Persistent Dissociation and Its Neural Correlates in Predicting Outcomes After Trauma Exposure
Abstract
Objective:
Dissociation, a disruption or discontinuity in psychological functioning, is often linked with worse psychiatric symptoms; however, the prognostic value of dissociation after trauma is inconsistent. Determining whether trauma-related dissociation is uniquely predictive of later outcomes would enable early identification of at-risk trauma populations. The authors conducted the largest prospective longitudinal biomarker study of persistent dissociation to date to determine its predictive capacity for adverse psychiatric outcomes following acute trauma.
Methods:
All data were part of the Freeze 2 data release from the Advancing Understanding of Recovery After Trauma (AURORA) study. Study participants provided self-report data about persistent derealization (N=1,464), a severe type of dissociation, and completed a functional MRI emotion reactivity task and resting-state scan 2 weeks posttrauma (N=145). Three-month follow-up reports were collected of posttraumatic stress, depression, pain, anxiety symptoms, and functional impairment.
Results:
Derealization was associated with increased ventromedial prefrontal cortex (vmPFC) activation in the emotion reactivity task and decreased resting-state vmPFC connectivity with the cerebellum and orbitofrontal cortex. In separate analyses, brain-based and self-report measures of persistent derealization at 2 weeks predicted worse 3-month posttraumatic stress symptoms, distinct from the effects of childhood maltreatment history and current posttraumatic stress symptoms.
Conclusions:
The findings suggest that persistent derealization is both an early psychological and biological marker of worse later psychiatric outcomes. The neural correlates of trauma-related dissociation may serve as potential targets for treatment engagement to prevent posttraumatic stress disorder. These results underscore dissociation assessment as crucial following trauma exposure to identify at-risk individuals, and they highlight an unmet clinical need for tailored early interventions.
Trauma-related pathological dissociation is a disruption or discontinuity in the typical integration of a person’s psychological functioning in the aftermath of a traumatic event (1). It encompasses a range of symptoms, including derealization—the focus of this study because it is a symptom that is, in particular, tied to impairment and disease severity (2, 3). Derealization is a feeling of detachment from people, places, or objects in one’s environment (4). When experiencing derealization, individuals sometimes report feeling foggy or like they are in a movie or a dream. By providing some psychological distance from the traumatic experience, this type of dissociation may help people cope during experiences of overwhelming threat and in the aftermath of trauma (5).
Unfortunately, persistent dissociative coping responses come at a high cost for both the individual and society, as dissociative symptoms are often linked to a more severe psychiatric course and protracted treatment across disorders (6, 7). Furthermore, dissociation is often associated with worse symptoms of posttraumatic stress, depression, anxiety, and pain and with greater functional impairment (7–10).
Still, evidence for the efficacy of leveraging dissociative symptoms to predict posttrauma psychiatric course is inconsistent. These inconsistencies are likely due to two interwoven issues: the duration of dissociation being measured (peritraumatic vs. persistent), and whether dissociation functions as an independent predictor of posttraumatic stress—that is, independent from variables like trauma history and current psychiatric symptoms. For example, peritraumatic dissociation that occurs during or in the immediate aftermath of a traumatic event predicts ongoing posttrauma stress, anxiety, depression, and pain, but this prediction is often diminished or eliminated when other variables are accounted for (11, 12). Alternatively, a growing body of both cross-sectional and prospective work demonstrates that persistent dissociation that lasts beyond the peritraumatic period is a much better indicator of long-term posttraumatic stress symptom severity compared to peritraumatic dissociation (13). Initial evidence also suggests that persistent dissociation appears to maintain its predictive capacity for posttraumatic stress even when accounting for other variables such as trauma type and current posttraumatic stress disorder (PTSD) symptoms (14). Notably, researchers have not yet tested whether persistent dissociation prospectively predicts posttrauma anxiety, depression, pain, and functional impairment while also accounting for variables such as trauma history and current posttraumatic stress symptoms. Determining whether persistent dissociation is predictive of later outcomes would be enormously beneficial by enabling early identification of at-risk trauma populations and those in need of targeted, early mental health intervention.
Given the potential predictive capacity of self-reported dissociation for various psychiatric outcomes and impairment, its biological correlates may also provide added value in identifying individuals at risk of worse outcomes. However, the biology of trauma-related dissociation has itself received limited attention, and its predictive capacity has not yet been studied. Work that has examined the biology of trauma-related dissociation demonstrates patterns of excessive corticolimbic inhibition for depersonalization/derealization in trauma spectrum disorders, suggesting that this kind of dissociation is a type of emotion and arousal overregulation (15, 16). In emotionally provocative contexts, individuals with high levels of dissociation exhibit hyperactivity in cortical regions related to emotion regulation (e.g., the ventromedial prefrontal cortex [vmPFC]) and hypoactivity in limbic regions related to salience detection and the visceral experience of one’s body (e.g., the amygdala and the insula). Hyperactivity in the medial prefrontal cortex, in particular, demonstrates the most prominent association with depersonalization/derealization symptoms of dissociation (17). Like self-report studies, longitudinal neurobiological studies of dissociation are limited, and none have used the neurobiology of dissociation to predict later outcomes.
To address these critical gaps, we conducted the largest prospective longitudinal biomarker study of dissociation to date focused on a specific type of dissociation: persistent derealization. We focused specifically on derealization because recent evidence suggests that this subtype of dissociation may, in particular, be associated with symptom and impairment severity in trauma spectrum disorders (2, 3). In a sample of adults who recently experienced trauma, we tested the unique predictive capacity of both self-report and neurobiological measures of persistent derealization.
