No Association Between Amygdala Responses to Negative Faces and Depressive Symptoms: Cross-Sectional Data from 28,638 Individuals in the UK Biobank Cohort
Neurocognitive models of depression emphasize the role of the amygdala in dysfunctional emotional processing (1, 2). Specifically, increased amygdala responsiveness to negative stimuli is posited as a mechanism underlying the aberrant low-level emotional processing in depressed individuals, manifesting as a negativity bias in the perception and recognition of emotional stimuli (3).
Early brain imaging studies supported the view that relative to control subjects, patients with depression show larger amygdala responses to negative faces (4–7), and a recent PubMed search for “amygdala AND depression” yielded >4,000 hits—supporting the significance that this brain region has been assigned in the context of depressive disorder. Four recent meta-analyses (the largest by Li and Wang (11) at N=2,383) also found increased amygdala activity during emotional tasks (including, but not limited to, facial stimuli) in depressed patients versus control subjects (8–11). Moreover, meta-analyses across psychiatric disorders suggest that amygdala hyperactivation is transdiagnostic, most pronounced in nonpsychotic illnesses (e.g., anxiety and depression) (12, 13). Amygdala reactivity to emotional (facial) stimuli is often used in studies investigating antidepressant treatment effects (14) and depression risk (15, 16) and is sometimes referred to as a biomarker for depression (17, 18). In summary, various lines of research support the view that depression is associated with amygdala responses and, vice versa, amygdala responses are associated with depression and risk of depression. While most previous studies compared individuals with clinical cases of depression to control subjects, it would be reasonable to expect such findings to translate to depressive symptoms in the general population, particularly considering the transdiagnostic nature of the association (12, 13).
In this Priority Data Letter, we report on the largest study to date to test the association between amygdala reactivity and depressive symptoms based on UK Biobank data. For transparency, we initially set out to investigate the association between sleep disruption and depression, focusing on amygdala reactivity as a key candidate mechanism linking the two associated variables (the details of our pre-registered study can be found at https://osf.io/xcv39). When completing our pre-defined analyses, we found no association between the proposed mediator (amygdala reactivity) and our key outcome (depressive symptoms). We judge this unexpected finding to be sufficiently important for the field to merit a standalone report.
Methods
The present study used cross-sectional data from the UK Biobank cohort (19), which includes adults from the U.K. general population recruited between 2006 and 2010. The target age range at recruitment was 40–69 years, and no other exclusion criteria were applied. Participation rate was approximately 5%. Three in-person follow-up visits have been performed in subsets of the participants. Here we report on data from the first imaging visit, initiated in 2014. The NHS National Research Ethics Service approved all procedures (Ref. 11/NW/0382) and all participants gave written informed consent. Data were downloaded from the Biobank in May 2020.
Participants
Inclusion criteria were the same as for the UK Biobank (19). We excluded participants if they reported a neurological condition (neurodegenerative disease, stroke, head injury, or epilepsy), if consent was withdrawn, or if data were missing for the outcome, predictor, or any of the covariates. The total number of participants included in analyses was 28,638.
Imaging Protocol and Study Variables
Details of the magnetic resonance imaging (MRI) acquisition protocol, image processing pipeline, and image data files from the UK Biobank have been previously described (20).
The dependent variable of interest was amygdala responses to faces relative to shapes from the so-called Hariri task (20–22). During functional MRI (fMRI), participants had to match one of two simultaneously presented images of negative emotional stimuli (angry and fearful facial expressions) with an identical target image (experimental block), or alternatively match geometric shapes (control block). An in-depth description of the imaging data processing can be found at https://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id=1977. The “Median BOLD effect (in group-defined amygdala activation mask) for faces-shapes contrast” (item 25052) served as the dependent variable in our analysis. This variable is derived through extracting the median BOLD effect for the faces versus shapes contrast from a group-defined region of interest (ROI)—the intersection of the original group-average ROI for this contrast and an amygdala mask derived from the Harvard-Oxford structural atlas. The ROI is shown in Figure S1 in the online supplement.
Current depressive symptoms were measured on the day of imaging with the following four questions: “Over the past two weeks, how often have you…” 1) “…felt down, depressed or hopeless?” (item 2050); 2) “…had little interest or pleasure in doing things?” (item 2060); 3) “…felt tired or had little energy?” (item 2080); and 4) “… felt tense, fidgety or restless?” (item 2070). The response alternatives for the questions were “Not at all,” “Several days,” “More than half the days,” and “Nearly every day,” coded from 0 to 3. Hence, the total score (here denoted “depression score”) ranged from 0 to 12. The first three questions match core items from the validated Patient Health Questionnaire (PHQ)-9 (23), while the fourth question is similar to items from the GAD-7 anxiety measure (24). The measure has been used in previous studies of current depressive symptoms in the UK Biobank cohort (25). Alternative item responses such as “I do not know” or “Prefer not to answer” were considered missing values. McDonald’s omega for the depression score in the current sample was 0.79.
