Evolution, Emotion, and Episodic Engagement
Abstract
Although rodent research provides important insights into neural correlates of human psychology, new cortical areas, connections, and cognitive abilities emerged during primate evolution, including human evolution. Comparison of human brains with those of nonhuman primates reveals two aspects of human brain evolution particularly relevant to emotional disorders: expansion of homotypical association areas and expansion of the hippocampus. Two uniquely human cognitive capacities link these phylogenetic developments with emotion: a subjective sense of participating in and reexperiencing remembered events and a limitless capacity to imagine details of future events. These abilities provided evolving humans with selective advantages, but they also created proclivities for emotional problems. The first capacity evokes the “reliving” of past events in the “here-and-now,” accompanied by emotional responses that occurred during memory encoding. It contributes to risk for stress-related syndromes, such as posttraumatic stress disorder. The second capacity, an ability to imagine future events without temporal limitations, facilitates flexible, goal-related behavior by drawing on and creating a uniquely rich array of mental representations. It promotes goal achievement and reduces errors, but the mental construction of future events also contributes to developmental aspects of anxiety and mood disorders. With maturation of homotypical association areas, the concrete concerns of childhood expand to encompass the abstract apprehensions of adolescence and adulthood. These cognitive capacities and their dysfunction are amenable to a research agenda that melds experimental therapeutic interventions, cognitive neuropsychology, and developmental psychology in both humans and nonhuman primates.
In this review, we explore two related topics. One is brain evolution and unique aspects of human cognition that depend on recent changes in hominin brains (1–5). The other concerns the role of primate research in understanding mental illness. To address these topics, we proceed in three sections. In the first, we introduce the idea that advantageous evolutionary changes can create vulnerabilities; in the second, we review research on social and mnemonic capacities reflecting unique features of human and nonhuman primate brains; and in the third, we describe a research agenda aimed at combining human and primate research to improve treatment and outcome prediction.
Evolutionary Perspectives
Phylogenetic Disease
Three decades ago, Rapoport (6) introduced the concept of a phylogenetic disease, in which cognitive and mnemonic vulnerabilities arise as by-products of evolutionary innovation. He chose as his example Alzheimer’s dementia, and he postulated that genetic factors underlying the evolution of large human brains introduced vulnerabilities along with their more obvious benefits. Specifically, he proposed (6, p. 148) that
neuropathological studies of Alzheimer patients demonstrate selective disease in the frontal, parietal, and temporal association neocortices, and in the posterior hippocampus, entorhinal cortex, basocortical amygdaloid complex, and nucleus basalis of Meynert, all of which underwent recent “integrated phylogeny.”
By “integrated phylogeny,” Rapoport meant that the brain structures he listed had undergone significant expansion and modification during human evolution and that these phylogenetic developments interacted with each other. Nonhuman primates, he concluded, could never suffer from Alzheimer’s dementia because they lack the evolutionary innovations that produce distinctively human forms of memory, which patients lose in this disease. More recently, Konopka et al. (7) advanced a similar idea regarding speech and language, and Pattabiraman et al. (8) have done so for other disorders.
The idea that vulnerabilities accompany advantageous evolutionary changes can be counterintuitive, but increases in fitness often come with inherent disadvantages. For example, during locomotion, homeothermic animals need to expend ∼20 times more energy than poikilotherms matched for body size; even at rest they burn 5–10 times more calories (9). So, compared with other vertebrates, homeotherms succumb to starvation much faster. Their evolutionary success attests to the net benefit of this trade-off, but this fact provides cold comfort to individuals facing famine. Likewise, trade-offs between the large neonatal head size of hominins and the pelvic architecture required for bipedal gait produced both advantages in cognition and vulnerabilities in childbirth (10).
Here we propose that two evolutionary innovations, both involving episodic memory, contribute to emotional disorders. One is a capacity for reexperiencing events (REE) with a subjective sense of personal participation; the other is flexible planning through constructive episodic simulation (CES), which empowers a temporally limitless imagination of events.
In The Evolution of Memory Systems (5), two of us linked these cognitive capacities to neural representations of the “self,” conspecifics, and rules governing social relationships. We reviewed evidence that these forms of memory evolved in hominins, building on anthropoid innovations. Cortical networks representing the “self” involve lasting memories of an individual’s attributes, capacities, past experiences, and social relationships. We proposed that 1) species-specific representations of the “self” and “others” evolved in tandem with new social systems during hominin evolution; and 2) a sense of participation in remembered and imagined events depends on these self-representations.