Approximately 2 weeks after the traumatic event, participants reported on symptoms of persistent derealization, and a subset of participants completed a functional MRI scan that included an emotion reactivity task (passive viewing of fearful faces) as well as a resting-state scan. Based on prior data, we expected that derealization would be related to hyperactivity in the vmPFC and hypoactivity in the amygdala and insula while participants were viewing fearful faces, potentially reflecting overmodulation of affective responses. Additionally, given the strength of medial PFC findings with dissociation in previous work, we hypothesized that both self-reported derealization and vmPFC activity during the fearful faces task would predict 3-month follow-up reports of posttraumatic stress, depression, pain, and anxiety symptoms and functional impairment, even when accounting for a history of childhood maltreatment and 2-week levels of posttraumatic stress symptoms.
Methods
Participants
Participants were a convenience sample recruited from 22 emergency departments across the United States as part of the Advancing Understanding of Recovery After Trauma (AURORA) multisite study on adverse posttraumatic psychiatric outcomes (18). To be eligible for the study, participants had to be 18–75 years of age, be fluent in English, be alert and oriented at the time of enrollment, and possess an iOS- or Android-compatible smartphone. Exclusion criteria included self-inflicted injury, occupational injury, prisoner status, pregnancy or breastfeeding, an ongoing domestic violence situation, and more severe physical injury. Exclusion criteria for the MRI session were contraindications to MRI (e.g., metal implants). MRI scans were conducted approximately 2 weeks after the emergency department visit at one of four sites: McLean Hospital, Emory University, Temple University, or Wayne State University. All data reported here were part of the Freeze 2 data release, which includes data collection from September 23, 2016, to July 31, 2019. All participants provided written informed consent. All data were collected in accordance with ethical guidelines pertaining to the use of human participants, and procedures were approved by each data collection site’s institutional review board.
Measures
Participants completed surveys on their own at home or over the telephone with an experimenter’s assistance. We measured history of childhood maltreatment using an abbreviated version of the Childhood Trauma Questionnaire (19) and persistent derealization severity at 2 weeks posttrauma using a two-item abbreviated version of the Brief Dissociative Experiences Scale (20). We measured posttraumatic stress symptom severity at 2 weeks and 3 months posttrauma using the PTSD Checklist for DSM-5 (21). At 3 months posttrauma, we also measured depression and anxiety symptom severity, pain extent, and functional impairment in work, family, and social life using the PROMIS Depression Short Form (22), an abbreviated version of the PROMIS Anxiety Bank (22), the Numeric Pain Rating Scale, and the Sheehan Disability Scale (23), respectively. Higher scores on all measures indicated more severe symptoms or impairment (see the online supplement for further details).
MRI
Detailed information on MRI sequence and task parameters and on preprocessing steps is provided in the online supplement. Briefly, brain scans were conducted approximately 2 weeks after the emergency department visit using Siemens 3-T MRI scanners with an MPRAGE T1-weighted image for structural scans and an EP2d-BOLD sequence for functional scans. The functional scans occurred in the following order: a 9.2-minute resting-state scan, a 4.9-minute emotion reactivity task, a 9.8-minute go/no-go inhibition task (not reported here), and a 9.7-minute monetary reward task (not reported here). The emotion reactivity task was designed to probe reactivity to social threat cues by having participants passively view fearful and neutral facial expressions (24). All data were preprocessed using a standardized pipeline via fMRIPrep, version 1.2.2.
We derived our vmPFC and insula region of interest from previous dissociation-related results in motor vehicle accident samples completing an emotionally provocative task (25). Specifically, we used a 5-mm sphere in the left vmPFC (MNI coordinates, −12, 50, 4) and in the right anterior insula (MNI coordinates, 38, 20, 0). We derived our amygdala region of interest from previous work using the fearful faces task in a traumatized civilian sample (24). Specifically, we used a 5-mm sphere in the right amygdala (MNI coordinates 20, 0, −16). We extracted the average value of the regions of interest from the first-level fearful > neutral face contrast in the emotion reactivity task. We also used the vmPFC region of interest to extract the average value from subject-level resting-state data. We correlated the vmPFC region of interest values with the whole brain voxel time courses for each subject and computed Fisher z-transformations to generate subject-level connectivity maps.
Statistical Analysis
Reported p values are two-tailed. All hypothesis tests and confidence intervals based on linear regression models were calculated using type HC3 robust standard errors in SPSS, version 28, which do not require assumptions of normality or constant variance for validity (26).
Neural correlates of derealization.
To test whether self-reported persistent derealization was associated with region-of-interest activity in the vmPFC, amygdala, and insula during the emotion reactivity task, we conducted three separate linear regressions, controlling for MRI scanner (McLean Hospital, Emory University, Temple University, or Wayne State University), sex assigned at birth (male, female), age, childhood maltreatment history, and 2-week posttraumatic stress symptoms. We applied a Hommel correction for multiple testing (27) using R, version 3.5.3, to account for our choice of three regions of interest with derealization; corresponding p values are designated “corrected” in the Results section. No correction for multiple testing was applied to results for control covariates. To test whether vmPFC connectivity was associated with derealization, we entered subject-level seed-based connectivity maps into a linear model using the 3dMVM program in AFNI, version 20.0.24 (28). Only results that survived correction for multiple comparisons are reported (see the online supplement for further details).
Derealization self-report and 3-month outcomes.
