Testing Quantitative and Qualitative Sex Effects in a National Swedish Twin-Sibling Study of Posttraumatic Stress Disorder
Abstract
Objective:
Twin studies have demonstrated that posttraumatic stress disorder (PTSD) is moderately heritable, and the pattern of findings across studies suggests higher heritability in females compared with males. Formal testing of sex differences has yet to be done in twin studies of PTSD. The authors sought to estimate the genetic and environmental contributions to PTSD, and to formally test for sex differences, in the largest sample to date of both sexes, among twins and siblings.
Methods:
Using the Swedish National Registries, the authors performed structural equation modeling to decompose genetic and environmental variance for PTSD and to formally test for quantitative and qualitative sex differences in twins (16,242 pairs) and in full siblings within 2 years of age of each other (376,093 pairs), using diagnostic codes from medical registries.
Results:
The best-fit model suggested that additive genetic and unique environmental effects contributed to PTSD. Evidence for a quantitative sex effect was found, such that heritability was significantly greater in females (35.4%) than males (28.6%). Evidence of a qualitative sex effect was found, such that the genetic correlation was high but less than complete (rg=0.81, 95% CI=0.73–0.89). No evidence of shared environment or special twin environment was found.
Conclusions:
This is the first demonstration of quantitative and qualitative sex effects for PTSD. The results suggest that unique environmental effects, but not the shared environment, contributed to PTSD and that genetic influences for the disorder are stronger in females compared with males. Although the heritability is highly correlated, it is not at unity between the sexes.
Nearly 70% of the population is exposed to at least one traumatic event in their lifetime (1), and a notable proportion develop posttraumatic stress disorder (PTSD). PTSD is characterized by symptoms of reexperiencing, avoidance, alterations in mood and cognition, and hyperarousal. Lifetime prevalence rates of PTSD are between 6.1% and 9.2% (2, 3). There are well-established sex differences in the prevalence of PTSD, whereby females are approximately twice as likely as males to develop PTSD (4, 5), a disparity that is robust against trauma type, diagnostic criteria, and methodology (4, 6), underscoring the need to examine genetic differences in risk across the sexes. PTSD is associated with deleterious consequences, including physical health problems (7), psychiatric comorbidities (8), and interpersonal difficulties (9). PTSD is associated with an annual productivity loss greater than $3 billion in the United States (10). Given the personal and societal impacts of PTSD, efforts to understand the etiology of this disorder are critical.
PTSD is influenced by both genetic and environmental risk, both of which may contribute to observed sex differences (11). PTSD has less of a research base in high-quality genetic epidemiologic studies compared with other psychiatric disorders, underscoring the need for high-quality genetic epidemiology work on this important disorder. A limited number of twin registries have assessed PTSD, none of which have included national population-based samples (12–14). Of the twin studies of PTSD, moderate genetic influence (i.e., heritability) has been demonstrated, with estimates based on both male and female samples ranging from 26% to 72% (12–14). These estimates may be impacted by the sex of participants included in the study sample. Indeed, True et al. (14), in an all-male sample of Vietnam-era veterans, found that ∼30% of the variance across PTSD symptom clusters was accounted for by genetic factors. In a later twin analysis of PTSD diagnosis in the Vietnam era (15), the heritability of PTSD was reported to be 26% (95% CI=12–40), an estimate that differs from findings reported by Sartor et al. (12), who found that genetic influences accounted for 71% (95% CI=41–85) of the variance in PTSD in an all-female twin sample. Stein et al. (13) reported PTSD to be 38% (95% CI=24–52) heritable in their sample, which included both male and female twin pairs. The pattern across twin studies supports the need to test to see if the degree of genetic influence is higher in females than males, termed a “quantitative sex effect.” However, a key limitation to the literature is that formal tests of differences in heritability between males and females have not been possible, either because single-sex samples were used or, in the only study of both male and female twins (13) (N=406 pairs), because of insufficient statistical power, highlighting the need for well-powered mixed-sex samples.
