Effects of Gabapentin on Dorsal Anterior Cingulate Cortex GABA and Glutamate Levels and Their Associations With Abstinence in Alcohol Use Disorder: A Randomized Clinical Trial
Abstract
Objective:
Although gabapentin has demonstrated efficacy in mitigating alcohol withdrawal symptoms and preventing relapse drinking in individuals with alcohol use disorder (AUD), the neurobiological mechanisms of action underlying these therapeutic effects remain unknown. The present study evaluated changes in GABA and glutamate levels in the dorsal anterior cingulate cortex (dACC) as candidate mechanisms of action.
Methods:
In a 16-week randomized clinical trial, 68 adults with AUD, including a history of alcohol withdrawal syndrome, received 1,200 mg/day of gabapentin (N=37) or placebo (N=31) and nine medical management visits after ≥72 hours of abstinence. Proton MR spectroscopy (1H-MRS) estimates of dACC levels of GABA (N=67) and glutamate (N=64) were acquired before start of treatment and again approximately 14 days after randomization. Percent days abstinent was reported via timeline followback interview.
Results:
The effects of gabapentin on GABA and glutamate levels were significantly associated with participants’ percent days abstinent during early treatment. Specifically, gabapentin was associated with greater increases in glutamate and greater decreases in GABA levels in participants who remained mostly or entirely abstinent, and yet the opposite in participants who drank on more than half of the days preceding the second scan. Furthermore, gabapentin-treated participants with greater increases in glutamate levels during early treatment had significantly more percent days abstinent across the remainder of the study, relative to placebo-treated participants.
Conclusions:
In addition to providing insight into the mechanisms through which gabapentin may promote abstinence in individuals with AUD, this study also provides evidence for a biomarker of efficacious treatment that may be used to evaluate other glutamatergic or GABAergic medications for AUD and related conditions.
Gabapentin, a safe and well-tolerated medication that is approved by the U.S. Food and Drug Administration to treat postherpetic neuralgia, partial seizures, and restless legs syndrome, has garnered considerable attention for its efficacy in mitigating alcohol withdrawal symptoms (1) and preventing relapse drinking in individuals with alcohol use disorder (AUD) (1–3). Although much research has focused on the neurobiological mechanisms of gabapentin’s (“on-label”) anticonvulsant, anxiolytic, and analgesic effects, few studies have focused on the mechanisms of its (“off-label”) therapeutic effects in AUD, which likely diverge as a result of the interacting effects of drinking and withdrawal on the brain (4). The present study represents the first controlled investigation of the neurobiological mechanisms of gabapentin in people with AUD.
Although gabapentin was designed as a structural analogue to γ-aminobutyric acid (GABA), it is inert at GABA receptors and synapses (5). Instead, gabapentin binds with high affinity to the α2δ-1 protein and exerts its primary therapeutic effects through selective blockade of presynaptic voltage-gated calcium channels containing the α2δ-1 subunit (5). Recent research has revealed that gabapentin’s therapeutic effects may also involve 1) α2δ-1 interaction with non-calcium-channel proteins, including N-methyl-d-aspartate (NMDA) receptors, neurexin-1α, and thrombospondin (6), as well as 2) α2δ-1-independent mechanisms, including activation of KCNQ3/5 voltage-gated potassium channels (7) and expression of δ-subunit-containing GABAA receptors (8). Each of these molecular mechanisms reduces excitatory/glutamatergic and/or enhances inhibitory/GABAergic neurotransmission.
Proton MR spectroscopy (1H-MRS) provides the opportunity to use MRI to better understand these issues in humans by measurement of brain GABA and glutamate levels in vivo. 1H-MRS studies in healthy volunteers (9, 10) and adults with epilepsy (11, 12) have confirmed that gabapentin increases brain GABA levels by 25%−50%, with lower baseline GABA levels and relatively higher doses of gabapentin associated with larger increases in GABA (9, 11).
Comparatively few studies have investigated the mechanisms of gabapentin’s effects on alcohol drinking and withdrawal in AUD. Because the neurobiological effects of drinking (i.e., inhibition of neuronal signaling via binding to GABA receptors and inhibiting glutamatergic synapses [13, 14]) involve the same neurochemical mechanisms as gabapentin, the literature on gabapentin’s mechanisms cannot be easily applied to AUD. A seminal preclinical investigation of gabapentin demonstrated decreased anxiogenic effects of withdrawal and dependence-induced self-administration of alcohol, mediated by decreased GABAergic transmission in the central amygdala (15). Subsequent studies have found that gabapentin 1) prevents excessive excitatory synaptogenesis by antagonizing the interaction of α2δ-1 with thrombospondins, upregulated after intermittent ethanol exposure (16), and 2) normalizes alcohol-induced deficits in delta (1–4 Hz) power during slow-wave sleep (17). Conversely, a recent 1H-MRS study found no association between parieto-occipital cortex GABA levels after ≥1 week of gabapentin treatment in a cross-sectional convenience sample of individuals with AUD in early recovery (18).