In this review, we discuss two aspects of hominin brain evolution that enabled REE and CES: expansion of homotypical association areas, including the granular prefrontal cortex, and expansion of the hippocampus. We explore clinical implications of the idea that prefrontal cortex expansion generated human-specific self-representations, while hippocampus expansion reflected the integration of these representations into episodic memories and mental simulations.
Cortical Evolution
About 6.3 million years ago, hominins diverged from the chimpanzee-bonobo lineage (Figure 1B). Until ∼3 million years ago, hominin brains differed little from those of modern chimpanzees in either relative size or sulcal pattern (11). Subsequently, hominin brains expanded dramatically, but nonuniformly: the granular prefrontal cortex came to dominate the frontal lobe (12, 13), and similar expansions occurred in homotypical association areas of the parietal and temporal lobes (13–15).
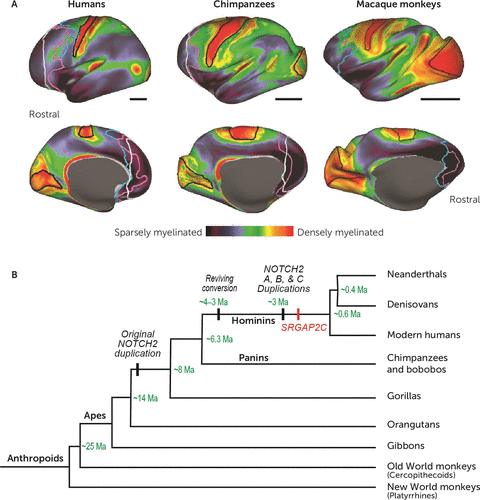
FIGURE 1. Expansion of homotypical association areas in the human braina
a Panel A shows a myeloarchitectonic analysis based on structural MRI in humans, chimpanzees, and macaque monkeys, plotted onto an inflated image of the cerebral cortex. White lines show the caudal boundary of the granular prefrontal cortex (PFC) based on using an arbitrary criterion, the genu of the corpus callosum, as the boundary. This practice led to a substantial underestimate of the PFC’s size. Purple and blue lines show the caudal boundary of the PFC based on cytoarchitectonics. This analysis identified homotypical areas, which have a reasonable proportion of the six major neocortical layers. (Note that despite the term “granular” in the granular PFC, this part of the PFC consists of homotypical and dysgranular cortex, which are considered “granular” in a frontal-lobe context.) Purple lines provide a conservative estimate, that is, the least plausible amount of granular PFC, and blue lines indicate the most likely boundary between the granular PFC and other parts of the frontal lobe. Black bars indicate relative scale. Reproduced from Donahue et al. (13). Panel B is a phylogenetic tree of relationships among anthropoids, with divergence and gene-duplication times in green type. Ma=million years ago. Adapted from Fiddes et al. (22).
Recently, Donahue et al. (13) used structural MRI to measure the density of cortical myelin in humans, chimpanzees, and macaque monkeys. Figure 1A shows that lightly myelinated areas (dark blue) occupy a much larger proportion of the frontal, parietal, and temporal lobes in humans than in either chimpanzees or macaques. The granular prefrontal cortex exemplifies these findings. Donahue et al. used cytoarchitectonic analysis to confirm that homotypical association areas make up the vast majority of lightly myelinated frontal cortex (12, 13), resolving a controversy on this point (16, 17). They observed, for example, that the granular prefrontal cortex is 9–10 times larger than the primary motor cortex in humans, but only 3 times larger in chimpanzees and macaques. Passingham and Smaers (18) came to similar conclusions based on a different data set.
Human brains show significant person-to-person differences in the volume of homotypical association areas (19). This variation correlates with overall brain volume, and areas that expanded more recently in evolution also develop later in ontogeny (15). The most dramatically expanded parts of the temporal, parietal, and frontal lobes have strong interconnections with each other, as revealed by resting-state covariance in functional MRI (fMRI) data (20). These areas also show selective fMRI activations and coupling during tasks requiring individuals to draw on knowledge from multiple cognitive domains (20), a hallmark of general intelligence (21).
