume of the prefrontal lobe (7). A study with [18F]fluorodeoxyglucose and positron emission tomography (54) showed lower glucose metabolism in anterior frontal regions of patients with childhood-onset schizophrenia than in normal subjects. In another preliminary 1H-MRS study of children with schizophrenia (55), NAA in the frontal gray matter was significantly lower than normal.
Although our results are consistent with those from previous imaging research in childhood-onset schizophrenia in revealing the anatomy of the circuitry involved in this disorder, some inconsistencies emerge. A recent longitudinal MRI investigation (8) showed disproportionate progressive changes over 2 years in children with childhood-onset schizophrenia, even an average of 4 years after illness onset. This series of MRI studies has shown progressive reduction in the volumes of temporal and mesial temporal structures (including the hippocampus), of the midsagittal thalamic area, and, indeed, of the whole brain, together with a progressive increase in the size of the ventricles. The large changes seen in these structures of patients with childhood-onset schizophrenia prompted the interpretation that these subjects were in a developmental period particularly sensitive to pathological effects (8). It was also suggested that this period of development may have been unique in revealing ventricular enlargement that would afterward remain stable (8). This speculation notwithstanding, the present data, obtained from a similarly aged group that contained patients from those earlier structural studies, show that NAA abnormalities are of similar magnitude to those seen in adult-onset schizophrenia and support the thesis of static pathology affecting neuronal integrity of the hippocampal area and the dorsolateral prefrontal cortex. It should be noted that the lack of a correlation between NAA measures in the hippocampal area and dorsolateral prefrontal cortex and their respective volumes may be an artifact of our relatively small number of subjects, but this negative result was also found in our earlier studies of other study groups (26, 27). Moreover, we also did not find any correlation between NAA measures and length of illness, which would be expected if the pathology were progressive. In this respect it is interesting that, as in our previous studies of patients with adult-onset schizophrenia (26-28), we also did not find any abnormality related to CHO signals. CHO signals can be influenced by several pathological, pharmacological (56), and physiological processes, including diet (57). Aware of the limitations associated with the interpretation of the CHO signal, we have previously noted that low NAA values and high CHO values (attributed to gliosis) can be found in neurodegenerative diseases (58, 59). The current finding of low NAA and normal CHO may imply the presence of neuronal damage unaccompanied by gliosis, such as might occur with a neurodevelopmental defect. Neurodevelopmental pathology would also be consistent with the fact that patients with childhood-onset schizophrenia do not seem to have more profound neuronal damage than patients with adult-onset schizophrenia (as shown by the similar effect sizes). Therefore, it is conceivable that the abnormalities identified in the structural studies (especially those of the hippocampal area) are unrelated to the neuronal compartment associated with NAA signals (i.e., neurons and their processes). These data, however, do not provide further insight into the earlier onset of disease in childhood-onset schizophrenia.
No correlation was found in this study between NAA measures in the hippocampal area and dorsolateral prefrontal cortex and the volume of the hippocampal area and prefrontal lobe. The lack of correlation may again reflect the relatively small number of subjects and the partial volume effects inherent in current spectroscopy techniques, i.e., the spectroscopic signal was measured from an area larger than the real volume. Thus, our MRSI of the hippocampal region and dorsolateral prefrontal cortex might have misrepresented the true anatomical signal. Alternatively, the lack of a correlation between volume and metabolites in our data might be consistent with emerging evidence suggesting that NAA reflects more than neuronal density (11–14, 60-64) and is sensitive to neuronal function.
As in earlier studies, we did not find correlations between NAA measures and treatment with neuroleptics. Although lack of a correlation does not exclude the possibility of some effect of neuroleptics on NAA, it is unlikely that the findings are solely explained by treatment with neuroleptics. In fact, we and others have shown that low NAA measures are found also in patients who have received only minimal exposure to neuroleptics (18, 28). Moreover, Okumura et al. (65) performed a study in rats and reported no effect of chlorpromazine on NAA levels. As we reported previously (26), we cannot exclude that the low NAA values are due to T1 or T2 relaxation effects. However, as argued before, such a regionally specific abnormality of NAA is unlikely to arise from such effects. Another limitation of this study is that we did not measure absolute concentrations of the metabolites, thus making the statements about single metabolites not definitive. However, as argued in our previous paper (26), the pattern of ratio differences (low ratio of NAA to CRE, CHO, and NAA in the centrum semiovale together with no difference in the ratio of CHO to CRE or to centrum semiovale CHO or in the ratio of CRE to centrum semiovale CRE) strongly suggests that the ratio abnormalities are due to NAA.