It is against this background that we report the results of the first controlled investigation of the neurobiological mechanisms of gabapentin treatment for AUD. In this MRI substudy of a previously reported clinical trial (2), individuals with AUD, including a history of alcohol withdrawal syndrome and ≥72 hours of abstinence, were randomly assigned to receive gabapentin (1,200 mg/day) or placebo treatment for 16 weeks. Eligible participants additionally completed two 1H-MRS/MRI scans, one immediately preceding the first dose of study drug and the other after approximately 2 weeks of treatment. Here, we report the prospective effects of 1) early gabapentin treatment on GABA and glutamate levels in the dorsal anterior cingulate cortex (dACC), in the context of variable relapse drinking among participants, and 2) GABAergic and glutamatergic medication effects on further drinking across the remainder of the trial.
Methods
Participants and Procedure
1H-MRS data for the present analysis were collected as part of a larger (N=96) 16-week, randomized, double-blind, placebo-controlled trial of gabapentin (titrated over 5 days to 1,200 mg/day) for individuals with AUD and a history of alcohol withdrawal syndrome. A history of posttraumatic stress disorder with stable symptoms was allowed given its comorbidity with AUD and potential response to gabapentin (19). The study protocol was approved by the Medical University of South Carolina Institutional Review Board. Briefly, after providing written informed consent and undergoing assessment, participants who met DSM-5 (20) criteria for AUD and a history of alcohol withdrawal syndrome, with no current major psychiatric illnesses, no other substance dependence, and no clinically significant alcohol withdrawal syndrome at baseline (defined as a score ≥10 on the Clinical Institute Withdrawal Assessment for Alcohol Scale–Revised [21]), were randomly assigned to receive gabapentin or placebo for 16 weeks, with drinking assessed during this period using a daily calendar method (the timeline followback) (22). The primary report found that significantly more individuals in the gabapentin group had no heavy drinking days and total abstinence compared with those in the placebo group (2). Consented and evaluable participants of the 1H-MRS substudy (N=68) (Figure 1) completed their first scan immediately prior to treatment randomization (following ≥3 days of abstinence from alcohol; mean=4.24 days, SD=2.03) and their second scan approximately 14 days after randomization (median=14 days, range=11–21). The initial 3-T Trio (N=47) MRI scanner was upgraded to a Prisma-Fit 3-T scanner (N=21) (Siemens, Germany) partway through the study, with the same 32-channel phased-array head coil used across scanners. Time from randomization and scanner were examined as covariates across statistical models, but were eliminated, as they were universally nonsignificant (p values >0.10).
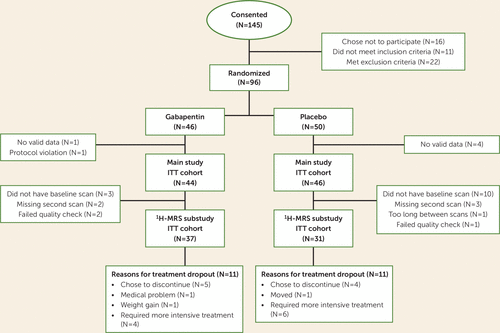
FIGURE 1. CONSORT diagram for a 1H-MRS study of gabapentin in alcohol use disordera
a1H-MRS=proton MR spectroscopy; ITT=intention-to-treat.
1H-MRS Acquisition and Processing
A structural scan was taken for 1H-MRS voxel placement and tissue segmentation (256 sagittal slices; 1 mm thick, 50% gap). A 2.5×2.5×3 cm3 dACC voxel was placed on midsagittal T1-weighted images, posterior to the genu of the corpus callosum with the ventral edge aligned to the dorsal edge of the callosum (23). The dACC was chosen because of its demonstrated role in brain response to alcohol cues, relapse to heavy drinking, and core neurobehavioral deficits associated with AUD, and because most previous 1H-MRS AUD studies have measured this region (24–27). See Figure 2 for visualization of the voxel and sample spectrum. After placement of saturation bands 1 cm away from voxel faces and shimming via FASTESTMAP (28), single-voxel water-suppressed 1H-MRS spectra were acquired using a MEGA-PRESS sequence (TR=2,000 ms, TE=68 ms, number of averages=256) with symmetric editing pulse frequencies for macromolecule suppression (1.9 ppm, 1.5 ppm) (29) and a PRESS sequence sensitive to glutamate (TR=2,000 ms, TE=40 ms, number of averages=128) (30). Each sequence was followed by a matched water-unsuppressed acquisition for phase and eddy current correction and concentration referencing. Common sequence parameters included TR=2,000 ms, 16-step phase cycling, spectral width=2.5 kHz, and 2,048 complex data points. MEGA-PRESS data were processed using the Gannet MATLAB toolbox (version 3.1), with frequency/phase correction applied prior to fitting (31). PRESS data were processed using LCModel, version 6.3, with a vendor-supplied GAMMA-simulated basis set and an analysis window of 0.2–4.0 ppm (32). Within-voxel tissue fractions of gray and white matter and CSF were calculated based on automated segmentation in SPM12 (Wellcome Department of Cognitive Neurology), using a volume mask generated in Gannet (33). Metabolite concentrations were normalized to unsuppressed water and corrected for within-voxel CSF fraction.