Evolutionary change depends on modifications of genetic programs. Fiddes et al. (22) concluded that ∼14 million years ago a replication error inserted an incomplete and functionally defective copy of the ancestral NOTCH2 gene into the genome of an ancestor shared by humans, gorillas, and chimpanzees (Figure 1B). A subsequent replication error inserted an additional nucleotide sequence from the same gene ∼3 million years ago, during human evolution, which restored the gene’s function. Later, that gene—NOTCH2NL—duplicated again, eventually producing three active versions on human chromosome 1. Suzuki et al. (23) showed that the NOTCH2NL protein blocks a signal that stops the division of neural stem cells. This blockade leads to the production of many more neurons, which augment the human neocortex.
The NOTCH2 gene is one of several that contribute to cortical development. Another gene on chromosome 1, SRGAP2, duplicated ∼2.4 million years ago (Figure 1B). Its paralogue, SRGAP2C, counteracts the effect of the original SRGAP2 gene, causing proliferation of dendritic spines and branching (24, 25). Two additional human-specific segmental duplications, HAR5 (26) and ARHGAP11B, made further contributions. Expression of the latter in mouse (27) or marmoset (28) embryos increases mitotic division in radial glial cells, which causes the subventricular zone of the developing cortex to increase in thickness by almost one-third. This summary only scratches the surface of the specific genes and genetic pathways involved in cerebral expansion, but it suffices to illustrate the principles at work.
Taken together, these findings support the idea that homotypical association areas expanded dramatically during human evolution, beginning ∼2–3 million years ago. Additional evidence from comparative neuroanatomy, reviewed by Murray et al. (5) and first recognized by Preuss and Goldman-Rakic (29), shows that several new homotypical association areas emerged during anthropoid primate evolution, including new parts of the granular prefrontal cortex. During hominin brain evolution, it was these areas that expanded preferentially.
Another phylogenetic change occurred in the hippocampus. It decreased in relative size during most of anthropoid evolution, but during hominin evolution this trend reversed (30, 31). These findings suggest that hippocampal functions became less important during most of anthropoid evolution. Later, during hominin evolution, one or more of its functions acquired new significance, and it is likely that this development provided novel selective advantages based on innovative forms of memory (5, 31).
Primate-Unique Neurocognitive Capacities
Social Representations in the Medial Prefrontal Cortex
Converging evidence implicates a large-scale cortical network in the two cognitive processes mentioned above: reexperiencing events (REE) and constructive episodic simulation (CES). This circuitry is variably described as the medial network or the larger default-mode network, which includes the medial prefrontal cortex, the hippocampus, and the cingulate cortex, among other areas. Despite some method-based disagreements about its components, all methods place the medial prefrontal cortex in these networks (32, 33).
In addition to a role in episodic memory, this area supports social cognition by storing a unique form of memory: features of conspecifics and of one’s self. After reviewing the evolution of the medial prefrontal cortex, Murray et al. (5) proposed that its self-representations endow the medial network with unique properties that characterize human episodic memories, especially a vivid sense of participation in the “here and now.” This proposal drew heavily on the work of Lau and colleagues (34, 35), who attributed human self-representation to successive rerepresentation of personal actions, intentions, goals, and states, which reside in the medial prefrontal cortex. This region encompasses the cortex extending from the medial frontal pole (area 10) to the caudal boundary of the anterior cingulate cortex (ACC; areas 24, 25, and 32). Comparative neuroanatomy suggests that its rostral, granular component originally evolved in anthropoids and expanded during human evolution (5). Rostral parts of the medial prefrontal cortex are thus well suited to establish rerepresentations of the “self” by drawing on information encoded in phylogenetically older areas. Rerepresentation involves the abstraction of information by higher-order prefrontal areas from lower-order ones. Rostral, granular prefrontal areas draw on representations in areas situated more caudally, and higher levels of rerepresentation emerged as the granular prefrontal cortex expanded during human evolution. Murray et al. reasoned that if each anthropoid species evolved a modified version of self-representation, suited to the social system of that species, then modern humans also have a species-specific form of self-representation.
Across anthropoids, including humans, the ACC represents social value in addition to other kinds of valuations (36). Single-neuron activity in the ACC encodes a monkey’s social preferences, acquired through vicarious reinforcement (37), and selective ACC lesions prevent monkeys from acquiring prosocial preferences (38). Phylogenetic analysis shows that social complexity increased during the evolution of anthropoids, which descended from primates with solitary social systems (39). The same analysis reveals that complex social systems evolved convergently in prosimians and anthropoids. So, notwithstanding the social functions of the ACC in other mammals (40), nonsocial functions of the medial prefrontal cortex were likely co-opted for social ones independently during anthropoid evolution (41).