In conclusion, patients with childhood-onset schizophrenia show low NAA measures in the hippocampal area and the dorsolateral prefrontal cortex, suggesting impairment of neuronal integrity in these areas. These results imply a common pattern of cortical pathology in patients with childhood-onset schizophrenia and patients with adult-onset schizophrenia, further indicating a common physiopathology in these disorders.
Received Dec. 1, 1997; revision received March 19, 1998; accepted April 9, 1998. From the Clinical Brain Disorders Branch, Intramural Research Programs, NIMH; the Child Psychiatry Branch, NIMH, Bethesda, Md.; the Neuroimaging Branch, National Institute of Neurological Disorders and Stroke, Rockville, Md.; and the Laboratory of Diagnostic Radiology Research, Office of the Director, NIH, Bethesda, Md.. Address reprint requests to Dr. Weinberger, Clinical Brain Disorders Branch, Intramural Research Programs, NIMH, Neurosciences Center at St. Elizabeths Hospital, 2700 Martin Luther King Jr. Ave., S.E., Washington, DC 20032; [email protected] (e-mail). The authors thank Jay Giedd, M.D., for help in scanning some of the patients and Maksim Shapiro, B.S., for help with data analysis.

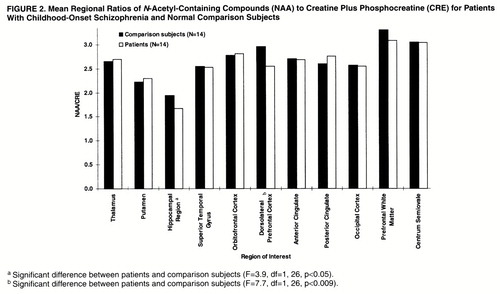
Figure 2. Mean Regional Ratios of N-Acetyl-Containing Compounds (NAA) to Creatine Plus Phosphocreatine (CRE) for Patients With Childhood-Onset Schizophrenia and Normal Comparison Subjects
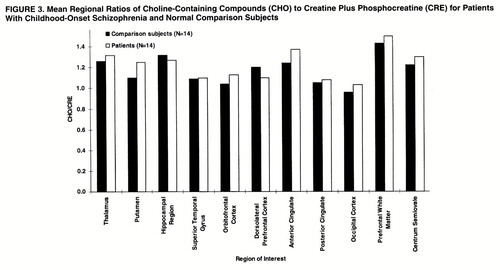
Figure 3. Mean Regional Ratios of Choline-Containing Compounds (CHO) to Creatine Plus Phosphocreatine (CRE) for Patients With Childhood-Onset Schizophrenia and Normal Comparison Subjects.
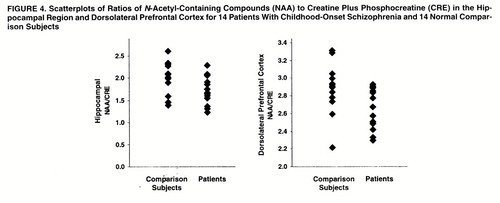
Figure 4. Scatterplots of Ratios of N-Acetyl-Containing Compounds (NAA) to Creatine Plus Phosphocreatine (CRE) in the Hippocampal Region and Dorsolateral Prefrontal Cortex for 14 Patients With Childhood-Onset Schizophrenia and 14 Normal Comparison Subjects.