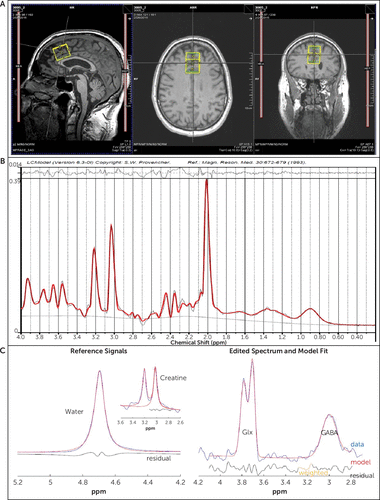
FIGURE 2. Dorsal anterior cingulate cortex (dACC) voxel and sample spectrum in a 1H-MRS study of gabapentin in alcohol use disordera
a Panel A shows a representative dorsal anterior cingulate cortex (dACC) voxel placement. Panel B shows a representative PRESS spectrum fitted in LCModel. Panel C shows a representative MEGA-PRESS GABA difference spectrum (right) along with reference water and creatine signals (left), fitted in Gannet. 1H-MRS=proton MR spectroscopy.
Data Analytic Plan
First, to evaluate the generalizability of our previously reported findings to the present subsample, the number of evaluable individuals with no drinking days (abstinent) over the entire 16-week trial was analyzed using a two-sample z test to produce a chi-square p value; individuals with missing drinking data were assumed to have been drinking (2). 1H-MRS quality control was conducted via visual inspection of spectra (34), by investigators blind to condition (J.J.P. and T.R.B.), along with evaluation of spectral fit errors relative to their observed distribution. Spectra with fit errors >3*interquartile range above the third quartile or below the first quartile were excluded from analysis. Change variables were created for both glutamate and GABA by subtracting initial scan values from those at the second scan. The ratio of gray matter to brain matter was calculated as the amount of within-voxel gray matter over the sum of within-voxel gray and white matter. The relationship between change in metabolite values (the dependent variable) and medication group, drinking levels between scans (percent days abstinent), and their interaction was estimated with linear models. Each model included covariates measuring baseline percent days abstinent, the metabolite value at the first scan, and ratio of gray matter to brain matter. To further examine the interaction between medication group and between-scan drinking, PROCESS model 1 was used to calculate Johnson-Neyman significance regions (35). For the second part of the analysis, linear mixed-effect models were estimated using REML in the R package nlme (36). These models predicted weekly percent days abstinent over the remainder of the study (seven 2-week periods) following each participant’s second scan and used all available longitudinal data from each participant, providing unbiased parameter estimates and standard errors when missing values were at least missing at random (37). Medication group, change in metabolite values, and their interaction were included as predictors. Baseline percent days abstinent was incorporated as a covariate. These models were reestimated with baseline metabolite values entered in place of change in metabolite values to evaluate whether baseline metabolite levels could serve a treatment response prediction function, similarly to change in metabolite levels.
Results
There were no significant differences between participants in the gabapentin and placebo groups on any demographic or clinical characteristic (Table 1). Sixty participants (88%) from the 16-week parent study (gabapentin group, N=33; placebo group, N=27) provided full longitudinal drinking data (see Table S3 in the online supplement for percent days abstinent by treatment group by week). Participants demonstrated good medication adherence, determined via pill counts, and adherence did not differ by treatment group (gabapentin: mean=92.7%, SD=8.8; placebo: mean=92.7%, SD=12.1; p=0.988). The previously reported effect of gabapentin on total abstinence (2) was upheld in the present subsample, with significantly more individuals in the gabapentin group abstinent (8 of 37; 22%) compared with the placebo group (1 of 31; 3%), a difference of 19% (p=0.026).
