Cerebral Phosphate Metabolism in First-Degree Relatives of Patients With Schizophrenia
Abstract
OBJECTIVE: Most phosphorus-31 magnetic resonance spectroscopy (31P-MRS) studies have described measures of lower membrane anabolism or greater catabolism in the frontal lobes of patients with schizophrenia. The purpose of the present study was to evaluate whether these findings can also be detected in young subjects at genetic risk for schizophrenia. METHOD: Fourteen children and siblings of patients with schizophrenia (mean age=16.7 years) and 14 comparison subjects (mean age=16.9 years) were included in a 31P-MRS study of the frontal lobe. RESULTS: The high-risk subjects had significantly lower mean ratios of phosphomonoesters to phosphodiesters (0.25 versus 0.31) and higher mean phosphodiester values (37.59% versus 34.87%) than comparison subjects. CONCLUSIONS: These findings suggest greater phospholipid breakdown even in young first-degree relatives of patients with schizophrenia. This suggestion is discussed with respect to the membrane phospholipid hypothesis of schizophrenia.
Schizophrenia is considered a neurobiological disorder. The examination of relatives of subjects with schizophrenia is one approach in the effort to find the fundamental neurobiological characteristics more directly linked to gene expression. In recent years, a neurobiochemical paradigm of disturbed membrane phospholipid metabolism has been established for schizophrenia (1). Corresponding to this hypothesis, most phosphorus-31 magnetic resonance spectroscopy (31P-MRS) studies have found lower levels of phosphomonoesters (suggesting reduced phospholipid building processes) and higher levels of phosphodiesters (indicating greater phospholipid breakdown) in the frontal lobes of adult patients experiencing their first episode of schizophrenia and neuroleptic-naive adult patients with schizophrenia (2, 3). In contrast, lower phosphodiester levels were observed in chronically ill but neuroleptic-naive patients with schizophrenia (4).
Early-onset schizophrenia has a dismal prognosis (5). Therefore, it is important to detect subjects at risk as early as possible. So far, only a few proton magnetic resonance spectroscopy (1H-MRS) studies in first-degree relatives of patients with schizophrenia (6, 7) and in young subjects with symptoms of schizophrenia spectrum disorders (8, 9) have shown results that can be interpreted as suggesting higher membrane catabolism in several brain regions. This possible vulnerability is a phenomenon of distinct dimensions and triggered by genetic conditions as well as environmental influences. Therefore, we hypothesize that young subjects at genetic risk for schizophrenia have a membrane phospholipid imbalance with higher phosphodiester levels and/or lower phosphomonoester levels.
Method
Fourteen children or siblings of patients hospitalized for a schizophrenic disorder diagnosed according to DSM-III-R criteria and 14 comparison subjects who were matched for age, sex, and diagnosis were examined. The mean age of the first-degree relatives of patients with schizophrenia was 16.7 years (SD=1.9); four were male and 10 were female. The mean age of the comparison subjects was 16.9 years (SD=2.6); four were male and 10 were female.
The psychiatric status of subjects in both groups was determined by using the Schedule for Affective Disorders and Schizophrenia (10) or the Schedule for Affective Disorders and Schizophrenia for School-Age Children (11). Potential comparison subjects with a first- or second-degree relative showing any schizophrenic symptoms were excluded, as were those suffering from an internal or neurological disorder, schizophrenic or affective symptoms, or substance abuse and subjects under dietary restrictions. Two subjects in each group had an adjustment disorder.
The study was approved by the ethical commission of the University of Jena. After a full explanation of the procedure, written informed consent was obtained from the children and their parents.
The 31P-MRS procedure was performed with a 1.5-T magnetic resonance tomograph (Philips Gyroscan ACS II); we used a 31P-quadrature head coil. An image-selected in vivo spectroscopy sequence (12) with double volume-selective adiabatic high-frequency pulses was performed. The standard sequence (repetition time=3000 msec, sampling rate=2 kHz, sampling time=0.5 seconds, number of sampled points=1,024) was used. The regions of interest were placed bilaterally in the frontal region with a mean size of 28 × 28 × 50 mm3 each. Magnetic field homogeneity was shimmed before the measurement over an area containing both acquired regions of interest with respect to the 1H line (full width at half maximum <15 Hz). The examination happened simultaneously in both volumes and was averaged over 768 measurements.
An iterative nonlinear least-square fit in the frequency domain (total line-shape analysis, implemented in Peak Research [PERCH] software [13]) was used to analyze all spectra. The spectra were evaluated independently by two investigators without any knowledge of the proband status (S.R. and S.K.). Interrater reliability for phosphodiester and phosphomonoester values was between 0.86 and 0.90. We used the nonparametric Mann-Whitney U test to determine statistical differences between the groups, focusing our special attention on phosphodiester and phosphomonoester measures.
Results
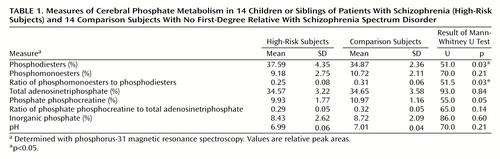
Table 1 presents the 31P-MRS measures for the two regions of interest combined. The total percent of phosphodiesters was significantly higher and the total phosphomonoesters-phosphodiesters ratio was significantly lower in the high-risk group than in the comparison group. The median values were not influenced by outliers, but the spread of phosphodiester values was greater in the high-risk group. The high-energy phosphate phosphocreatine level, ratio of phosphate phosphocreatine to adenosinetriphosphate, inorganic phosphate, and pH values were similar in both groups.