1. Green WH, Padron-Gayol M, Hardesty AS, Bassiri M: Schizophrenia with childhood onset: a phenomenological study of 38 cases. J Am Acad Child Adolesc Psychiatry 1992; 31:976–986Crossref, Google Scholar
2. Werry JS: Child and adolescent (early onset) schizophrenia: a review in light of DSM-III-R. J Autism Dev Disorder 1992; 22:601–624Crossref, Medline, Google Scholar
3. Zahn TP, Jacobsen LK, Gordon CT, McKenna K, Frazier JA, Rapoport JL: Autonomic nervous system markers of psychopathology in childhood onset schizophrenia. Arch Gen Psychiatry 1997; 54:904–912Crossref, Medline, Google Scholar
4. Jacobsen LK, Hong WL, Hommer DW, Hamburger SD, Castellanos FX, Frazier JA, Giedd JN, Gordon CT, Karp BI, McKenna K, Rapoport JL: Smooth pursuit eye movements in childhood-onset schizophrenia: comparison with attention-deficit hyperactivity disorder and normal controls. Biol Psychiatry 1996; 40:1144–1154Crossref, Medline, Google Scholar
5. Asarnow RF, Sherman T, Strandburg R: The search for the psychobiological substrate of childhood onset schizophrenia. J Am Acad Child Psychiatry 1986; 25:604–614Crossref, Google Scholar
6. Goldberg TE, Gold JM: Neurocognitive deficits in schizophrenia, in Schizophrenia. Edited by Hirsch SR, Weinberger DR. Oxford, England, Blackwell Science, 1995, pp 142–162Google Scholar
7. Frazier JA, Giedd JN, Hamburger SD, Albus KE, Kaysen D, Vaituzis AC, Rajapakse JC, Lenane MC, McKenna K, Jacobsen LK, Gordon CT, Breier A, Rapoport JL: Brain anatomic magnetic resonance imaging in childhood-onset schizophrenia. Arch Gen Psychiatry 1996; 53:617–624Crossref, Medline, Google Scholar
8. Rapoport JL, Giedd J, Kumra S, Jacobsen L, Smith A, Lee P, Nelson J, Hamburger S: Childhood-onset schizophrenia: progressive ventricular change during adolescence. Arch Gen Psychiatry 1997; 54:897–903Crossref, Medline, Google Scholar
9. Urenjak J, Williams SR, Gadian DG, Noble M: Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci 1993; 13:981–989Crossref, Medline, Google Scholar
10. Toft PB, Leth H, Lou HC, Pryds O, Henriksen O: Metabolite concentrations in the developing brain estimated with proton MR spectroscopy. J Magn Reson Imaging 1994; 4:674–680Crossref, Medline, Google Scholar
11. Arnold DL, Matthews PM, Francis G, Antel J: Proton magnetic resonance spectroscopy of human brain in vivo in the evaluation of multiple sclerosis: assessment of the load of disease. Magn Reson Med 1990; 14:154–159Crossref, Medline, Google Scholar
12. De Stefano N, Matthews PM, Arnold DL: Reversible decreases in N-acetylaspartate after acute brain injury. Magn Reson Med 1995; 34:721–727Crossref, Medline, Google Scholar
13. Hugg JW, Kuzniecky RI, Gilliam FG, Morawetz RB, Fraught RE, Hetherington HP: Normalization of contralateral metabolic function following temporal lobectomy demonstrated by 1H magnetic resonance spectroscopic imaging. Ann Neurol 1996; 40:236–239Crossref, Medline, Google Scholar
14. Cheng LL, Ma MJ, Becerra L, Ptak T, Tracey I, Lackner A, Gonzalez RG: Quantitative neuropathology by high resolution magic angle spinning proton magnetic resonance spectroscopy. Proc Natl Acad Sci USA 1997; 94:6408–6413Crossref, Medline, Google Scholar
15. Miller BL: A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed 1991; 4:47–52Crossref, Medline, Google Scholar
16. Barker PB, Breiter SN, Soher BJ, Chatham JC, Forder JR, Samphilipo MA, Magee CA, Anderson JH: Quantitative proton spectroscopy of canine brain: in vivo and in vitro correlations. Magn Reson Med 1994; 32:157–163Crossref, Medline, Google Scholar