We conducted Pearson correlation analyses to test associations between derealization at 2 weeks and symptom outcomes at 3 months. We then conducted a series of multiple linear regression analyses to test whether derealization self-report data predicted 3-month symptom outcomes when controlling for sex, age, childhood maltreatment history, and 2-week posttraumatic stress symptoms. We applied a Hommel correction for multiple testing (27) to account for our choice of five clinical associations of interest with derealization. No correction for multiple testing was applied to results for control covariates.
vmPFC and 3-month outcomes.
We conducted Pearson correlation analyses to test associations between vmPFC activity and 3-month symptom outcomes. To test whether vmPFC activity during the emotion reactivity task also predicted 3-month outcomes that were found to be significant in the self-report sample, we then conducted a series of linear regressions controlling for MRI scanner, sex, age, childhood maltreatment history, and 2-week posttraumatic stress symptoms. We conducted two separate regressions (one for each significant 3-month outcome in the self-report sample). We applied a Hommel correction for multiple testing (27) to account for our choice of two clinical associations of interest with vmPFC activity. No correction for multiple testing was applied to results for control covariates.
Results
Sample Characteristics
Participants were 1,464 adults who presented in the emergency department within 72 hours after a trauma exposure. A subset of these individuals (N=145) also completed an MRI scan approximately 2 weeks after their emergency department visit. See the online supplement for details on quality control exclusion. Participants’ demographic characteristics and acute trauma exposure types are summarized in Table 1. Approximately 55% of self-report participants (N=798) and 50% of MRI participants (N=72) endorsed some level of persistent derealization at 2 weeks. See the online supplement for details on demographic variables associated with derealization.
| Characteristic | Self-Report Sample (N=1,464) | MRI Sample (N=145) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 35.39 | 12.94 | 34.66 | 12.62 |
| N | % | N | % | |
| Sex assigned at birth | ||||
| Female | 954 | 65.2 | 97 | 66.9 |
| Male | 510 | 34.8 | 48 | 33.1 |
| Race/ethnicity | ||||
| Asian, Hawaiian, American Indian, other | 49 | 3.3 | 6 | 4.1 |
| Black/African American | 765 | 52.3 | 59 | 40.7 |
| Hispanic/Latino | 174 | 11.9 | 26 | 17.9 |
| White | 472 | 32.2 | 54 | 37.2 |
| Missing | 4 | 0.3 | 0 | 0.0 |
| Education | ||||
| Less than high school | 171 | 11.7 | 5 | 3.4 |
| High school diploma/GED | 369 | 25.2 | 37 | 25.5 |
| Some college | 412 | 28.1 | 50 | 34.5 |
| Associate’s degree | 213 | 14.5 | 14 | 9.7 |
| Bachelor’s degree | 192 | 13.1 | 25 | 17.2 |
| Master’s degree | 79 | 5.4 | 11 | 7.6 |
| Professional/doctoral degree | 22 | 1.5 | 2 | 1.3 |
| Missing | 6 | 0.4 | 0 | 0.0 |
| Annual family income | ||||
| <$19,000 | 497 | 33.9 | 37 | 25.5 |
| $19,001–$35,000 | 434 | 29.6 | 43 | 29.7 |
| $35,001–$50,000 | 194 | 13.3 | 25 | 17.2 |
| $50,001–$75,000 | 114 | 7.8 | 13 | 9.0 |
| $75,001–$100,000 | 84 | 5.7 | 10 | 6.9 |
| >$100,000 | 88 | 6.0 | 16 | 11.0 |
| Missing | 53 | 3.6 | 1 | 0.7 |
| Trauma type | ||||
| Motor vehicle collision | 1,138 | 77.7 | 107 | 73.8 |
| Physical assault | 139 | 9.5 | 16 | 11.0 |
| Sexual assault | 12 | 0.8 | 2 | 1.4 |
| Fall, >10 feet | 16 | 1.1 | 1 | 0.7 |
| Fall, <10 feet | 48 | 3.3 | 4 | 2.8 |
| Incident causing traumatic stress exposure to many people | 5 | 0.3 | 1 | 0.7 |
| Nonmotorized collision (e.g., bicycle, skateboard) | 17 | 1.2 | 5 | 3.4 |
| Burns | 7 | 0.5 | 1 | 0.7 |
| Animal-related | 29 | 2.0 | 3 | 2.1 |
| Other | 53 | 3.6 | 5 | 3.4 |
TABLE 1. Demographic characteristics and acute trauma exposure type
Neural Correlates of Persistent Derealization
Emotion reactivity task activity.
A linear regression controlling for MRI scanner, sex, age, childhood maltreatment history, and 2-week posttraumatic stress symptoms revealed that higher levels of derealization were associated with greater vmPFC region of interest activity during fearful > neutral face conditions (B=0.03, SE=0.01, t=2.60, pcorrected=0.030, η2p=0.03) (Figures 1A and 1B). This relationship was robust to MRI scanner effects (see the online supplement). A second and third linear regression tested the same model, but now predicting amygdala and insula region of interest activity, respectively, during fearful > neutral face conditions. The relationship between derealization and amygdala and insula activity in these models was not significant (amygdala: B=−0.01, SE=0.03, t=−0.35, pcorrected=0.730, η2p=0.00; insula: B=−0.01, SE=0.02, t=0.36, pcorrected=0.730, η2p=0.00). Finally, we conducted an exploratory whole-brain voxelwise correlation analysis with persistent derealization in the fearful > neutral contrast controlling for the same covariates as those in the region-of-interest analyses; however, no activity survived correction for multiple comparisons.