| Characteristic | Total (N=68) | Gabapentin (N=37) | Placebo (N=31) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 49.3 | 10.6 | 49.6 | 10.4 | 48.9 | 10.9 |
| N | % | N | % | N | % | |
| PTSD (current or past) | 18 | 26.5 | 9 | 24.3 | 9 | 29.0 |
| Nicotine use | 31 | 45.6 | 17 | 45.9 | 14 | 45.2 |
| Antidepressant use | 18 | 26.5 | 9 | 24.3 | 9 | 29.0 |
| Male | 50 | 73.5 | 28 | 75.7 | 22 | 71.0 |
| Married or cohabitating | 29 | 42.6 | 19 | 51.4 | 10 | 32.3 |
| Education ≤12 years | 8 | 11.8 | 5 | 13.5 | 3 | 9.7 |
| Employed | 49 | 72.1 | 26 | 70.3 | 23 | 74.2 |
| White | 65 | 95.6 | 35 | 94.6 | 30 | 96.8 |
| Mean | SD | Mean | SD | Mean | SD | |
| Alcohol use and severity indicators | ||||||
| Drinks per dayb | 11.0 | 4.6 | 10.8 | 4.3 | 11.2 | 5.0 |
| Drinks per drinking dayb | 13.0 | 4.8 | 13.2 | 4.9 | 12.8 | 4.8 |
| Days abstinentb (%) | 14.1 | 21.3 | 14.9 | 22.7 | 13.3 | 19.8 |
| Heavy drinking daysb (%) | 83.0 | 22.3 | 82.8 | 23.6 | 83.2 | 21.0 |
| Days abstinent prior to randomization | 4.2 | 2.0 | 4.2 | 2.1 | 4.2 | 1.9 |
| Alcohol Dependence Scale scorec | 18.6 | 7.5 | 19.7 | 7.8 | 17.4 | 7.1 |
| Obsessive Compulsive Drinking Scale scored | 27.4 | 9.4 | 27.9 | 9.1 | 26.7 | 9.9 |
| Alcohol Withdrawal Symptom Checklist score | 10.5 | 7.0 | 10.9 | 7.4 | 9.9 | 6.4 |
| DSM-5 alcohol withdrawal items positive | 4.5 | 1.3 | 4.7 | 1.1 | 4.4 | 1.5 |
| Clinical Institute Withdrawal Assessment for Alcohol Scale–Revised score | 2.8 | 1.8 | 2.8 | 2.0 | 2.8 | 1.6 |
| N | % | N | % | N | % | |
| Past alcohol treatments and measures | ||||||
| Treatments | 19 | 27.9 | 13 | 35.1 | 6 | 19.4 |
| Detoxifications | 9 | 13.2 | 7 | 18.9 | 2 | 6.5 |
| Alcohol blood tests (biomarkers) | ||||||
| Carbohydrate-deficient transferrin ≥1.7% | 50 | 74.6 | 26 | 72.2 | 24 | 77.4 |
| GGT >36 U/L | 52 | 76.5 | 29 | 78.4 | 23 | 74.2 |
TABLE 1. Demographic and drinking characteristics of participants in a 1H-MRS study of gabapentin in alcohol use disordera
Effects of Gabapentin on Early Treatment Changes in Glutamate and GABA Levels
Four participants (three in the gabapentin group, one in the placebo group) were excluded from the glutamate analyses and one (in the gabapentin group) from the GABA analyses because their 1H-MRS scans failed quality control. Within-voxel gray matter–to–brain matter tissue fraction did not significantly differ between participants in gabapentin group (mean=0.65, SD=0.043) and the placebo group (mean=0.67, SD=0.039; p=0.081). (See Table S1 in the online supplement for 1H-MRS quality control metrics and Table S2 for summary statistics of metabolite levels organized by scan and by group.)
Controlling for pretreatment glutamate level (β=−0.57, p<0.001) and percent days abstinent over the 90 days preceding screening (β=−0.14, p=0.745), as well as percent days abstinent between pretreatment and posttreatment scans (β=−0.81, p=0.069) and gray matter–to–brain matter tissue fraction (β=0.92, p=0.703), participants in the gabapentin group had greater decreases (and/or smaller increases) in glutamate between scans (β=−1.26, p=0.008). However, this main effect of treatment was qualified by a significant interaction with percent days abstinent between scans (β=1.68, p=0.005). Specifically, Johnson-Neyman analyses demonstrated that participants in the gabapentin group with <50% of days abstinent between scans had greater decreases (and/or smaller increases) in glutamate relative to those in the placebo group (p values <0.05), whereas participants in the gabapentin group with >95% of days abstinent between scans had greater increases (and/or smaller decreases) in glutamate relative to those in the placebo group (p=0.08) (Figure 3A). (See Figure S1 in the online supplement for spaghetti plots of glutamate levels by between-scan drinking by treatment group.)