In humans, meta-analysis shows that a large part of the medial prefrontal cortex has significant fMRI activations while people make judgments about themselves and others. There are at least two clusters of self-related sites: one in the pregenual cortex (area 32), the other immediately rostral to the first, in the medial frontal pole cortex (area 10) (42). A meta-analysis of fMRI covariance confirms that these rostral areas process social representations (43), with three subdivisions: dorsomedial, ventromedial (including subgenual), and pregenual cortex. Performance on both episodic memory tasks and social tasks significantly predicts fMRI activations in all three subdivisions (43), which points to these areas as key sites for combining the representations of one’s self and events.
REE and CES: Self-Representation and Shared Neural Substrates
From an origin in the medial prefrontal cortex, corticocortical connections can distribute conjunctive self-event representations to other components of the medial network, in which they are thought to infuse episodic memories with a “here-and-now” quality. A subjective sense of REE results: “what it was like” when the event occurred (5). Self-representations can also establish a sense of participation in events imagined via CES, which generally lacks “here-and-now” qualities. This distinction arises because reality monitoring allows people to differentiate imaginary from actual events, although this function can fail in mental illness (44).
Episodic memories and imagined events might seem distantly related, but four lines of evidence suggest that the neural networks supporting REE also underlie CES. First, when people either remember events or imagine future ones, overlapping brain regions manifest activations in fMRI studies, implicating a core network in REE and CES (45, 46). This network includes the medial prefrontal cortex and other medial network areas, such as the hippocampus and retrosplenial cortex (Figure 2A). Second, remembering and imagining events share properties (47), such as a positivity bias—that is, a preponderance of memories and imagined events with a positive affective valence. The extent of this positivity bias correlates significantly for reexperiencing past events and imagining future ones (48). Third, patients with impairments in remembering events also have a poor capacity for imagining future events (49, 50). And fourth, individual differences in precommissural (but not postcommissural) fornix microstructure correlate with the episodic richness of both past and future autobiographical narratives (51).
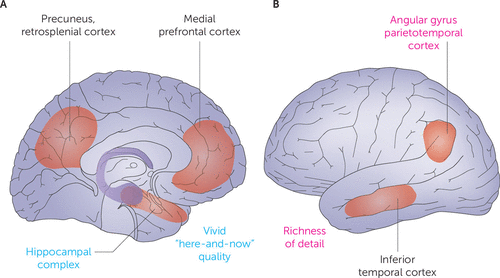
FIGURE 2. Core network for reexperiencing events and constructive episodic simulationa
a Panel A shows medial areas, and panel B shows lateral areas. (Adapted from reference 45 by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Nature Reviews Neuroscience, 2007; 8:657–661, Schacter et al., “Remembering the past to imagine the future: the prospective brain.” Copyright 2007 Springer Nature.)
An overlap in the neural circuitry for REE and CES probably reflects their common dependence on mechanisms for mental construction of event sequences and boundaries (49, 52, 53). Furthermore, memories of past events populate imagined ones.
The medial prefrontal cortex, by rerepresenting one’s self and others at the highest hierarchical level, enables the incorporation of self-representations into event memories, in part via interaction with the hippocampus. The medial prefrontal cortex and the hippocampus play related but specialized roles in this process. Damage to the hippocampus causes an impairment in constructing detailed episodic simulations, but patients with such damage incorporate themselves normally. Patients with medial prefrontal damage show the opposite pattern; they can construct detailed mental simulations but rarely incorporate themselves (54).
Emotion and the Amygdala
Emotional responses are prominent features of both REE and CES, an observation with several clinical implications. Research on macaques reveals important circuitry underlying emotional features of REE and CES in humans.
Specifically, this work shows that the medial prefrontal cortex (Figure 3) and the hippocampus have dense interconnections with the amygdala. According to Price and Drevets (55), the amygdala and medial prefrontal cortex are reciprocally connected, especially the latter’s caudal parts. The hippocampus and the amygdala also have dense interconnections with each other, mostly involving the temporal (anterior) hippocampus (56, 57), which underlies mental scene construction (58). The hippocampus also sends dense inputs to the medial prefrontal cortex, which, like amygdala inputs, concentrate in its caudal aspects (59). Thus, inputs from the amygdala and hippocampus overlap in the medial prefrontal cortex, although they terminate mostly in different laminae (60). Through these inputs and local circuitry, the medial prefrontal cortex has the connections needed to integrate information from the amygdala and hippocampus with representations of one’s self.