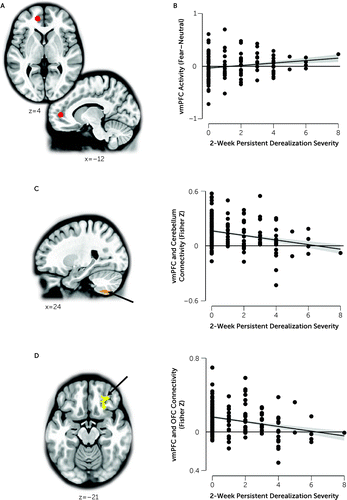
FIGURE 1. Neural correlates of derealization after trauma exposurea
aIn panel A, the red 5-mm sphere represents the Hopper et al. (25) left ventromedial prefrontal cortex (vmPFC) region of interest used in the emotion reactivity (fearful faces) task and resting-state connectivity analysis. In panel B, vmPFC activity during the emotion reactivity task was positively correlated with self-report derealization severity 2 weeks posttrauma (r=0.18, N=143, p=0.030). In panel C, connectivity between the left vmPFC and the right cerebellar lobule VIIIa (orange) was negatively correlated with 2-week derealization scores (r=−0.26, N=143, p=0.001). In panel D, connectivity between the left vmPFC and the right orbitofrontal cortex (OFC) (yellow) was negatively correlated with 2-week derealization scores (r=−0.27, N=143, p<0.001). Scatterplots and correlation values are for zero-order correlations. Scatterplot dots represent individual participants’ scores for activity/connectivity and derealization severity. Lines represent the linear line of best fit. Shaded error bands represent standard error of the mean.
Resting-state connectivity.
To further understand how the vmPFC region of interest was related to derealization, we conducted a whole-brain resting-state seed-based connectivity analysis with the same vmPFC region of interest (25) as the seed. When controlling for MRI scanner, sex, age, childhood trauma, and 2-week posttraumatic stress symptom severity, derealization was associated with decreased connectivity between the vmPFC and two regions: the right lobule VIIIa in the cerebellum (cluster volume=92, peak t=−4.14, MNI coordinates for the peak t=26, −63, −57) (Figure 1C) and an area in the right orbitofrontal cortex (OFC) (Brodmann area 47, cluster volume=74, peak t=−4.11, MNI coordinates for the peak t=26, 26, −15) (Figure 1D). In post hoc analyses, this connectivity was not linked to levels of activity in the vmPFC during the emotional reactivity task (see the online supplement).
Prediction of 3-Month Outcomes by Self-Reported and Neural Correlates of Persistent Derealization
Self-reported derealization.
Higher levels of derealization at 2 weeks posttrauma were associated with higher levels of 3-month PTSD, anxiety, and depression symptoms and of extent of pain and impairment (Table 2). A series of multiple linear regressions revealed that 2-week derealization predicted 3-month PTSD (pcorrected=0.040) and depression symptoms (pcorrected=0.020) even when accounting for sex, age, childhood maltreatment history, and 2-week posttraumatic stress symptoms (Table 3).
| Correlation | ||||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Mean | SD | 1 | 2 | 3 | 4 | 5 | 6 |
| Derealization self-report at 2 weeks and outcomes at 3 months | ||||||||
| 1.Derealization at 2 weeks | 1.73 | 2.12 | ||||||
| 2.PTSD at 3 months | 25.13 | 19.13 | 0.53** | |||||
| 3.Pain extent at 3 months | 7.54 | 6.50 | 0.32** | 0.55** | ||||
| 4.Impairment at 3 months | 11.87 | 9.37 | 0.29** | 0.66** | 0.52** | |||
| 5.Anxiety at 3 months | 6.63 | 4.70 | 0.42** | 0.83** | 0.45** | 0.62** | ||
| 6.Depression at 3 months | 53.34 | 11.09 | 0.42** | 0.67** | 0.31** | 0.48** | 0.63** | |
| Ventromedial prefrontal cortex activity and outcomes at 3 months | ||||||||
| 1.vmPFC fear > neutral | −0.01 | 0.23 | ||||||
| 2.PTSD at 3 months | 23.40 | 16.86 | 0.23* | |||||
| 3.Pain extent at 3 months | 7.10 | 6.08 | 0.14 | 0.63** | ||||
| 4.Impairment at 3 months | 11.93 | 10.06 | 0.05 | 0.65** | 0.57** | |||
| 5.Anxiety at 3 months | 6.46 | 4.58 | 0.11 | 0.84** | 0.52** | 0.64** | ||
| 6.Depression at 3 months | 53.31 | 10.66 | 0.19* | 0.78** | 0.49** | 0.62** | 0.82** | |
TABLE 2. Correlations of self-reported derealization and ventromedial prefrontal cortex activity with 3-month outcomesa
| Measure | B | Robust SE | t | p | η2p | Adj. R2 | Model Without Derealization Adj. R2 | F | df | p |
|---|---|---|---|---|---|---|---|---|---|---|
| PTSD at 3 months | ||||||||||
| Overall model | 0.519 | 0.515 | 222.71 | 5, 1022 | <0.001 | |||||
| Sex | 0.47 | 0.89 | 0.52 | 0.601 | 0.00 | |||||
| Age | 0.09 | 0.03 | 3.25 | 0.001 | 0.01 | |||||
| Childhood maltreatment | 0.13 | 0.05 | 2.64 | 0.009 | 0.01 | |||||
| Posttraumatic stress at 2 weeks | 0.62 | 0.03 | 19.86 | <0.001 | 0.28 | |||||
| Derealization at 2 weeks | 0.82 | 0.32 | 2.58 | 0.010b | 0.01 | |||||
| Pain extent at 3 months | ||||||||||
| Overall model | 0.223 | 0.220 | 58.69 | 5, 1000 | <0.001 | |||||