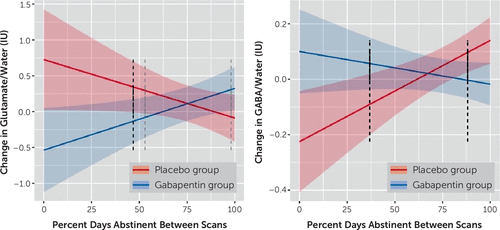
FIGURE 3. Change in dorsal anterior cingulate cortex (dACC) glutamate (N=64) and GABA (N=67) levels, by percent days abstinent between scans, by treatment groupa
a Results are normalized to water and expressed in international units. Error bands represent 95% confidence intervals. Dark and light dotted lines represent the p<0.05 and p<0.10 thresholds, respectively, of Johnson-Neyman significance regions.
Conversely, controlling for pretreatment GABA level (β=−0.77, p<0.001) and pretreatment percent days abstinent (β=0.09, p=0.445), as well as percent days abstinent between scans (β=0.37, p=0.002) and gray matter–to–brain matter fraction (β=−1.23, p=0.038), participants in the gabapentin group had greater increases (and/or smaller decreases) in GABA between scans (β=0.33, p=0.009). However, similar to the glutamate measures, this main effect of treatment was qualified by a significant interaction with percent days abstinent between scans (β=−0.48, p=0.002). Specifically, Johnson-Neyman analyses demonstrated that participants in the gabapentin group with <40% of days abstinent between scans had greater increases (and/or smaller decreases) in GABA relative to those in the placebo group (p values <0.05), whereas participants in the gabapentin group with >85% of days abstinent between scans had greater decreases (and/or smaller increases) in GABA relative to those in the placebo group (p values <0.05) (Figure 3B). (See Figure S2 in the online supplement for spaghetti plots of GABA levels by between-scan drinking by treatment group.)
Effects of Gabapentin-Induced Changes in Glutamate and GABA Levels on Subsequent Abstinence
After controlling for pretreatment percent days abstinent (β=0.57, p=0.003) and time in study (averaged over 2-week blocks) (β=−0.01, p=0.268), there was a significant interaction between medication group (main effect, β<−0.01, p=0.909) and change in between-scan glutamate levels (main effect, β=−0.14, p=0.051; interaction, β=0.28, p=0.007) on percent days abstinent during the remaining study treatment period. Specifically, Johnson-Neyman analyses demonstrated that participants in the gabapentin group who had decreased glutamate levels between scans (i.e., <−0.88 IU; Z<−1.24 or 7.8% of the sample) had fewer percent days abstinent for the remainder of the study relative to those in the placebo group (p values <0.05), whereas participants in the gabapentin group who had increased glutamate levels between scans (i.e., >0.79 IU; Z>0.86 or 18.8% of the sample) had more percent days abstinent relative to those in the placebo group (p values <0.05) (Figure 4A). Substituting between-scan changes in glutamate levels for baseline glutamate levels, we found a significant interaction between medication group (main effect, β=3.24, p=0.040) and baseline glutamate levels on percent days abstinent during the remaining study treatment period (main effect, β=0.22, p=0.028; interaction, β=−0.29, p=0.040) (see Figure S2 in the online supplement).
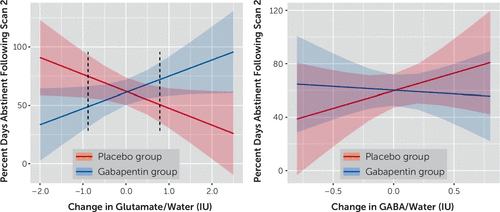
FIGURE 4. Percent days abstinent following scan 2 (i.e., early treatment), by change in dorsal anterior cingulate cortex (dACC) glutamate (N=64) and GABA (N=67) levels, by treatment groupa
a Results are normalized to water and expressed in international units. Error bands represent 95% confidence intervals. Dark dotted lines represent the p<0.05 threshold of Johnson-Neyman significance regions.