The following covariates were used in analyses: Age at assessment (item 21003), treated as a continuous variable; Sex (item 31); Education (item 6138), dichotomized into the categories “college/university degree” versus “no college/university degree”; Townsend deprivation index at recruitment (item 189); and Body Mass Index (BMI) (item 21001), treated as a continuous variable.
Statistical Analyses
Data were analyzed with linear regression. In a first model, we investigated the crude association between depressive symptoms and amygdala responses. In the second model (considered our primary analysis), the following covariates were added: age, sex, education, Townsend deprivation index, and BMI. Additional analyses were conducted with only the mood and anhedonia depression items (first two questions analogous with the PHQ-2 [26]). Because of the zero-inflated/skewed data for the main predictor (low depression scores in the sample), we also performed the following sensitivity analyses: 1) we removed all participants with depression score=0 and repeated the same aforementioned analyses; and 2) we compared participants with depression score=12 with those scoring 0, as well as participants with depression score>6 compared with participants scoring 0, using independent sample t tests. To explore possible age effects, we stratified the main analysis by age (≤ vs. > median), and investigated the interaction between below/above median age and depression score as a predictor of amygdala responses (Table S10 in the online supplement). We also performed follow-up analyses using self-reported depression diagnosis, as well as use of antidepressant medication and a lifetime depression diagnosis based on a mental health follow up in a subset of patients in 2017, see online supplement, and an analysis looking at predictors of depression score, including the aforementioned covariates, as well as amygdala responses, to capture the potential bidirectional association (Table S10 in the online supplement). Furthermore, we performed a sensitivity analysis excluding 170 participants with a potential signal dropout within the amygdala mask (Table S11 in the online supplement) and lastly to quantify the evidence in favor of the null hypothesis (i.e., no association between depression score and amygdala responses), we present the data using Bayesian methodology (Figure S3 in the online supplement). Analyses were performed in R (27) with the lme4 package (28) and ggplot2 (29) was used for illustration. All analysis scripts can be found at doi.org/10.5281/zenodo.5126666.
Results
The sample included 28,638 participants (53% female), median age of 64 years (range=44–82), mean BMI of 26.4 kg/m2 (SD=4.3), and 52% having attended college. Depression scores ranged from 0 to 12 (median=0, IQR=0–2). Lower age, female sex, higher Townsend deprivation index, lower education, and higher BMI were all significant predictors of higher depression scores (Table S10 in the online supplement). There were 1,744 participants using antidepressant medication (as classified as Anatomical Therapeutic Chemical Classification System [ATC] code N06).
In the unadjusted model, depressive symptoms were not associated with amygdala responses (β=0.0006 [SE=0.0004]; p=0.1206; see part A of Figure 1, Table 1); however, lower age, male sex, college education, and higher BMI all showed significant associations (parts B-F of Figure 1, Table 1).
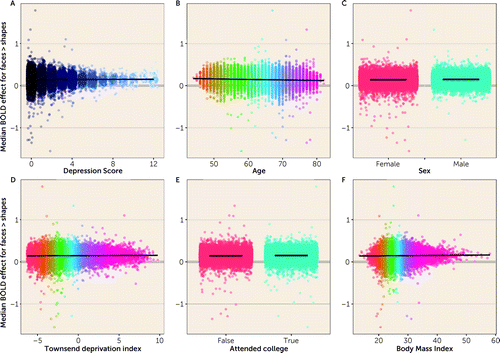
FIGURE 1. Relationships of depression score (A) and other individual predictors (B–F) with amygdala responsesa
aDots represent individual datapoints and lines represent regression lines for continuous predictors and mean values for each group for dichotomous predictors.
| Predictor | Univariable analysis | Multivariable-adjusted | ||||
|---|---|---|---|---|---|---|
| β-coefficient | SE | p | β-coefficient | SE | p | |
| Depression score | 0.0006 | 0.0004 | 0.1206 | −0.0001 | 0.0004 | 0.7891 |
| Age | −0.0012 | 0.0001 | <0.0001 | −0.0012 | 0.0001 | <0.0001 |
| Sex | 0.0080 | 0.0013 | <0.0001 | 0.0089 | 0.0014 | <0.0001 |
| Townsend deprivation index | 0.0008 | 0.0002 | 0.0025 | 0.0005 | 0.0003 | 0.0700 |
| Attended college | 0.0091 | 0.0013 | <0.0001 | 0.0083 | 0.0013 | <0.0001 |
| Body mass index | 0.0005 | 0.0002 | 0.0005 | 0.0004 | 0.0002 | 0.0046 |
TABLE 1. Strength of univariable and multivariable-adjusted relationships in linear regression between depression score and amygdala responses to adverse emotional facial stimuli in UK Biobank participantsa
In the covariate-adjusted model, there was no evidence of a relationship between depressive symptoms and amygdala responses (β=−0.0001 [SE=0.0004]; p=0.7891 [Table 1]). The results were consistent across younger/older participants (Tables S6–S8 in the online supplement).