Evidence from molecular genetic studies on PTSD also support the need to formally test for sex differences in twin studies of PTSD. For example, molecular-based heritability of PTSD across subsets in a large international meta-analysis was consistently higher for females compared with males (16). Furthermore, the molecular literature has identified some sex-specific genetic variants. For example, studies of the pituitary adenylate cyclase-activating polypeptide receptor 1 gene (PACAP) show that the single-nucleotide polymorphism rs2267735 in the PACAP gene predicts PTSD symptoms and diagnosis in females only (17). This finding has been further supported by Lind and colleagues’ meta-analysis (18) demonstrating that an allele of rs2267735 conferred significant risk for PTSD in the combined sex data and in the subsample of females, but not in the subsample of males. Thus, our aim in the present study was to test whether different genes influence the heritable risk among males and females, termed a “qualitative sex effect.”
PTSD has emerged as an important disorder, and it has less of a research base in high-quality genetic epidemiologic studies compared with major depression, bipolar disorder, and schizophrenia. Thus, the purpose of the present study was to estimate the genetic and environmental contributions to PTSD in the largest sample to date of both sexes among twins (N=16,242 pairs) and siblings (N=376,093 pairs), using the Swedish registries. The use of the registry data provides several key methodological advantages. First, the national registry-based design addresses prior shortfalls in representativeness of the samples. Second, all previous twin studies relied on self-report or interviews, which have limitations (e.g., reporting bias, recall bias); we would be able to test for replication of prior findings using registry-based diagnostic codes. Third, we extended the analyses to include sibling pairs in addition to twin pairs, allowing us to test whether there was an influence of twin-specific environment in the etiology of PTSD. Lastly, given that limitations in sample composition and sample size prevented previous studies from formally testing sex effects, this study extends the literature on sex effects on PTSD by testing these effects in a well-powered national sample. PTSD has substantial sex differences in prevalence (4, 5), and clarification of etiologic sex differences in this particular diagnosis is of broad interest to the field. Existing studies in the literature (e.g., 12–14, 16, 18) led us to hypothesize a greater influence of genetic risk in females compared with males, as well as differences between the sexes in the constitution of the heritable risk.
Methods
Data Sources
We linked nationwide Swedish registers via the unique 10-digit identification number assigned at birth or immigration to all Swedish residents. The identification number was replaced by a serial number to ensure anonymity; informed consent was not required for this study as the secondary data are nationwide register data. All procedures contributing to this work complied with the ethical standards of the relevant national and institutional regulations and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects or patients were approved by the Regional Ethical Review Board of Lund University (No. 2008/409 and later amendments). The following sources were used to create our data set: the Multi-Generation Register, containing information about year of birth and legal sex and linking individuals born after 1932 to their relatives; the Swedish Twin Registry; the Hospital Discharge Register, containing data on hospitalizations for Swedish inhabitants from 1964 to 2018; and the Outpatient Care Register, containing information from all outpatient clinics from 2001 to 2018. In addition, we had medical diagnoses from primary health care clinics from the counties throughout Sweden, although the time periods vary because of the regions’ different timing of digitalizing patient records (for details, see the online supplement). We defined 148,823 unique individuals with PTSD, of whom 128,794 (86.54%) were diagnosed in primary health care settings, 32,714 (21.98%) in outpatient care settings, and 15,553 (10.45%) in hospitalizations. Most individuals were found in only one of the registers (N=124,065, 83.36%), but there were some overlaps, where individuals were found in two of the registers (N=21,278, 14.30%) or in all three (N=2,480, 1.67%).
Sample
The study population consisted of males and females (according to legal sex as reported in the registry) who were born in Sweden between 1955 and 1980. Twin pairs were identified from the Swedish Twin Registry and were included in the analysis if the zygosity was known. Full siblings were identified from the Multi-Generation Register and were included in the analysis if they were born less than 2 years apart. To assign zygosity in the same-sex twin pairs, standard self-report items from mailed questionnaires were used, which were 95%–99% accurate when compared with biological markers (for more details, see reference 19). This is also an indirect screening for level of cooperation because at least one of the twins in the pair had to return a questionnaire to the Twin Registry.