In contrast, when pretreatment percent days abstinent (β=0.54, p=0.017) and time in study (β=−0.01, p=0.299) were controlled for, there were no significant main effects of medication group (β=0.004, p=0.959) or change in between-scan GABA levels (β=0.26, p=0.287), nor of their interaction (β=−0.32, p=0.327), on percent days abstinent during the remaining study treatment period (Figure 4B). Substituting between-scan changes in GABA levels for baseline GABA levels provided similarly null findings (p values >0.50)
Discussion
Following recent demonstration of the antidrinking efficacy of gabapentin in individuals with AUD and a history of alcohol withdrawal syndrome (2), the present analysis provides insight into the neurobiological mechanisms through which gabapentin may have worked to promote abstinence in that study. This preplanned investigation was based on the knowledge that adaptations in brain GABA and glutamate systems underlie AUD in general (4) and alcohol withdrawal syndrome in particular (23), and that potential gabapentin effects on those systems might explain its treatment efficacy (15). Consistent with the preclinical and 1H-MRS gabapentin literature, gabapentin treatment was associated with significantly increased GABA levels and significantly decreased glutamate levels in the dACC, a fronto-cortical brain region. However, these associations were significantly moderated by (i.e., depended on) participants’ percentage of abstinent days during the first few weeks of treatment, which is not surprising given the large and dynamic impact that drinking itself has on brain glutamate and GABA levels (14). Specifically, gabapentin (relative to placebo) was associated with 1) greater increases in glutamate and greater decreases in GABA levels in participants who remained mostly, or entirely, abstinent, but 2) greater decreases in glutamate and greater increases in GABA levels in participants who drank on more than approximately half of the days preceding the second scan. Furthermore, participants in the gabapentin group who had greater increases in glutamate (but not GABA) levels during the early weeks of treatment had significantly more percent days abstinent across the remaining study treatment period relative to those in the placebo group. Finally, lower baseline metabolite levels were associated with greater-magnitude metabolite-level changes across the first few weeks of treatment, and participants in the gabapentin group who had lower baseline glutamate levels had significantly more percent days abstinent across the study treatment period.
Consistent with the inhibitory effects of alcohol drinking on glutamatergic synapses, AUD has been consistently associated with reduced fronto-cortical glutamate levels in 1H-MRS studies (38–42), with the notable exception of during acute, clinically significant alcohol withdrawal, which has been associated with transient spikes in glutamate levels (23). Although the present study focused on individuals with AUD and a history of alcohol withdrawal syndrome, those experiencing acute, clinically significant alcohol withdrawal were excluded from participation to ensure their safety (i.e., if they were receiving placebo). As a result, gabapentin-induced increases in dACC glutamate levels in the present study likely represented neurochemical shifts in the direction of abstinence-induced “normalization” rather than acute alcohol withdrawal effects, and this normalization depended on maintaining abstinence during the early treatment period. In contrast, a 1H-MRS investigation of acamprosate in individuals with AUD undergoing inpatient detoxification treatment for clinically significant alcohol withdrawal symptoms (with >60% of participants receiving benzodiazepines) found that acamprosate significantly decreased ACC glutamate levels relative to placebo, which was interpreted as a therapeutic “normalization” from the transient spike in glutamate levels that accompanies clinically significant alcohol withdrawal (43). Unlike that study, however, we additionally demonstrated that medication-induced increases in glutamate prospectively predicted future abstinence.
Consistent with the facilitatory effects of alcohol drinking on GABAergic synapses, 1H-MRS-measured GABA levels were initially reported to be increased in individuals with AUD relative to healthy volunteers (44), although subsequent studies failed to replicate this finding (40, 45). The GABA findings from the present study could be viewed as consistent with results from that initial study, in that decreases in GABA levels were associated with increased percent days abstinent in participants in the gabapentin group, albeit only during the early treatment period. The GABA findings from the present study are also consistent with the seminal preclinical investigation of gabapentin for AUD, in which gabapentin was found to reduce excessive alcohol drinking by decreasing GABAergic transmission, thereby normalizing the alcohol-induced effect, in the central amygdala (15).
Although the present study affirms 1H-MRS as a potentially valuable tool for exploring neurochemical drug effects, the interpretability of 1H-MRS findings is fundamentally limited by the methodology’s relatively low spatiotemporal resolution (46, 47). Studies using 1H-MRS as a translational bridge (i.e., including both rodents and people) could overcome these limitations (23) and provide more detailed molecular explanations for the gabapentin effects observed in people with AUD. Given the dynamic nature of neurochemical adaptation to alcohol drinking and withdrawal (14, 48), more frequent 1H-MRS scanning during the initial days or weeks of gabapentin treatment could help disentangle the temporal interaction of changes in glutamate and GABA levels and percent days abstinent during the early phase of treatment. For example, consistent with findings from placebo-treated participants in the present study, we recently demonstrated that treatment-naive individuals with AUD had depleted dACC GABA levels, which normalized within 72 hours of monitored abstinence and which remained normal across a subsequent 5-day period of abstinence (25). Although we found in the present study that both baseline and changes in dACC glutamate levels were associated with gabapentin’s promotion of abstinence, the findings involving changes in glutamate levels were nearly six times more statistically reliable than those involving baseline glutamate levels.