| Sex | −0.77 | 0.39 | 0.92 | 0.355 | 0.00 | |||||
| Age | 0.36 | 0.01 | 5.79 | <0.001 | 0.03 | |||||
| Childhood maltreatment | 0.08 | 0.02 | 3.68 | <0.001 | 0.01 | |||||
| Posttraumatic stress at 2 weeks | 0.11 | 0.01 | 8.06 | <0.001 | 0.06 | |||||
| Derealization at 2 weeks | 0.27 | 0.13 | 2.08 | 0.038c | 0.00 | |||||
| Impairment at 3 months | ||||||||||
| Overall model | 0.246 | 0.246 | 68.59 | 5, 1029 | <0.001 | |||||
| Sex | 0.75 | 0.54 | 1.39 | 0.165 | 0.00 | |||||
| Age | 0.10 | 0.02 | 5.22 | <0.001 | 0.03 | |||||
| Childhood maltreatment | 0.11 | 0.03 | 3.92 | <0.001 | 0.02 | |||||
| Posttraumatic stress at 2 weeks | 0.23 | 0.02 | 11.97 | <0.001 | 0.12 | |||||
| Derealization at 2 weeks | −0.22 | 0.17 | −1.28 | 0.202 | 0.00 | |||||
| Anxiety at 3 months | ||||||||||
| Overall model | 0.372 | 0.373 | 125.64 | 5, 1046 | <0.001 | |||||
| Sex | 0.25 | 0.24 | 1.06 | 0.291 | 0.00 | |||||
| Age | 0.02 | 0.01 | 2.32 | 0.020 | 0.01 | |||||
| Childhood maltreatment | 0.04 | 0.01 | 3.01 | 0.003 | 0.01 | |||||
| Posttraumatic stress at 2 weeks | 0.14 | 0.01 | 16.16 | <0.001 | 0.20 | |||||
| Derealization at 2 weeks | 0.05 | 0.08 | 0.59 | 0.557 | 0.00 | |||||
| Depression at 3 months | ||||||||||
| Overall model | 0.394 | 0.389 | 138.32 | 5, 1050 | <0.001 | |||||
| Sex | −0.77 | 0.58 | −1.33 | 0.183 | 0.01 | |||||
| Age | 0.02 | 0.02 | 1.20 | 0.231 | 0.00 | |||||
| Childhood maltreatment | 0.14 | 0.03 | 4.87 | <0.001 | 0.02 | |||||
| Posttraumatic stress at 2 weeks | 0.29 | 0.02 | 14.36 | <0.001 | 0.16 | |||||
| Derealization at 2 weeks | 0.56 | 0.19 | 2.88 | 0.004b | 0.01 |
TABLE 3. Prediction of 3-month outcomes from self-reported derealizationa
vmPFC activity.
More vmPFC activity in the fear > neutral face contrast during the emotion reactivity task was associated with higher 3-month PTSD and depression symptoms (Table 2). Given that self-report derealization predicted 3-month PTSD and depression symptoms, we wanted to test whether vmPFC activity would also predict these outcomes. A series of multiple linear regressions revealed that 2-week vmPFC activity predicted 3-month PTSD symptoms (pcorrected=0.022) even when accounting for MRI scanner, sex, age, childhood maltreatment history, and 2-week posttraumatic stress symptoms (Table 4).
| Measure | B | Robust SE | t | p | η2p | Adj. R2 | Model Without vmPFC Activity Adj. R2 | F | df | p |
|---|---|---|---|---|---|---|---|---|---|---|
| PTSD at 3 months | ||||||||||
| Overall model | 0.390 | 0.366 | 10.34 | 8, 109 | <0.001 | |||||
| MRI scanner | ||||||||||
| 1 vs. 4 | 4.93 | 3.31 | 1.49 | 0.139 | 0.02 | |||||
| 2 vs. 4 | 1.53 | 5.89 | 0.26 | 0.795 | 0.00 | |||||
| 3 vs. 4 | 1.16 | 3.21 | 0.36 | 0.719 | 0.00 | |||||
| Sex | −2.26 | 2.82 | −0.80 | 0.425 | 0.01 | |||||
| Age | 0.01 | 0.11 | 0.07 | 0.943 | 0.00 | |||||
| Childhood maltreatment | <0.001 | 0.14 | 0.003 | 0.998 | 0.00 | |||||
| Posttraumatic stress at 2 weeks | 0.61 | 0.09 | 7.10 | <0.001 | 0.32 | |||||
| vmPFC activity | 12.55 | 4.88 | 2.57 | 0.011b | 0.06 | |||||
| Depression at 3 months | ||||||||||
| Overall model | 0.298 | 0.288 | 7.20 | 8, 109 | <0.001 | |||||
| MRI scanner | ||||||||||
| 1 vs. 4 | 0.09 | 2.45 | 0.04 | 0.971 | 0.00 | |||||
| 2 vs. 4 | 0.12 | 5.00 | 0.02 | 0.981 | 0.00 | |||||
| 3 vs. 4 | −1.13 | 2.10 | −0.54 | 0.593 | 0.00 | |||||
| Sex | 0.19 | 1.93 | 0.10 | 0.920 | 0.00 | |||||
| Age | −0.01 | 0.07 | −0.15 | 0.882 | 0.00 | |||||
| Childhood maltreatment | 0.11 | 0.11 | 1.06 | 0.290 | 0.01 | |||||
| Posttraumatic stress at 2 weeks | 0.32 | 0.07 | 4.93 | <0.001 | 0.18 | |||||
| vmPFC activity | 5.80 | 2.28 | 1.77 | 0.080 | 0.03 |
TABLE 4. Prediction of 3-month outcomes from ventromedial prefrontal cortex activity in fear > neutral face contrasta
The above-reported results predict dimensional symptom outcomes. We also conducted a set of post hoc analyses to predict PTSD and depression diagnosis using self-report derealization and vmPFC activity (see the online supplement). Models with 2-week derealization alone as a predictor had adequate discrimination for both depression and PTSD diagnosis at 3 months. However, improvement in overall discrimination and in sensitivity and specificity were minimal when 2-week derealization score was combined with other clinical predictors. Discrimination for vmPFC activity alone was below acceptable levels for both depression and PTSD diagnosis, and addition of vmPFC to other clinical predictors only modestly improved overall discrimination, sensitivity, and specificity.