Discussion
The higher phosphodiester levels and lower phosphomonoesters/phosphodiesters ratios could correspond to a greater phospholipid breakdown in the subjects at genetic risk for schizophrenia. This main result is in accordance with the findings in unmedicated, first-episode patients with schizophrenia and in subjects genetically at risk for schizophrenia assessed by 1H-MRS (6, 7). To our knowledge, this is the first study to detect an altered phospholipid membrane metabolism in a young genetic high-risk group. Under the assumption that the higher phosphodiester levels are caused by phospholipid breakdown products (contradictory results were presented in references 14 and 15), the higher phospholipid catabolism may be an intermediate phenotype of schizophrenia. However, this evidence is limited because of the small number of subjects investigated. Obviously, only longitudinal studies would be able to show whether these alterations are subclinical phenomena of the schizophrenic prodromal phase or vulnerability indicators. It is also necessary to determine the specific and the potential relative contribution of these alterations to the schizophrenic disorder.
 |
Received June 8, 2000; revision received Jan. 4, 2001; accepted Jan. 11, 2001. From the Department of Child and Adolescent Psychiatry, Department of Psychiatry, Institute of Diagnostic and Interventional Radiology, University of Jena. Address reprint requests to Dr. Klemm, Department of Child and Adolescent Psychiatry, University of Jena, Philosophenweg 3-5, D-07740 Jena, Germany; [email protected] (e-mail). Supported by Deutsche Forschungsgemeinschaft (DFG) grant BL 435/4-1.
1. Horrobin DF: The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr Res 1998; 30:193–208Crossref, Medline, Google Scholar
2. Pettegrew JW, Keshavan MS, Panchalingam K, Strycho S, Kaplan DB, Tretta MG, Allen M: Alterations in brain high-energy phosphate and membrane metabolism in first-episode, drug-naive schizophrenics. Arch Gen Psychiatry 1991; 48:563–568Crossref, Medline, Google Scholar
3. Stanley JA, Williamson PC, Drost DJ, Carr TJ, Rylett J, Malla A, Thomson RT: An in vivo study of the prefrontal cortex of schizophrenic patients at different stages of illness via phosphorus magnetic resonance spectroscopy. Arch Gen Psychiatry 1995; 52:399–406Crossref, Medline, Google Scholar
4. Volz HP, Rößger G, Riehemann S, Hübner G, Maurer I, Wenda B, Rzanny R, Kaiser WA, Sauer H: Increase of phosphodiesters during neuroleptic treatment of schizophrenics: a longitudinal 31P-magnetic resonance spectroscopic study. Biol Psychiatry 1999; 45:1221–1225Google Scholar
5. Lay B, Blanz B, Hartmann M, Schmidt MH: The psychosocial outcome of adolescent-onset schizophrenia: a 12-year followup. Schizophr Bull 2000; 26:801–816Crossref, Medline, Google Scholar
6. Keshavan MS, Montrose DM, Pierri JN, Dick EL, Rosenberg D, Talagala L, Sweeney JA: Magnetic resonance imaging and spectroscopy in offspring at risk for schizophrenia: preliminary studies. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21:1285–1295Google Scholar
7. Callicott J, Egan M, Bertolino A, Mattay V, Langheim F, Frank J, Weinberger D: Hippocampal N-acetyl aspartate in unaffected siblings of patients with schizophrenia: a possible intermediate neurobiological phenotype. Biol Psychiatry 1998, 44:941–950Google Scholar
8. Bertolino A, Kumra S, Callicott JH, Mattay VS, Lestz RM, Jacobsen L, Barnett IS, Duyn JH, Frank JA, Rapoport JL, Weinberger DR: Common pattern of cortical pathology in childhood-onset and adult-onset schizophrenia as identified by proton magnetic resonance spectroscopic imaging. Am J Psychiatry 1998; 155:1376–1383Google Scholar
9. Brooks WM, Hodde-Vargas J, Vargas LA, Yeo RA, Ford CC, Hendren RL: Frontal lobe of children with schizophrenia spectrum disorders: a proton magnetic resonance spectroscopic study. Biol Psychiatry 1998; 43:263–269Crossref, Medline, Google Scholar
10. Endicott J, Spitzer RL: A diagnostic interview: the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry 1978; 35:837–844Crossref, Medline, Google Scholar
11. Delmo C, Weiffenbach O, Gabriel M, Marchio E, Poustka F: Kiddie-SADS—Present and Lifetime Version (K-SADS-PL): Deutsche Forschungsversion. Frankfurt, Germany, University of Frankfurt/M, 1998Google Scholar
12. Ordige RJ, Connelly A, Lohman JA: Image-selected in vivo spectroscopy: a new technique for spatially selective NMR spectroscopy. J Magn Reson 1986; 66:283–294Google Scholar
13. Laatikainen R, Niemitz M, Malaisse WJ, Biesemans M, Willem R: A computational strategy for the deconvolution of NMR spectra with multiplet structures and constraints: analysis of overlapping 13C-2H multiplets of 13C enriched metabolites from cell suspensions incubated in deuterated media. Magn Reson Med 1996; 36:359–365Crossref, Medline, Google Scholar
14. Potwarka JJ, Drost DJ, Williamson PC, Carr T, Canaran G, Rylett WJ, Neufeld RWJ: A 1H-decoupled 31P chemical shift imaging study of medicated schizophrenic patients and healthy controls. Biol Psychiatry 1999; 45:687–693Crossref, Medline, Google Scholar
15. Blüml S, Tan J, Harris K, Adatia N, Karme A, Sproull T, Ross B: Quantitative proton-decoupled 31P MRS of the schizophrenic brain in vivo. J Comput Assist Tomogr 1999; 23:272–275Crossref, Medline, Google Scholar