A notable strength of this study was that, unlike most 1H-MRS GABA studies (49), we used a specialized MEGA-PRESS acquisition sequence that eliminated coedited “macromolecules,” which comprise 50% of the “GABA” signal (therefore denoted “GABA+”) acquired via traditional MEGA-PRESS acquisition (29). Other strengths included a relatively large sample (e.g., more than twice that of Umhau et al. [43]) and a relatively long duration of gabapentin treatment (i.e., 16 weeks, to address the often protracted nature of alcohol withdrawal symptoms [50]), delivered in the context of a randomized double-blind placebo-controlled trial.
Limitations of the study include acquisition of 1H-MRS data from a single brain region, precluding conclusions concerning the regional specificity of findings; an MRI scanner upgrade that occurred partway through the study, although analyses demonstrated that results did not differ by scanner; exclusion of individuals with AUD who were experiencing acute, clinically significant alcohol withdrawal at the time of scanning; primary reliance on self-report alcohol consumption data; and inability to predict, at the individual level, who might respond best to gabapentin.
In sum, the results from this study suggest that gabapentin treatment promotes early abstinence partly by increasing dACC glutamate levels that are subsequently associated with gabapentin’s efficacy in reducing drinking over an extended period in individuals with AUD and a history of alcohol withdrawal syndrome. These novel findings contribute significantly to our understanding of how gabapentin may work to prevent relapse drinking in certain individuals with AUD who attempt abstinence, and are consistent with our previous report of gabapentin’s efficacy (2). They also provide evidence for a biomarker of efficacious treatment (i.e., increased dACC glutamate levels) that may be used to evaluate other glutamatergic and/or GABAergic medications for individuals with AUD, and potentially other conditions marked by dACC glutamate and/or GABA deficiency (e.g., cannabis use disorder [51] and co-occurring bipolar disorder and substance use disorder [52]).
1. : A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction 2019; 114:1547–1555Crossref, Medline, Google Scholar
2. : Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med 2020; 180:728–736Crossref, Medline, Google Scholar
3. : Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med 2014; 174:70–77Crossref, Medline, Google Scholar
4. : Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 2016; 3:760–773Crossref, Medline, Google Scholar
5. : The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol 2006; 6:108–113Crossref, Medline, Google Scholar
6. : Analgesia with gabapentin and pregabalin may involve N-methyl-d-aspartate receptors, neurexins, and thrombospondins. J Pharmacol Exp Ther 2020; 374:161–174Crossref, Medline, Google Scholar
7. : Gabapentin is a potent activator of KCNQ3 and KCNQ5 potassium channels. Mol Pharmacol 2018; 94:1155–1163Crossref, Medline, Google Scholar
8. : Gabapentin increases expression of δ subunit-containing GABAA receptors. EBioMedicine 2019; 42:203–213Crossref, Medline, Google Scholar
9. : The impact of gabapentin administration on brain GABA and glutamate concentrations: a 7T 1H-MRS study. Neuropsychopharmacology 2012; 37:2764–2771Crossref, Medline, Google Scholar
10. : Modulation of cerebral GABA by topiramate, lamotrigine, and gabapentin in healthy adults. Neurology 2002; 58:368–372Crossref, Medline, Google Scholar
11. : Effects of gabapentin on brain GABA, homocarnosine, and pyrrolidinone in epilepsy patients. Epilepsia 2000; 41:675–680Crossref, Medline, Google Scholar
12. : The effect of gabapentin on brain gamma-aminobutyric acid in patients with epilepsy. Ann Neurol 1996; 39:95–99Crossref, Medline, Google Scholar
13. :
14. : Synaptic targets: chronic alcohol actions. Neuropharmacology 2017; 122:85–99Crossref, Medline, Google Scholar
15. : Cellular and behavioral interactions of gabapentin with alcohol dependence. J Neurosci 2008; 28:5762–5771Crossref, Medline, Google Scholar
16. : Adolescent ethanol exposure enhances NMDA receptor-mediated currents in hippocampal neurons: reversal by gabapentin. Sci Rep 2017; 7:13133Crossref, Medline, Google Scholar
17. : Effect of gabapentin on sleep and delta and theta EEG power in adult rats exposed to chronic intermittent ethanol vapor and protracted withdrawal during adolescence. Psychopharmacology (Berl) 2018; 235:1783–1791Crossref, Medline, Google Scholar
18. : Brain GABA and glutamate concentrations following chronic gabapentin administration: a convenience sample studied during early abstinence from alcohol. Front Psychiatry 2018; 9:78Crossref, Medline, Google Scholar
19. : Use of gabapentin in the treatment of substance use and psychiatric disorders: a systematic review. Front Psychiatry 2019; 10:228Crossref, Medline, Google Scholar
20.
21. : Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for Alcohol Scale (CIWA-Ar). Br J Addict 1989; 84:1353–1357Crossref, Medline, Google Scholar
22. : Alcohol Timeline Followback Users’ Manual. Toronto, Addiction Research Foundation, 1995Google Scholar
23. : Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry 2012; 71:1015–1021Crossref, Medline, Google Scholar
24. : Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J Psychiatry Neurosci 2014; 39:149–169Crossref, Medline, Google Scholar
25. : Intraindividual changes in brain GABA, glutamate, and glutamine during monitored abstinence from alcohol in treatment-naive individuals with alcohol use disorder. Addict Biol 2020; 25:e12810Crossref, Medline, Google Scholar
26. : Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol 2013; 18:121–133Crossref, Medline, Google Scholar
27. : Cingulate cortex functional connectivity predicts future relapse in alcohol dependent individuals. Neuroimage Clin 2016; 13:181–187Crossref, Medline, Google Scholar
28. : Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med 2000; 43:319–323Crossref, Medline, Google Scholar
29. : Absence of changes in GABA concentrations with age and gender in the human anterior cingulate cortex: a MEGA-PRESS study with symmetric editing pulse frequencies for macromolecule suppression. Magn Reson Med 2013; 69:317–320Crossref, Medline, Google Scholar
30. : Comparative reliability of proton spectroscopy techniques designed to improve detection of J-coupled metabolites. Magn Reson Med 2008; 60:964–969Crossref, Medline, Google Scholar
31. : Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J Magn Reson Imaging 2014; 40:1445–1452Crossref, Medline, Google Scholar
32. : Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993; 30:672–679Crossref, Medline, Google Scholar
33. : Tissue correction for GABA-edited MRS: considerations of voxel composition, tissue segmentation, and tissue relaxations. J Magn Reson Imaging 2015; 42:1431–1440Crossref, Medline, Google Scholar
34. : Issues of spectral quality in clinical 1H-magnetic resonance spectroscopy and a gallery of artifacts. NMR Biomed 2004; 17:361–381Crossref, Medline, Google Scholar
35. : Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach, 2nd ed. Edited by Little TD. New York, Guilford, 2017Google Scholar
36. : Linear and Nonlinear Mixed Effects Models. 2017 (https://CRAN.R-project.org/package=nlme)Google Scholar
37. : Statistical Analysis With Missing Data. Hoboken, NJ, Wiley, 2002Crossref, Google Scholar
38. : Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology 2013; 38:1401–1408Crossref, Medline, Google Scholar
39. : Loss of control of alcohol use and severity of alcohol dependence in non-treatment-seeking heavy drinkers are related to lower glutamate in frontal white matter. Alcohol Clin Exp Res 2013; 37:1643–1649Medline, Google Scholar
40. : Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend 2012; 125:27–36Crossref, Medline, Google Scholar
41. : Associations between recent heavy drinking and dorsal anterior cingulate N-acetylaspartate and glutamate concentrations in non-treatment-seeking individuals with alcohol dependence. Alcohol Clin Exp Res 2016; 40:491–496Crossref, Medline, Google Scholar
42. : Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacology 2011; 36:1359–1365Crossref, Medline, Google Scholar
43. : Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry 2010; 67:1069–1077Crossref, Medline, Google Scholar
44. : Preliminary evidence of low cortical GABA levels in localized 1H-MR spectra of alcohol-dependent and hepatic encephalopathy patients. Am J Psychiatry 1999; 156:952–954Link, Google Scholar
45. : Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol Psychiatry 2006; 59:85–93Crossref, Medline, Google Scholar
46. : The potential of 1H-MRS in CNS drug development. Psychopharmacology (Berl) 2021; 238:1241–1254Crossref, Medline, Google Scholar
47. : Neuropharmacological and neurobiological relevance of in vivo 1H-MRS of GABA and glutamate for preclinical drug discovery in mental disorders. Neuropsychopharmacology 2014; 39:2331–2339Crossref, Medline, Google Scholar
48. : Chronic alcohol consumption, abstinence, and relapse: brain proton magnetic resonance spectroscopy studies in animals and humans. Curr Top Behav Neurosci 2013; 13:511–540Crossref, Medline, Google Scholar
49. : Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage 2014; 86:43–52Crossref, Medline, Google Scholar
50. : The recognition and management of protracted alcohol withdrawal may improve and modulate the pharmacological treatment of alcohol use disorder. J Psychopharmacol 2020; 34:1171–1175Crossref, Medline, Google Scholar
51. : γ-Amino butyric acid and glutamate abnormalities in adolescent chronic marijuana smokers. Drug Alcohol Depend 2013; 129:232–239Crossref, Medline, Google Scholar
52. : Unique prefrontal GABA and glutamate disturbances in co-occurring bipolar disorder and alcohol dependence. Transl Psychiatry 2017; 7:e1163Crossref, Medline, Google Scholar