Measures
PTSD was assessed from medical registers, using ICD-9 codes 308 and 309 and ICD-10 codes F431, F430, F438, F439, and F620.
Statistical Model
We used an extended version of the classical twin model and included full siblings born up to 2 years apart. As shown in Figure S1 in the online supplement, we assumed a liability threshold model with four possible sources of liability to PTSD: additive genetic (A), shared environment (C), special twin environment (T), and unique environment (E). The model assumes that monozygotic twins share 100% of their genes, while dizygotic twins and full siblings share 50% of their genes identical by descent. Shared environment reflects family and community experiences that render the twins and siblings more similar for the phenotype in question. The special twin environment reflects environmental experiences that are uniquely shared by twin pairs. Unique environment includes developmental effects and other environmental experiences not shared by siblings or twins, as well as a random error. The path components in the model are represented by lowercase letters and the variance components—that is, the squared path components—are denoted by capital letters. To denote the male and female specific components, we utilize the subscripts m and f, respectively. We allowed for different prevalence of PTSD for the males and females, as well as for twins and siblings. In a liability threshold model, the prevalences are captured by different thresholds, and to account for the variation in birth year in the sample, which is associated with risk of having a PTSD diagnosis, we allowed the threshold to linearly depend on birth year, the so-called age regression (see Table S2 and Figure S4 in the online supplement).
In a first set of analyses, we included only same-sex pairs and investigated whether we could simplify the model by dropping the T paths for males (tm) and females (tf). The models being investigated (see Figure S2 in the online supplement) were compared using likelihood ratio tests as well as the Akaike information criterion (AIC), which balances explanatory power and parsimony. After dropping model components that did not improve model fit in terms of AIC, we included opposite-sex pairs and investigated quantitative (i.e., differences in A between males and females) and qualitative (i.e., differences in the genes underlying the heritability, measured by the genetic correlation, rg, and the common environment correlation, rc) sex differences in the constrained model. Opposite-sex pairs, from dizygotic twins and full siblings, provide information on rg and rc, and the models being estimated are presented in Figure S3 in the online supplement. Because we included full-sibling pairs, we obtained a larger sample size and thereby an improved power to investigate quantitative and qualitative sex differences. We performed this two-step procedure to facilitate the convergence of the likelihood function and to obtain reliable estimates.
Qualitative sex differences were investigated by including estimation of a genetic correlation (rg) and a shared environmental correlation (rc), which reflect, respectively, the similarity of the genetic and shared environmental effects affecting PTSD risk in males and females. We first set rc to unity and allowed rg be free, and next, we constrained rg to unity and allowed rc to be free (see Figure S3 in the online supplement for a graphical illustration). Quantitative sex differences were investigated by constraining the A and C path coefficients for males and females. The models were compared using the AIC, with the model with the lowest AIC being chosen as the best-fit model.
We present estimated path coefficients and variance components with likelihood-based 95% confidence intervals for the full and best-fit models.
SAS, version 9.4 (20), was used for data processing and descriptive statistics, and OpenMx (21) was used to fit the ACE models and provide maximum likelihood estimates of the parameters.
Results
Sample
Table 1 presents demographic data for our registry sample. The lifetime prevalence of PTSD was 19.6% in our population sample, and prevalence was higher among females than males (27.8% vs. 11.8%). We included eight types of twin-sibling relationships in our analysis; Table 2 presents the sample sizes, PTSD prevalences, and tetrachoric correlations. The sample sizes were smallest for the twins, and largest for opposite-sex sibling pairs. The prevalence of PTSD was higher in females than in males across all samples. Two noteworthy patterns were found in the tetrachoric correlations: Monozygotic pairs had higher correlations than all other samples, and female pairs had consistently higher correlations than male pairs.