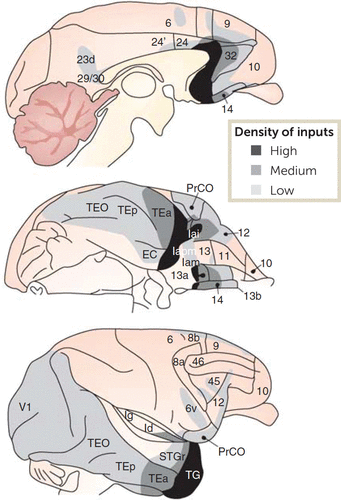
FIGURE 3. Amygdala-cortical connectivity in macaque monkeysa
a Amygdala projections to the neocortex are ranked according to the density of inputs—high, medium, or low. EC=entorhinal cortex; Id, Ig, Iam, Iapm, Iai=parts of insular cortex; PrCO=frontal opercular proisocortex; TEO, TEp, TEa, and TG=parts of inferior temporal cortex; STGr=rostral superior temporal cortex; V1=primary (striate) visual cortex. Rostral is to the right in all three images; in the top and bottom images, dorsal is up, and in the middle image, lateral is up. (Adapted from reference 55 by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Neuropsychopharmacology, 2010; 35:192–216, Price and Drevets, “Neurocircuitry of mood disorders.” Copyright 2010 Springer Nature.)
The understanding of neuroanatomy in this rich detail depends on research in macaque monkeys. These species and other anthropoids can provide this information for a simple reason: they have a homologue of the granular prefrontal cortex, but other common laboratory animals, such as rats and mice, do not (5, 29). For this reason, insights about brain structures conserved among anthropoids can, by applying an evolutionary perspective, contribute to understanding of human-specific cognitive functions and their relations with mental illness.
Several findings have implicated these connections in memories of emotional events, whether imagined or reexperienced. For example, neuroimaging studies have identified regions with greater activation for emotional events than for neutral events. Two meta-analyses using this approach have implicated the amygdala and hippocampus, along with the entorhinal, perirhinal, and visual cortex, in the encoding and retrieval of emotional episodic memories (61, 62). Another fMRI study found that functional connectivity between the amygdala and hippocampus increased during the retrieval of emotional contextual information (63).
Figure 4 presents a conceptual model of the amygdala’s hippocampal and medial prefrontal inputs, which contribute to emotional responses during REE and CES. These inputs combine with those from sensory (and other) areas of the cortex to regulate the amygdala’s net output. Reciprocal projections from the amygdala amplify these responses. Before discussing this model, we highlight some current ideas about amygdala function.
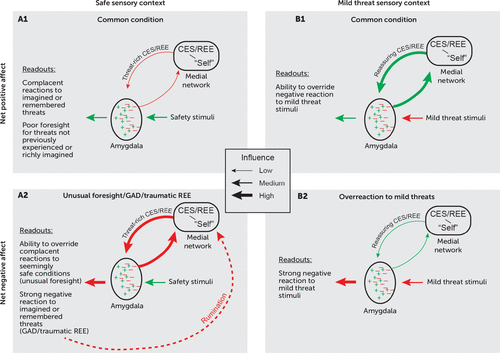
FIGURE 4. Model of amygdala output regulationa
a Emotional responses evoked by threat and safety signals can have either a positive (green) or a negative (red) valence. Inputs to the amygdala from the hippocampus and other parts of the medial network convey signals associated with remembered or imagined events. In panel A, reexperiencing events (REE) or constructive episodic simulation (CES) convey threat-rich memories or mental simulations to the amygdala, which contradict inputs from sensory areas signaling safety. In panel B, REE or CES convey reassuring signals to the amygdala, which contradict sensory cortex inputs signaling mild threats. In panels A1 and B1, positive emotional responses dominate mood and behavior; in panels A2 and B2, negative emotional responses dominate mood and behavior. Note that the depiction of inputs from sensory areas omits the influence of top-down attention and other processes that affect the flow of sensory information to the amygdala. CES=constructive episodic simulation; GAD=generalized anxiety disorder; REE=reexperiencing events retrieved from memory.
It is generally accepted that the amygdala biases behavioral outputs to enhance Darwinian fitness. This function involves exploiting resources (e.g., nutrients, fluids, warmth), avoiding predators, producing progeny, and learning about sensory cues that signal resources, threats, or safety (56). It is crucial that such biases adapt to an individual’s current state and motivations, and experiments on both macaque monkeys and rodents have shown that the amygdala plays a necessary role in value updating based on current biological needs (64).