Discussion
Despite foundational work on the psychological and neural basis of dissociation, the field has yet to definitively determine whether dissociation is a unique predictor of worse posttrauma psychiatric course. We examined whether the psychological and neural correlates of derealization, a specific type of dissociation, predicted worse psychiatric symptoms in the largest prospective longitudinal biomarker study of dissociation to date. Here, we demonstrated that self-report and the neural manifestations of persistent (2-week) derealization both predicted 3-month PTSD symptom severity, distinct from a history of childhood maltreatment history, and 2-week posttraumatic stress symptoms. This suggests that persistent derealization following acute trauma is an early sign of likely having worse mental health as time goes on.
In terms of the underlying neural markers, we found that persistent derealization was associated with increased vmPFC activity during an emotion reactivity task when controlling for the severity of childhood maltreatment and current posttraumatic stress symptoms. This finding partially replicates previous work demonstrating a pattern of increased medial PFC activity in the dissociative subtype of PTSD and dissociative identity disorder (15, 16), especially for consciously perceived fearful facial stimuli (29). The vmPFC is consistently involved in emotion regulation, and specifically emotion regulation that is automatic and outside awareness or conscious control (30). This neural activity may, in part, underlie emotion overregulation and the experience of feeling detached from one’s surroundings in the aftermath of traumatic events.
Interestingly, we did not find an association between derealization and amygdala or insula activation in the emotion reactivity task. Models of dissociation implicate corticolimbic inhibition and decreased amygdala/insula activity in emotionally arousing contexts when individuals are feeling dissociative (15, 16). However, a recent systematic review found the direction and strength of amygdala and insula findings in relation to dissociation to be mixed (17). The relationship between dissociation and activity in these structures may be highly dependent on the task context and type of dissociation being studied. More work is necessary to understand the nuances of these potential neural markers of dissociation.
Moreover, we found that at rest, derealization was associated with decreased resting-state connectivity between the vmPFC and the posterior lobe of the cerebellum (lobule VIIIa). This region of the cerebellum is largely involved in sensorimotor function and includes a secondary somatotopic representation of the human body (31). Lobule VIII typically demonstrates functional connectivity with the anterior prefrontal cortex and primary motor cortex (32). Recent work on the dissociative subtype of PTSD found decreased connectivity in other sensorimotor regions of the cerebellum and cortical regions involved in multisensory integration (33). Contemporary theories of cerebellar function hypothesize that the cerebellum is a master regulator across sensorimotor, cognitive, and affective domains, helping to maintain allostasis (34). Given this, we speculate that decreased synchronization between the vmPFC and this region of the cerebellum may contribute to perceptual and affective distortions experienced during derealization (e.g., feelings that surroundings are fading away, unreal, or strange). Given our cerebellum findings and those in prior work, the cerebellum is emerging as a key region that has been understudied in trauma-related dissociation.
Additionally, we observed an association between derealization and decreased resting-state connectivity between the vmPFC and OFC. Both regions have recently been identified as potential biomarkers of pathological dissociation, although their functional connectivity remains understudied in relation to dissociation (17). The OFC receives a wide array of sensory and visceral input and may serve as a major integration area for this information (35). It has also been implicated in decision making, emotional processing, and assigning a reward value and valence to experience (35). It has also been linked to individual differences in anxiety through its role in amygdala regulation (36, 37). The vmPFC is a hub of the default network involved in emotion processing and self-related thinking (38). Typically, the OFC has strong connections with cingulate regions, and the vmPFC receives inputs from both the OFC and cingulate regions (39). This communication is thought to facilitate an assessment of a stimulus’s value and can influence goal-directed action (39). Moreover, altered communication between the OFC and cingulate regions can impact the output of all medial PFC regions (39). Given this, we hypothesize that decreased synchronization between the OFC and vmPFC may also be connected to the increased vmPFC activity during derealization. Furthermore, we hypothesize that these altered networks may also contribute to perceptual and affective distortions experienced during derealization (e.g., feelings that familiar surroundings are unreal, unfamiliar, strange, or puzzling).