| Group | Number of Pairs | Birth Year | Parental Immigration Status | Parental Education Level | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Both Swedish | One Swedish Born | Both Born Abroad | Low | Middle | High | ||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | ||||
| MZ male | 2,292 | 1967.7 | 7.7 | 2,112 | 92.15 | 100 | 4.36 | 80 | 3.49 | 518 | 22.60 | 962 | 41.97 | 807 | 35.21 |
| DZ male | 2,407 | 1965.2 | 7.4 | 2,224 | 92.40 | 102 | 4.24 | 81 | 3.37 | 711 | 29.54 | 967 | 40.17 | 723 | 30.04 |
| MZ female | 2,707 | 1968.0 | 7.6 | 2,507 | 92.61 | 115 | 4.25 | 85 | 3.14 | 641 | 23.69 | 1,169 | 43.18 | 888 | 32.80 |
| DZ female | 2,577 | 1965.8 | 7.5 | 2,387 | 92.63 | 87 | 3.38 | 103 | 4.00 | 747 | 28.99 | 1,074 | 41.68 | 744 | 28.87 |
| DZ male–female | 6,259 | 1965.9 | 7.3 | 5,768 | 92.16 | 238 | 3.80 | 253 | 4.04 | 1857 | 29.67 | 2532 | 40.45 | 1,851 | 29.57 |
| FS male | 99,923 | 1966.5 | 6.8 | 85,149 | 85.21 | 4,327 | 4.33 | 10,447 | 10.46 | 25,606 | 25.63 | 40,966 | 41.00 | 33,098 | 22.12 |
| FS female | 88,496 | 1966.4 | 6.8 | 75,262 | 85.05 | 3,870 | 4.37 | 9,364 | 10.58 | 23,018 | 26.01 | 36,997 | 41.81 | 28,266 | 31.94 |
| FS male–female | 187,674 | 1966.4 | 6.8 | 159,941 | 85.22 | 8,136 | 4.34 | 19,597 | 10.44 | 47,866 | 25.50 | 77,956 | 41.54 | 61,477 | 32.76 |
TABLE 1. Demographic characteristics of the sample in a study of the heritability of PTSD in males and females, by sibling pairsa
| Group | Number of Pairs | N | With PTSD | Tetrachoric Correlation for PTSD | SE | |
|---|---|---|---|---|---|---|
| N | % | |||||
| MZ male | 2,292 | 4,584 | 489 | 10.67 | 0.283 | 0.051 |
| DZ male | 2,407 | 4,814 | 520 | 10.80 | 0.120 | 0.054 |
| MZ female | 2,707 | 5,414 | 1,437 | 26.54 | 0.351 | 0.031 |
| DZ female | 2,577 | 5,154 | 1,333 | 25.86 | 0.167 | 0.035 |
| DZ male–female | 6,259 | Male: 6,259 | Male: 675 | 10.78 | 0.056 | 0.028 |
| | | Female: 6,259 | Female: 1,644 | 26.27 | | |
| FS male | 99,923 | 199,846 | 22,796 | 11.41 | 0.145 | 0.008 |
| FS female | 88,496 | 176,992 | 48,313 | 27.30 | 0.181 | 0.006 |
| FS male–female | 187,674 | Male: 187,674 | Male: 22,259 | 11.86 | 0.133 | 0.005 |
| | | Female: 187,674 | Female: 51,883 | 27.65 | | |
TABLE 2. Sample sizes (N=760,661 unique individuals) and tetrachoric correlations in a study of the heritability of PTSD in males and femalesa
Same-Sex Modeling
We began model fitting in the same-sex samples to determine whether the unique twin environmental factor (T) was needed. As shown in Table 3, the ACTE model had a higher AIC than the ACE model (36,6057.5 vs. 36,6053.5). Thus, our further model fitting was done from an ACE model (Table 4).