17. Yurgelun-Todd DA, Renshaw PF, Gruber SA, Waternaux CM, Cohen BM: Proton magnetic resonance spectroscopy of the temporal lobes in schizophrenics and normal controls. Schizophr Res 1996; 19:55–59Crossref, Medline, Google Scholar
18. Renshaw PF, Yurgelun-Todd DA, Tohen M, Gruber S, Cohen BM: Temporal lobe proton magnetic resonance spectroscopy of patients with first-episode psychosis. Am J Psychiatry 1995; 152:444–446Link, Google Scholar
19. Nasrallah HA, Skinner TE, Schmalbrock P, Robitaille PM: Proton magnetic resonance spectroscopy of the hippocampal formation in schizophrenia: a pilot study. Br J Psychiatry 1994; 165:481–485Crossref, Medline, Google Scholar
20. Maier M, Ron MA, Barker GJ, Tofts PS: Proton magnetic resonance spectroscopy: an in vivo method of estimating hippocampal neuronal depletion in schizophrenia. Psychol Med 1995; 25:1201–1209Crossref, Medline, Google Scholar
21. Buckley PF, Moore C, Long H, Larkin C, Thompson P, Mulvany F, Redmond O, Stack JP, Ennis JT, Waddington JL: 1H Magnetic resonance spectroscopy of the left temporal and frontal lobes in schizophrenia: clinical neurodevelopmental and cognitive correlates. Biol Psychiatry 1994; 36:792–800Crossref, Medline, Google Scholar
22. Fukuzako H, Takeuchi K, Hokazono Y, Fukuzako T, Yamada K, Hashiguchi T, Obo Y, Ueyama K, Takigawa M, Fujimoto T: Proton magnetic resonance spectroscopy of the left medial temporal and frontal lobes in chronic schizophrenia: preliminary report. Psychiatry Res 1995; 61:193–200Crossref, Medline, Google Scholar
23. Stanley JA, Williamson PC, Drost DJ, Rylett RJ, Carr TJ, Malla A, Thompson RT: An in vivo proton magnetic resonance spectroscopy study of schizophrenia patients. Schizophr Bull 1996; 22:597–609Crossref, Medline, Google Scholar
24. Bartha R, Williamson PC, Drost DJ, Malla A, Carr TJ, Cortese L, Canaran G, Rylett RJ, Neufeld RWJ: Measurement of glutamate and glutamine in the medial prefrontal cortex of never treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 1997; 54:959–965Crossref, Medline, Google Scholar
25. Duyn JH, Gillen J, Sobering G, van Zijl PC, Moonen CTW: Multisection proton MR spectroscopic imaging of the brain. Radiology 1993; 188:277–282Crossref, Medline, Google Scholar
26. Bertolino A, Nawroz S, Mattay VS, Barnett AS, Duyn JH, Moonen CTW, Frank JA, Tedeschi G, Weinberger DR: Regionally specific pattern of neurochemical pathology in schizophrenia as assessed by multislice proton magnetic resonance spectroscopic imaging. Am J Psychiatry 1996; 153:1554–1563Link, Google Scholar
27. Bertolino A, Callicott JH, Nawroz S, Mattay VS, Duyn JH, Tedeschi G, Frank JA, Weinberger DR: Reproducibility of proton magnetic resonance spectroscopic imaging in patients with schizophrenia. Neuropsychopharmacology 1998; 18:11–19Crossref, Google Scholar
28. Bertolino A, Callicott JH, Elman I, Mattay VS, Tedeschi G, Frank JA, Breier A, Weinberger DR: Regionally specific neuronal pathology in untreated patients with schizophrenia: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry 1998; 43:641–648Crossref, Medline, Google Scholar
29. Deicken RF, Zhou L, Corwin F, Vinogradov S, Weiner MW: Decreased left frontal lobe N-acetylaspartate in schizophrenia. Am J Psychiatry 1997; 154:688–690Link, Google Scholar
30. Bertolino A, Saunders RC, Mattay VS, Bachevalier J, Frank JA, Weinberger DR: Proton magnetic resonance spectroscopic imaging in monkeys with mesial temporo-limbic lesions. Cereb Cortex 1997; 7:740–748Crossref, Medline, Google Scholar