All sensitivity analyses as well as analyses involving only the two core depression symptom items showed consistent null effects (results available in the online supplement), and the Bayesian model indicated strong evidence in favor of the null hypothesis (Figure S3 in the online supplement).
Discussion
Our analyses show that amygdala responses to angry and fearful faces are not associated with depressive symptoms in 28,638 individuals from the UK Biobank cohort. The results stand in contrast to most previous studies, including four recent meta-analyses (8–11), although we note consistency with three other meta-analyses (30–32) that 1) similarly failed to observe an association and 2) found marked between-study heterogeneity in task design, facial expression (combinations of sad/angry/fearful), instructions (labeling emotion, matching faces), and analysis pipelines (33). In light of these inconsistencies, our data do not preclude the possibility that amygdala differences are present in general (i.e., for all types of tasks) between depressed patients and control subjects, or that other methods may reveal differences in brain structure, activity, or connectivity. It is possible that altered perception of negative facial expressions might be an important feature of depression (34) but in light of the present data, it seems highly unlikely that amygdala responses to negative faces are of fundamental importance for the pathophysiology or symptomatology of depression.
The present study has a few limitations. The sample consisted of older, relatively healthy participants with generally low depression scores. Since most previous work has been conducted in younger samples, and taking into account the age effect seen in our analysis, we cannot exclude the possibility that amygdala reactivity to negative facial expression might be related to depression in younger people, or that depressive symptoms in older adults might be etiologically or qualitatively different in some way (35). However, exploratory stratified analyses showed no indications of such an effect. It has also been suggested that amygdala function might decline over the lifespan (36, 37) (albeit to a lesser extent than for many other brain regions) (38), which could potentially mask an effect in the current sample. Furthermore, depressive symptoms were assessed with only a subset of the questions from the validated PHQ questionnaire, and depressive symptoms might not be comparable to the case/control studies included in the previously mentioned meta-analyses. However, it is important to note that additional supplementary analyses using other prior indicators of risk for depression (e.g., lifetime diagnosis of major depressive disorder) showed consistent null effects. Therefore, our findings imply that a potential effect of depressive symptoms on amygdala reactivity to negative faces in nonclinical samples might not be reliably present, or at the very least is smaller than previously thought (we observed a partial f2 of 0.000002 in the adjusted analysis), but as noted caution should be exercised when extrapolating from our findings to clinical samples.
The fMRI task lasted only 5 minutes, without any behavioral outcomes. The amygdala is also treated as a single entity in the analyses, which does not take into account the region’s functionally and anatomically heterogeneous collection of nuclei. Furthermore, since the UK Biobank does not provide amygdala-specific data for individual task conditions, we cannot exclude the possibility that the result is due to the specific contrast (i.e., the comparison with shapes). However, task results point to an overall amygdala effect for faces compared with shapes as indicated by a positive intercept in the models—supporting task validity. Finally, a broader methodological reflection—relevant to the entire field of task-related fMRI—relates to growing evidence of low within-subject task reliability (39) and challenges of using regional brain activity for the study of individual differences (40).
Our study has several strengths. Importantly, our sample size is markedly larger than any other previous meta-analysis of the topic—indeed by a factor of 10—and the standardized imaging protocol in UK Biobank, including analysis pipeline, overcomes several methodological issues that may impact reproducibility in neuroimaging research (41).
Conclusions
We conclude that amygdala responses to negative emotional facial stimuli are not associated with depressive symptoms in a middle-aged and older population-based sample. While clinical cases of depression might be qualitatively different from depressive symptoms in the general population, an association between depression and amygdala responses to negative faces is not likely to be as large as previously suggested. Our analyses suggest that amygdala responses to negative facial expressions should not be considered an important feature/biomarker of depressive symptoms, at least not in the general population.