| Model | Description | −2log Likelihood | Number of Parameters | AIC | Difference in AIC | p (LR test) |
|---|---|---|---|---|---|---|
| 1 | Full model | 36,6033.5 | 12 | 36,6057.5 | | |
| 2 | Drop tm | 36,6033.5 | 11 | 36,6055.5 | −2 | 0.9996 |
| 3 | Drop tf | 36,6033.5 | 11 | 36,6055.5 | −2 | 0.9995 |
| 4 | Drop tm and tf | 36,6033.5 | 10 | 36,6053.5 | −4 | 1 |
TABLE 3. Model-fitting results in same-sex pairs in a study of the heritability of PTSD in males and femalesa
| Model | Description | −2log Likelihood | Number of Parameters | AIC |
|---|---|---|---|---|
| 1 | rg free, rc free | 734,242.5 | 14 | 734,270.5 |
| 2 | rg free, rc=1 | 734,242.5 | 13 | 734,268.5 |
| 3 | rg=1, rc free | 734,243.1 | 13 | 734,269.1 |
| 4 | rg=1, rc=1 | 734,243.3 | 12 | 734,267.3 |
| 5 | am=af, cm=cf | 734,288.5 | 11 | 734,310.5 |
| 6 | rg free, cm=cf=0 | 734,242.5 | 11 | 734,264.5 |
| 7 | rg=1, cm=cf=0 | 734,948.04 | 10 | 734,968.0 |
TABLE 4. Model-fitting results in the full sample in a study of the heritability of PTSD in males and femalesa
Full-Sample Modeling
We conducted a series of ACE models to test for sex effects and to determine the most parsimonious model. We began with a full model with both rg and rc parameters estimated (Table 4). Results from the submodels showed evidence for a quantitative sex effect (model 5, which constrained components to equality across the sexes, had the worst model fit, based on AIC) and a qualitative sex effect (model 7, which constrained rg to 1, had a worse fit than model 6, based on AIC). Model 6, which included both sex effects and dropped C, fit the data best, as indicated by having the lowest AIC. Additional sensitivity testing results (see Table S1 in the online supplement) support the conclusion that rg is not 0.
Best-Fit Model Parameters
Based on the model-fitting procedures above, the best-fit model parameter estimates are presented in Figure 1, and are also provided in Table S2 in the online supplement. Heritability of PTSD was higher in females than males (35.42% [95% CI=33.30–37.54] vs. 28.60% [95% CI=25.59–31.60]), suggesting a quantitative sex difference. The rg of 0.81 (SE=0.04) suggests that although the sets of genes contributing to PTSD for females and males are highly correlated, there are some unique effects (i.e., evidence of a qualitative sex difference). Unique environmental effects accounted for the remaining variance: 64.58% (95% CI=62.46–66.70) for females and 73.40% (95% CI=68.40–74.41) for males.
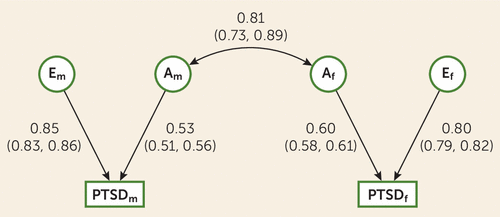
FIGURE 1. Path estimates for the best-fit model in a study of the heritability of PTSD in males and femalesa
aNumbers in parentheses are 95% confidence intervals. A=additive genetic; E=unique environment; f=female; m=male; PTSD=posttraumatic stress disorder.
Discussion
With over 16,000 twin pairs and 376,000 sibling pairs, this is the largest study to date to examine the genetic and both the shared and unique environmental influences on PTSD. Previous twin studies of PTSD have been conducted with substantially smaller sample sizes, with the largest consisting of 4,042 pairs (14). The large sample size in the present study, driven by the inclusion of full siblings, allowed us to model the etiologic components for PTSD with great power to test qualitative and quantitative sex differences. This allowed for estimation of etiologic components with higher precision than was possible in previous twin studies, all of which have had large confidence intervals surrounding the heritability estimates (e.g., 12%–40% [15]). Our results indicate moderate heritability for PTSD (females, 35.42%; males, 28.60%). The moderate heritability estimates found in our analyses are in line with previous studies in all males (14) and combined sex pairs (13) and are within the confidence interval range (41%–85%) of the heritability estimated in an all-female sample (12). Our findings are also consistent with a previous twin study by our group conducted in Norwegian young adults that found that familial factors accounted for 32% (95% CI=0.18–0.65) of variance in PTSD (22); the sample was, however, underpowered to distinguish genetic from shared environmental sources of influence.