Concerning the relationship between persistent derealization and later symptoms, we found that more severe persistent derealization was associated with increased PTSD, anxiety, and depression symptom severity, pain extent, and functional impairment 3 months after trauma exposure. However, when accounting for sex, age, childhood maltreatment history, and concurrent posttraumatic stress symptom severity, persistent derealization only predicted later PTSD and depression symptoms. PTSD and depression often co-occur in the aftermath of trauma (40). There is both evidence that co-occurring PTSD and depression may represent a more general traumatic stress factor and, in contrast, that these disorders can be separate constructs with unique predictors and outcomes (41). It is as yet unclear whether derealization predicts PTSD and depression as separate entities or whether it predicts a more general traumatic stress reaction in this population, which we hope can be further addressed in future studies. Nonetheless, these results suggest that knowing an individual’s levels of derealization in the weeks following trauma exposure will help predict their future depression and PTSD symptoms, distinct from knowing their sex, age, childhood maltreatment history, and current posttraumatic stress symptoms. This highlights the importance of assessing for dissociation in the aftermath of traumatic events to identify individuals who are at risk of a worse psychiatric course.
Similarly, brain activity associated with persistent derealization was related to more severe PTSD and depression symptoms later on (i.e., increased vmPFC activity in an emotionally provocative context). When accounting for MRI scanner, sex, age, childhood maltreatment history, and concurrent posttraumatic stress symptom severity, vmPFC activity still significantly predicted future PTSD symptoms. This suggests that after acute trauma, increased vmPFC activity during an emotionally provocative task may be a signal of worse PTSD symptoms to come.
This result is consistent with other recent findings implicating the vmPFC as a central node in prolonged posttraumatic psychopathology. For example, less vmPFC activation during an emotionally provocative task before a difficult combat training was linked to more PTSD symptoms after training (42). A “Goldilocks” level of vmPFC activity during emotionally charged contexts may exist (43). This optimal level of activity may be representative of successful emotion regulation that protects against future PTSD symptoms. In contrast, either an excess or a deficiency in this activity may lead to worse PTSD symptoms later on. Alternatively, the vmPFC may comprise subregions, some of which are associated with persistent dissociative symptoms (and worse PTSD symptoms later on) while others are associated with successful emotion regulation (and fewer PTSD symptoms later on).
Several limitations constrain the interpretations of our findings. We have demonstrated that the neural correlates and self-report measures of derealization predict later psychiatric outcomes; however, we have not tested whether derealization causes these outcomes directly. Moreover, it could be that some derealization preceded the specific traumatic event that brought individuals to the emergency department in this study. Further work is needed to test the causal relationship between trauma-related persistent dissociation and later psychiatric outcomes. Pathological dissociation is a multidimensional construct encompassing a wide range of experiences; however, we focused solely on derealization given its link to impairment severity (2, 3). The AURORA study prioritized measuring many posttraumatic sequelae, which limited the depth of assessment for any one construct. Therefore, our findings may not be generalizable to other types of dissociation. Also, our measure of persistent derealization was limited to a self-report 2 weeks after the acute trauma. Researchers in future work may wish to use clinician-administered measures to explore dissociation that persists beyond 2 weeks. In addition, the requirement of smartphone ownership for study participation may limit the generalizability of our findings. Moreover, we excluded individuals in ongoing domestic violence situations and those with self-inflicted injuries. Interpersonal trauma is associated with more dissociative symptoms and self-harm (44). Additionally, dissociative disorder diagnosis is strongly associated with measures of self-harm, suicidality, and numbers of suicide attempts (45). Therefore, our findings may suffer from a restriction in the range of acuity in participants’ dissociative symptoms. Specifically, the prevalence of derealization in our sample might have been even higher if individuals in domestic violence situations and those with self-inflicted injuries were eligible for enrollment. We recommend that future work include such individuals, both to better understand the full range of dissociative symptomatology and to better aid those with more severe pathology.
Conclusions
In the largest prospective longitudinal biomarker study of dissociation to date, our findings suggest that persistent derealization is both a psychological and biological marker of worse later psychiatric outcomes. Additionally, we have replicated previous neurobiological work and introduced novel findings demarcating the neural correlates of trauma-related dissociation. Importantly, the activity and connectivity of these regions may serve as potential neural targets for treatment engagement to prevent PTSD.
Previous work suggests that dissociation may undermine seeking treatment for PTSD, highlighting that there may be a need to screen for and treat individuals with prominent dissociative symptoms differently (7). Our work lends urgency to these claims, as persistent derealization is associated with more severe depression, anxiety, pain, and PTSD symptoms and greater functional impairment 3 months after trauma. Clinicians may wish to screen for dissociation in the weeks following trauma, and perhaps in other contexts (e.g., primary care) to identify individuals potentially at risk of a chronic, more severe psychiatric course. Ultimately, we hope this would in turn address a currently unmet clinical need for early intervention.