1 : A neurocognitive model for understanding treatment action in depression. Philos Trans R Soc Lond B Biol Sci 2015; 370:20140213Crossref, Medline, Google Scholar
2 : Neurocognitive mechanisms in depression: implications for treatment. Annu Rev Neurosci 2009; 32:57–74Crossref, Medline, Google Scholar
3 : Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci 2011; 12:467–477Crossref, Medline, Google Scholar
4 : Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry 2001; 58:1057–1063Crossref, Medline, Google Scholar
5 : A functional anatomical study of unipolar depression. J Neurosci 1992; 12:3628–3641Crossref, Medline, Google Scholar
6 : Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry 2001; 50:651–658Crossref, Medline, Google Scholar
7 : Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry 2004; 61:877–889Crossref, Medline, Google Scholar
8 : A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp 2008; 29:683–695Crossref, Medline, Google Scholar
9 : Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry 2012; 169:693–703Link, Google Scholar
10 : A meta-analysis of changes in brain activity in clinical depression. Front Hum Neurosci 2015; 8:1045Crossref, Medline, Google Scholar
11 : Abnormal neural activities in adults and youths with major depressive disorder during emotional processing: a meta-analysis. Brain Imaging Behav 2021; 15:1134–1154Crossref, Medline, Google Scholar
12 : Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am J Psychiatry 2020; 177:411–421Link, Google Scholar
13 : Shared neural phenotypes for mood and anxiety disorders: a meta-analysis of 226 task-related functional imaging studies. JAMA Psychiatry 2020; 77:172–179Crossref, Medline, Google Scholar
14 : Neural effects of antidepressant medication and psychological treatments: a quantitative synthesis across three meta-analyses. Br J Psychiatry 2021; 219:546–550Crossref, Medline, Google Scholar
15 : Functional and structural brain correlates of risk for major depression in children with familial depression. Neuroimage Clin 2015; 8:398–407Crossref, Medline, Google Scholar
16 : Elevated amygdala activity in young adults with familial risk for depression: a potential marker of low resilience. Biol Psychiatry Cogn Neurosci Neuroimaging 2020; 5:194–202Crossref, Medline, Google Scholar
17 : Promising neuroimaging biomarkers in depression. Psychiatry Investig 2019; 16:662–670Crossref, Medline, Google Scholar
18 : A neural biomarker of psychological vulnerability to future life stress. Neuron 2015; 85:505–511Crossref, Medline, Google Scholar
19 : UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015; 12:e1001779Crossref, Medline, Google Scholar
20 : Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci 2016; 19:1523–1536Crossref, Medline, Google Scholar
21 : The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage 2002; 17:317–323Crossref, Medline, Google Scholar
22 : Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage 2013; 80:169–189Crossref, Medline, Google Scholar
23 : The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613Crossref, Medline, Google Scholar
24 : A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006; 166:1092–1097Crossref, Medline, Google Scholar
25 : Understanding cognitive impairment in mood disorders: mediation analyses in the UK Biobank cohort. Br J Psychiatry 2019; 215:683–690Crossref, Medline, Google Scholar
26 : The Patient Health Questionnaire 2-item is a rapid, sensitive and specific screening tool for identifying adolescents with major depression. Evid Based Ment Health 2010; 13:104Crossref, Medline, Google Scholar
27 . R: A Language and Environment for Statistical Computing, Vol. 55. Vienna, Austria, R Foundation for Statistical Computing, 2015, pp 275–286. ISBN 3-900051-07-0. www.R-project.org/Google Scholar
28 : Fitting linear mixed-effects models using lme4. J Stat Softw 2015; 67; 1–48Crossref, Google Scholar
29 : ggplot2: Elegant Graphics for Data Analysis. New York, Springer-Verlag, 2009Google Scholar
30 : Patterns of cortico-limbic activations during visual processing of sad faces in depression patients: a coordinate-based meta-analysis. J Neuropsychiatry Clin Neurosci 2014; 26:34–43Crossref, Medline, Google Scholar
31 : Altered brain activity in unipolar depression revisited: meta-analyses of neuroimaging studies. JAMA Psychiatry 2017; 74:47–55Crossref, Medline, Google Scholar
32 : A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage 2012; 61:677–685Crossref, Medline, Google Scholar
33 : Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord 2011; 1:10Crossref, Medline, Google Scholar
34 : Processing of facial emotion expression in major depression: a review. Aust N Z J Psychiatry 2010; 44:681–696Crossref, Medline, Google Scholar
35 : Depression among older adults: a 20-year update on five common myths and misconceptions. Am J Geriatr Psychiatry 2018; 26:107–122Crossref, Medline, Google Scholar
36 : Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychol Sci 2004; 15:259–263Crossref, Medline, Google Scholar
37 : Age-differential patterns of brain activation during perception of angry faces. Neurosci Lett 2005; 386:99–104Crossref, Medline, Google Scholar
38 : The affective neuroscience of aging. Annu Rev Psychol 2016; 67:213–238Crossref, Medline, Google Scholar
39 : What is the test-retest reliability of common task-functional MRI measures? New empirical evidence and a meta-analysis. Psychol Sci 2020; 31:792–806Crossref, Medline, Google Scholar
40 : The reliability paradox: why robust cognitive tasks do not produce reliable individual differences. Behav Res Methods 2018; 50:1166–1186Crossref, Medline, Google Scholar
41 : Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013; 14:365–376Crossref, Medline, Google Scholar