Given the markedly different methodology between this study, being registry based and representative of the entire country, and previous studies, based on either personal interview (12) or self-report (13, 14), the close agreement of heritability estimates is reassuring. Our data are derived from a national sample using medical registries, which addresses potential sources of bias in self-report or interview studies (e.g., retrospective memory biases). The consistency of findings across diverse methods, diagnostic approaches, samples, and so on, suggests that the findings are robust against sources of error (23). Moreover, this is the first study to include both siblings and twins, which allowed testing for the influence of special twin environment (e.g., shared womb, unique twin relationship and family environment). We did not find evidence of a twin-specific environmental component. The best-fit model was an AE model, suggesting robust replication among siblings compared to previous twin studies. Our findings are consistent with previous studies that did not find a role of common environment in the etiology of PTSD (12–14).
We found that PTSD was moderately heritable, and, as hypothesized, we found a significant sex difference in that the degree of heritability was higher among females than males, which is evidence of a quantitative sex difference. Although this is the first formal test of a quantitative sex effect for PTSD, this finding of greater genetic influence among females compared with males is consistent with the overall pattern of findings across twin studies. Additionally, the molecular heritability of PTSD is higher in females than males (16). Analyses of major depression, also from Sweden, found similar sex differences of higher heritability estimates in females than males (42% [95% CI=36–47] vs. 29% [95% CI=19–38]) (24), as have other, smaller twin studies on major depression (25, 26).
We also found support for a modest qualitative sex effect (rg=0.81, 95% CI=0.73–0.89), suggesting that that the genes that contribute to PTSD in females and males, although highly correlated, are not entirely the same. The model fit worsened when the genetic correlation was set to unity (i.e., when the model forced the genetic correlation between males and females to be equal). Because this is the first formal test of a qualitative sex effect in a behavioral genetic study of PTSD, we do not have prior twin literature on this phenotype with which to compare our finding. However, in line with our qualitative sex difference finding, the molecular genetic literature on PTSD has found evidence of some sex-specific variant associations. Specifically, rs2267735 in ADCYAP1R1, which is critical in estrogen regulation, is associated with PTSD in females but not in males (17), a finding supported by a meta-analysis across studies (18). Studies of major depressive disorder, both in Sweden (rg=0.63, 95% CI=0.31–0.99) (24) and in the United States (rg=0.55, 95% CI=0.31–0.75) (26), have also reported qualitative sex effects, and the confidence intervals overlap with those found in the present study.
Given our findings of heritability differences as well as evidence for different genes influencing the heritability between the sexes, future studies should investigate the genetic underpinnings of sex hormones. Previous research suggests that testosterone, estrogen, and progesterone are all involved in the development of PTSD (for a review, see reference 27). Specific to females, variations in estrogen and progesterone during the menstrual cycle have been found to impact the hypothalamic-pituitary-adrenal (HPA) axis in response to stress (28, 29) as well as the cognitive-emotional processes underlying PTSD, such as fear conditioning and extinction (30). Contrastingly, testosterone has been found to have anxiolytic effects via reducing the HPA axis’s reactivity to stress (31, 32), whereas the constantly varied hormonal environment of females is thought to increase the reactivity of the HPA axis to stress, making females more susceptible to PTSD (33). Sex hormones may also influence PTSD development through epigenetic modification. For example, estrogen has been shown to regulate the expression of both ADCYAP1R1 (34) and HDAC4 (29), a gene that is linked to long-term memory formation and behavior, demonstrating that estrogen levels are influential in the stress response of females and that this system is vulnerable to genetic disruptions.
Front-line treatments for PTSD, suggested by various treatment guidelines, include trauma-focused therapies (e.g., prolonged exposure, cognitive processing therapy) (35). These treatments were found to be effective for both sexes in a recent large-scale meta-analysis (36). However, the effect sizes for females were found to be higher than those for males at posttreatment and follow-up assessments. Although the source of these differential psychosocial treatment effects is not yet known, they constitute a growing area of research; one such future direction includes the exploration of genetic influences on treatment response. In contrast to trauma-focused therapies, a meta-analysis of treatment with selective serotonin reuptake inhibitors for PTSD did not reveal differential effects by sex (37).