1. : Dissociative disorders in DSM-5. Depress Anxiety 2011; 28:824–852Crossref, Medline, Google Scholar
2. : Dissociative symptoms mediate the relation between PTSD symptoms and functional impairment in a sample of military members, veterans, and first responders with PTSD. Eur J Psychotraumatology 2018; 9:1463794Crossref, Medline, Google Scholar
3. : Depersonalization and derealization in self-report and clinical interview: the spectrum of borderline personality disorder, dissociative disorders, and healthy controls. J Trauma Dissociation 2017; 18:490–506Crossref, Medline, Google Scholar
4. : Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Association, 2013Crossref, Google Scholar
5. : Pierre Janet and modern views of dissociation. J Traum Stress 1989; 2:413–429Crossref, Google Scholar
6. : Deployment and the use of mental health services among US Army wives. N Engl J Med 2010; 362:101–109Crossref, Medline, Google Scholar
7. : Dissociation in posttraumatic stress disorder: evidence from the World Mental Health Surveys. Biol Psychiatry 2013; 73:302–312Crossref, Medline, Google Scholar
8. : Dissociation and dissociative mechanisms in panic disorder, obsessive-compulsive disorder, and depression: a review and heuristic framework. Psychol Conscious Theor Res Pract 2014; 1:243–270Google Scholar
9. : Dissociation and posttraumatic stress symptoms in patients with chronic pain. Int J Rehabil Health 2000; 5:129–139Crossref, Google Scholar
10. : The contributions of emotion regulation difficulties and dissociative symptoms to functional impairment among civilian inpatients with posttraumatic stress symptoms. Psychol Trauma 2020; 12:739–749Crossref, Medline, Google Scholar
11. : Socio-demographic and trauma-related predictors of PTSD within 8 weeks of a motor vehicle collision in the AURORA study. Mol Psychiatry 2021; 26:3108–3121Crossref, Medline, Google Scholar
12. : The independent predictive value of peritraumatic dissociation for PTSD symptomatology after type I trauma: a systematic review of prospective studies. Clin Psychol Rev 2008; 28:1009–1020Crossref, Medline, Google Scholar
13. : Peritraumatic and persistent dissociation in the presumed etiology of PTSD. Am J Psychiatry 2005; 162:2295–2301Link, Google Scholar
14. : Peritraumatic and persistent dissociation as predictors of PTSD symptoms in a female cohort. J Traum Stress 2012; 25:401–407Crossref, Medline, Google Scholar
15. : Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry 2010; 167:640–647Link, Google Scholar
16. : Opposite brain emotion-regulation patterns in identity states of dissociative identity disorder: a PET study and neurobiological model. Psychiatry Res 2014; 223:236–243Crossref, Medline, Google Scholar
17. : Biomarkers of pathological dissociation: a systematic review. Neurosci Biobehav Rev 2020; 123:120–202Crossref, Medline, Google Scholar
18. : The AURORA study: a longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Mol Psychiatry 2020; 25:283–296Crossref, Medline, Google Scholar
19. : Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry 1997; 36:340–348Crossref, Medline, Google Scholar
20. : Severity of Dissociative Symptoms–Adult (Brief Dissociative Experiences Scale [DES-B]–Modified). Washington, DC, American Psychiatric Association, 2010Google Scholar
21. : The PTSD Checklist for DSM-5 (PCL-5). 2013. https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.aspGoogle Scholar
22. : Item banks for measuring emotional distress from the patient-reported outcomes measurement information system (PROMIS®): depression, anxiety, and anger. Assessment 2011; 18:263–283Crossref, Medline, Google Scholar
23. : Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med 1997; 27:93–105Crossref, Medline, Google Scholar
24. : Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res 2013; 47:1469–1478Crossref, Medline, Google Scholar
25. : Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Traum Stress 2007; 20:713–725Crossref, Medline, Google Scholar
26. : Using heteroscedasticity consistent standard errors in the linear regression model. Am Stat 2000; 54:217–224Google Scholar
27. : A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika 1988; 75:383–386Crossref, Google Scholar
28. : Applications of multivariate modeling to neuroimaging group analysis: a comprehensive alternative to univariate general linear model. Neuroimage 2014; 99:571–588Crossref, Medline, Google Scholar
29. : Dissociative responses to conscious and non-conscious fear impact underlying brain function in post-traumatic stress disorder. Psychol Med 2008; 38:1771–1780Crossref, Medline, Google Scholar
30. : The neural bases of emotion regulation. Nat Rev Neurosci 2015; 16:693–700Crossref, Medline, Google Scholar
31. : Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 2010; 46:831–844Crossref, Medline, Google Scholar
32. : Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex 2009; 19:2485–2497Crossref, Medline, Google Scholar
33. : The cerebellum after trauma: resting-state functional connectivity of the cerebellum in posttraumatic stress disorder and its dissociative subtype. Hum Brain Mapp 2018; 39:3354–3374Crossref, Medline, Google Scholar
34. : The neuropsychiatry of the cerebellum: insights from the clinic. Cerebellum 2007; 6:254–267Crossref, Medline, Google Scholar
35. : The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 2005; 6:691–702Crossref, Medline, Google Scholar
36. : Mechanisms underlying the early risk to develop anxiety and depression: a translational approach. Eur Neuropsychopharmacol 2017; 27:543–553Crossref, Medline, Google Scholar
37. : The prefrontal cortex, pathological anxiety, and anxiety disorders. Neuropsychopharmacology 2022; 47:260–275Crossref, Medline, Google Scholar
38. : The brain’s default mode network. Annu Rev Neurosci 2015; 38:433–447Crossref, Medline, Google Scholar
39. : The cingulate cortex and limbic systems for action, emotion, and memory. Handb Clin Neurol 2019; 166:23–37Crossref, Medline, Google Scholar
40. : Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995; 52:1048–1060Crossref, Medline, Google Scholar
41. : Posttraumatic stress disorder and depression following trauma: understanding comorbidity. Am J Psychiatry 2004; 161:1390–1396Link, Google Scholar
42. : Neural indicators of interpersonal anger as cause and consequence of combat training stress symptoms. Psychol Med 2017; 47:1561–1572Crossref, Medline, Google Scholar
43. : Neural contributors to trauma resilience: a review of longitudinal neuroimaging studies. Trans Psychiatry 2021; 11:508Crossref, Medline, Google Scholar
44. : Dissociation, somatization, and affect dysregulation: the complexity of adaptation of trauma. Am J Psychiatry 1996; 153:83–93Link, Google Scholar
45. : Dissociative disorders and suicidality in psychiatric outpatients. J Nerv Ment Dis 2008; 196:29–36Crossref, Medline, Google Scholar