31. Orvaschel H, Tabrizi MA, Chambers W: Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version, 3rd ed. New York, New York State Psychiatric Institute and Yale University School of Medicine, 1980Google Scholar
32. Reich W, Welner Z: Diagnostic Interview for Children and Adolescents-RC (DSM-III-R Version). St Louis, Washington University, 1988Google Scholar
33. Hollingshead A, Frederick RC: Social stratification and psychiatric disorders. Am Sociological Rev 1958; 18:163–169Crossref, Google Scholar
34. Talaraich J, Tournoux P: A Co-Planar Sterotaxic Atlas of a Human Brain. Stuttgart, Germany, Theime, 1988Google Scholar
35. Duvernoy HM, Cabnis EA: The Human Brain: Surface, Three-Dimensional Sectional Anatomy, and MRI. New York, Springer-Verlag, 1991Google Scholar
36. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1983Google Scholar
37. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
38. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
39. Weinberger DR: Anteromedial temporal-prefrontal connectivity: a functional neuroanatomical system implicated in schizophrenia, in Psychopathology and the Brain. Edited by Carroll BJ, Barrett JE. New York, Raven Press, 1991, pp 25–43Google Scholar
40. Falkai P, Bogerts B: Cell loss in the hippocampus of schizophrenics. Eur Arch Psychiatry Neurol Sci 1986; 236:154–161Crossref, Medline, Google Scholar
41. Jakob H, Beckman H: Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J Neural Transm 1989; 65:303–326Crossref, Google Scholar
42. Jeste DV, Lohr JB: Hippocampal pathologic findings in schizophrenia: a morphometric study. Arch Gen Psychiatry 1989; 46:1019–1024Crossref, Medline, Google Scholar
43. Arnold SE, Hyman BT, Van Hoesen GW, Damasio AR: Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Arch Gen Psychiatry 1991; 48:625–632Crossref, Medline, Google Scholar
44. Selemon LD, Rajkowska G, Goldman-Rakic PS: Abnormally high neuronal density in the schizophrenic cortex: a morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry 1995; 52:805–818Crossref, Medline, Google Scholar
45. Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR: Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med 1990; 322:789–794Crossref, Medline, Google Scholar
46. Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Cellad F: Brain morphology and schizophrenia: a magnetic resonance imaging study of limbic prefrontal cortex and caudate structures. Arch Gen Psychiatry 1992; 49:921–926Crossref, Medline, Google Scholar
47. Bogerts B, Lieberman JA, Ashtari M, Bilder RM, Degreef G, Lerner G, Johns C, Masiar S: Hippocampus-amygdala volumes and psychopathology in chronic schizophrenia. Biol Psychiatry 1993; 33:236–246Crossref, Medline, Google Scholar
48. Marsh L, Suddath R, Higgins N, Weinberger DR: Medial temporal lobe structures in schizophrenia: relationship of size to duration of illness. Schizophr Res 1994; 11:225–238Crossref, Medline, Google Scholar
49. Zipursky RB, Marsh L, Lim KO, DeMent S, Shear PK, Sullivan EV, Murphy GM, Csernansky JG, Pfefferbaum A: Volumetric MRI assessment of temporal lobe structures in schizophrenia. Biol Psychiatry 1994; 35:501–516Crossref, Medline, Google Scholar
50. Andreasen NC, Flashman L, Flaum M, Arndt S, Swayze V, O"Leary DS, Ehrhardt JC, Yuh WT: Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. JAMA 1994; 272:1763–1769Crossref, Medline, Google Scholar