Two key limitations of the present study stem from the reliance on registry-based ICD codes for PTSD. First, we are unable to disentangle the effects of trauma exposure from that of PTSD. Studies using self-report history of trauma exposure have examined the gene-environment correlation for trauma exposure itself and found that exposure is moderately heritable (13, 38, 39) and more so for assaultive traumatic events (13, 38). Females are more likely to report experiencing sexual violence as compared with males, who are more likely to experience accidents, non-sexual assaults, and combat exposure (4, 40). Sex differences in trauma type exposure are an important consideration in the context of sex differences in rates of PTSD given that sexual violence has been shown to be the most pathogenic type of trauma exposure in terms of developing PTSD (5). Our group has estimated the degree of overlap of etiologic factors for self-reported trauma exposure with that of PTSD, finding that one-fifth of the familial influences on PTSD overlap with those that influence trauma (22). Thus, it is likely that part of the genetic and environmental influence of PTSD observed in this study is also capturing risk for exposure to trauma itself. Second, we are unable to examine item-level data underlying the dichotomous diagnoses. Thus, there is a possibility that sex invariance exists in the diagnoses being made in males and females. Among other limitations of our study is that the main source of our PTSD case subjects was nationwide primary care data, where diagnoses were made mainly by primary care physicians rather than psychologists or psychiatrists. Additionally, because sibling and twin data are sparse for immigrants in Sweden, our findings’ generalizability to populations beyond native-born Swedes is limited. However, slightly more than 5% of the study population are first-generation immigrants. In future analyses, examination of data from immigrants should be included to expand the generalizability of the findings. Additionally, studies utilizing other national registries should be conducted to determine whether the findings replicate.
In summary, PTSD has emerged as an important disorder, yet the genetic epidemiologic research base remains scant compared with other psychiatric disorders. This study represents the largest twin study of PTSD to date, the first to include siblings, and the first to test for sex differences within a twin modeling framework, which is critical given the substantial sex differences in prevalence. Our hypotheses of both quantitative and qualitative sex effects were supported. We note that the quantitative sex differences were more robust than the qualitative sex differences. Given the high comorbidity of PTSD with other disorders across the internalizing and externalizing spectrum, future analyses should incorporate multivariate modeling to test the degree of heritable overlap between PTSD and other common posttraumatic conditions. In addition, replication of findings with diverse ancestries is needed.
1. : The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychol Med 2016; 46:327–343Crossref, Medline, Google Scholar
2. : Posttraumatic stress disorder in the World Mental Health Surveys. Psychol Med 2017; 47:2260–2274Crossref, Medline, Google Scholar
3. : The epidemiology of DSM-5 posttraumatic stress disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions–III. Soc Psychiatry Psychiatr Epidemiol 2016; 51:1137–1148Crossref, Medline, Google Scholar
4. : Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull 2006; 132:959–992Crossref, Medline, Google Scholar
5. : National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress 2013; 26:537–547Crossref, Medline, Google Scholar
6. : Risk factors predict post-traumatic stress disorder differently in men and women. Ann Gen Psychiatry 2008; 7:24Crossref, Medline, Google Scholar
7. : PTSD and physical health. Curr Psychiatry Rep 2018; 20:116Crossref, Medline, Google Scholar
8. : Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry 2012; 69:372–380Crossref, Medline, Google Scholar
9. : Posttraumatic stress disorder and intimate relationship problems: a meta-analysis. J Consult Clin Psychol 2011; 79:22–33Crossref, Medline, Google Scholar
10. : Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry 2000; 61 Suppl 5:4–12Medline, Google Scholar
11. : Using the tools of genetic epidemiology to understand sex differences in neuropsychiatric disorders. Genes Brain Behav 2020; 19:e12660Crossref, Medline, Google Scholar
12. : Common genetic and environmental contributions to posttraumatic stress disorder and alcohol dependence in young women. Psychol Med 2011; 41:1497–1505Crossref, Medline, Google Scholar
13. : Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry 2002; 159:1675–1681Link, Google Scholar
14. : A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry 1993; 50:257–264Crossref, Medline, Google Scholar
15. : Common genetic liability to major depression and posttraumatic stress disorder in men. J Affect Disord 2008; 105:109–115Crossref, Medline, Google Scholar
16. : International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun 2019; 10:4558Crossref, Medline, Google Scholar
17. : Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 2011; 470:492–497Crossref, Medline, Google Scholar
18. : Association of posttraumatic stress disorder with rs2267735 in the ADCYAP1R1 gene: a meta-analysis. J Trauma Stress 2017; 30:389–398Crossref, Medline, Google Scholar
19. : The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med 2002; 252:184–205Crossref, Medline, Google Scholar
20. : Stata Statistical Software: Release 9.4. College Station, Tex, StataCorp, 2020 Google Scholar
21. : OpenMx: an open source extended structural equation modeling framework. Psychometrika 2011; 76:306–317Crossref, Medline, Google Scholar
22. : A population-based study of familial and individual-specific environmental contributions to traumatic event exposure and posttraumatic stress disorder symptoms in a Norwegian twin sample. Twin Res Hum Genet 2012; 15:656–662Crossref, Medline, Google Scholar
23. : Robust research needs many lines of evidence. Nature 2018; 553:399–401Crossref, Medline, Google Scholar
24. : A Swedish national twin study of lifetime major depression. Am J Psychiatry 2006; 163:109–114Link, Google Scholar
25. : Major depressive disorder in a community-based twin sample: are there different genetic and environmental contributions for men and women? Arch Gen Psychiatry 1999; 56:557–563Crossref, Medline, Google Scholar
26. : Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychol Med 2001; 31:605–616Crossref, Medline, Google Scholar
27. : Gender- and sex-based contributors to sex differences in PTSD. Curr Psychiatry Rep 2020; 22:19Crossref, Medline, Google Scholar
28. : Menstrual cycle effects on psychological symptoms in women with PTSD. J Trauma Stress 2015; 28:1–7Crossref, Medline, Google Scholar
29. : Estrogen-dependent association of HDAC4 with fear in female mice and women with PTSD. Mol Psychiatry 2018; 23:658–665Crossref, Medline, Google Scholar
30. : Prepulse inhibition deficits in women with PTSD. Psychophysiology 2016; 53:1377–1385Crossref, Medline, Google Scholar
31. : Sex differences in anxiety and depression: role of testosterone. Front Neuroendocrinol 2014; 35:42–57Crossref, Medline, Google Scholar
32. : The effect of deployment to a combat zone on testosterone levels and the association with the development of posttraumatic stress symptoms: a longitudinal prospective Dutch military cohort study. Psychoneuroendocrinology 2015; 51:525–533Crossref, Medline, Google Scholar
33. : Mediation and moderation effects of sex and gender in PTSD, in Comprehensive Guide to Post-Traumatic Stress Disorder. Edited by Martin C, Preedy V, Patel V. Cham, Switzerland, Springer, 2016, pp 1465–1481 Crossref, Google Scholar
34. : Functional evaluation of a PTSD-associated genetic variant: estradiol regulation and ADCYAP1R1. Transl Psychiatry 2016; 6:e978Crossref, Medline, Google Scholar
35. : Gender issues in PTSD, in Handbook of PTSD Science and Practice, 3rd ed. Edited by Friedman MJ, Schnurr PP, Keane TM. New York, Guilford, 2021, pp 229–245 Google Scholar
36. : Chromosomes to social contexts: sex and gender differences in PTSD. Curr Psychiatry Rep 2018; 20:114Crossref, Medline, Google Scholar
37. : AHRQ Comparative Effectiveness Reviews: Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder: A Systematic Review Update. Rockville, Md, Agency for Healthcare Research and Quality, 2018 Crossref, Google Scholar
38. : Common heritable contributions to low-risk trauma, high-risk trauma, posttraumatic stress disorder, and major depression. Arch Gen Psychiatry 2012; 69:293–299Crossref, Medline, Google Scholar
39. : Do genes influence exposure to trauma? A twin study of combat. Am J Med Genet 1993; 48:22–27Crossref, Medline, Google Scholar
40. : Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry 1998; 55:626–632Crossref, Medline, Google Scholar