51. Kelsoe JR, Cadet JL, Pickar D, Weinberger DR: Quantitative neuroanatomy in schizophrenia. Arch Gen Psychiatry 1988; 45:533–541Crossref, Medline, Google Scholar
52. Weinberger DR, Berman KF: Prefrontal function in schizophrenia: confounds and controversies. Philos Trans R Soc Lond B Biol Sci 1996; 351:1495–1503Crossref, Medline, Google Scholar
53. Tsai G, Passani LA, Slusher BS, Carter R, Baer L, Kleinman JE, Coyle JT: Abnormal excitatory neurotransmitter metabolism in schizophrenic brains. Arch Gen Psychiatry 1995; 52:829–836Crossref, Medline, Google Scholar
54. Jacobsen LK, Hamburger SD, Van Horn JD, Vaituzis AC, McKenna K, Frazier JA, Gordon CT, Lenane MC, Rapoport JL, Zametkin AJ: Cerebral glucose metabolism in childhood onset schizophrenia. Psychiatry Res 1997; 75:131–144Crossref, Medline, Google Scholar
55. Thomas AM, Ke Y, Caplan R, Levitt J, Azarnow R, Curran J, McCracken J: Frontal lobe 1H-MR spectroscopy of children with schizophrenia. International Society of Magnetic Resonance in Medicine Abstracts 1996; 3:1000Google Scholar
56. Satlin A, Bodick N, Offen WW, Renshaw PF: Brain proton magnetic resonance spectroscopy (1H-MRS) in Alzheimer"s disease: changes after treatment with xanomeline, an M1 selective cholinergic agonist. Am J Psychiatry 1997; 154:1459–1461Link, Google Scholar
57. Stoll A, Renshaw PF, De Micheli E, Wurtmann R, Pillay SS, Cohen B: Choline ingestion increases the resonance of choline-containing compounds in human brain: an in vivo proton magnetic resonance study. Biol Psychiatry 1995; 37:170–174Crossref, Medline, Google Scholar
58. Gadian DG, Connelly A, Duncan JS, Cross JH, Kirkham FJ, Johnson CL, Vargha-Khadem F, Neville BGR, Jackson GD: 1H Magnetic resonance spectroscopy in the investigation of intractable epilepsy. Acta Neurol Scand Suppl 1994; 152:116–121Crossref, Medline, Google Scholar
59. Hetherington H, Kuzniecky R, Pan J, Mason G, Morawetz R, Harris C, Faught E, Vaughan T, Pohost G: Proton nuclear magnetic resonance spectroscopic imaging of human temporal lobe epilepsy at 4.1 T. Ann Neurol 1995; 38:396–404Crossref, Medline, Google Scholar
60. Brenner RE, Munro PM, Williams SC, Bell JD, Barker GJ, Hawkins CP, Landon DN, McDonald WI: The proton NMR spectrum in acute EAE: the significance of the change in the Cho:Cr ratio. Magn Reson Med 1993; 29:737–745Crossref, Medline, Google Scholar
61. De Stefano N, Matthews P, Antel JP, Preul M, Francis G, Arnold DL: Chemical pathology of acute demyelinating lesions and its correlations with disability. Ann Neurol 1995; 38:901–909Crossref, Medline, Google Scholar
62. Vion-Dury J, Salvan AM, Confort-Gouny S, Dhiver C, Cozzone P: Reversal of brain metabolic alterations with zidovudine detected by proton localised magnetic resonance spectroscopy. Lancet 1995; 345:60–61Crossref, Medline, Google Scholar
63. Rango M, Spagnoli D, Tomei G, Bamonti F, Scarlato G, Zetta L: Central nervous system transsynaptic effects of acute axonal injury: a 1H magnetic resonance spectroscopy study. Magn Reson Med 1995; 33:595–600Crossref, Medline, Google Scholar
64. Falconer JC, Liu SJ, Abbe RA, Narayana PA: Time dependence of N-acetyl-aspartate, lactate, and pyruvate concentrations following spinal cord injury. J Neurochem 1996; 66:717–722Crossref, Medline, Google Scholar
65. Okumura N, Otsuki S, Nasu H: The influence of insulin hypoglycaemic coma, repeated electroshocks, and chlorpromazine or beta-phenylisopropylmethylamine administration on the free amino acids in the brain. J Biochem 1959; 46:247–252Crossref, Google Scholar