Two widespread misunderstandings have hampered attempts to reach consensus concerning amygdala function in this context. First, some authors have argued that the amygdala functions primarily, if not exclusively, in threat processing and negative affect (65). The vast literature on fear conditioning has contributed to this misconception, and many discussions of amygdala function continue to focus exclusively on negative affect. However, experimental evidence from both rodents and macaques has overturned this idea conclusively. Holland and Gallagher (66) reviewed evidence from several laboratories showing that the rat amygdala plays an important role in reward processing and positive affect, and Janak and Tye (67) reached the same conclusion more recently. And in macaque monkeys, the amygdala plays a necessary role in updating valuations that have a positive affective valence (68, 69).
Second, the idea that the prefrontal cortex predominantly “inhibits” amygdala output is contradicted by neuroanatomical evidence from macaques. These studies show that cortical projections to the amygdala, which are excitatory, terminate on both excitatory and inhibitory interneurons (70–72). Thus, cortical influences on the amygdala can modulate its outputs both positively and negatively, depending on the precise circuitry engaged. Figure 4 depicts this idea with green symbols for circuits contributing to positive-valence emotional readouts and red symbols for negative-valence readouts. In both cases, inputs from the medial prefrontal cortex and the hippocampus engage amygdala circuits that influence emotional responses to both remembered and imagined events. Inputs from sensory areas of the cortex, including homotypical association areas such as the inferior temporal cortex, provide current sensory contexts.
In Figure 4A2, inputs to the amygdala from both the hippocampus and medial prefrontal cortex convey threat-rich signals generated via retrieved episodic memories or by CES, which strongly activates threat-avoidance circuits via the amygdala. According to this model, when threat-rich signals from the medial network override signals reflecting a safe sensory context, a negative affective state results. For traumatic REE, this means that after a trigger prompts the recall of a harrowing event, a currently safe sensory context does little to mitigate a patient’s emotional responses. For CES, a powerful emotional reaction occurs for imagined harmful events despite concurrent safety signals. Most research on CES stems from studies of episodic memory, but CES also is highly relevant for studies of anxiety because overactive CES represents a cardinal feature of generalized anxiety disorder, an illness defined by excessive worry. In both generalized anxiety disorder and worries that occur in other mental disorders, maladaptive feedback loops (dashed red line in Figure 4A2) can arise, in which rumination about future harms and failures generates persistent anxiety.
In Figure 4B2, a negative affective state prevails for a different reason. Mild threats should cause little anxiety, if any, and in most people they do not. However, if reassuring REE or CES have insufficient influence over the amygdala’s output, a negative affective state will predominate despite a low or nonexistent risk.
Note that Figure 4A1 and 4B1 do not necessarily reflect optimal emotional responses. Some people respond inadequately to threats they have not experienced or are incapable of imagining in rich, predictive detail (Figure 4A1). In contrast, individuals with a highly cultivated capacity for CES (unusual foresight) can act in accordance with their imagination rather than their experience, thereby avoiding danger (Figure 4A2). Of course, excessive imagination of risks can create problems of its own.
REE, CES, and Emotional Vulnerabilities
The topics discussed thus far establish the foundation for a novel research agenda. This agenda melds clinical and primate research to seek improved treatment and prediction of anxiety and stress-related disorders. Creating such an agenda raises a question rarely posed in discussions of primate research: If certain mental illnesses result from vulnerabilities that developed during human evolution, how can studying nonhuman primates help us understand these illnesses? The answer is that monkeys have anthropoid-unique homologues of the cortical areas that malfunction in mental illnesses (73, 74). Although the functions of these areas may have changed during human evolution, nonhuman primate research is necessary both for understanding these phylogenetic changes and for discovering the fundamental neural functions that anthropoid-unique areas perform. Knowing how they enhance evolutionary fitness in monkeys provides irreplaceable insight into their modified functions in humans.
While the proposed research agenda calls for studies of brain circuits and their functions, research on the localization and timing of CNS gene expression provides additional insight. Notable studies compare the transcriptional underpinnings of brain development in rodents, nonhuman primates, and humans (75, 76). These reports identify genetic pathways and developmental processes more strongly shared by humans and macaques than by humans and rodents. Moreover, other gene-expression profiles differentiating humans and nonhuman primates manifest in adolescence, a period during which humans also display unique mnemonic capacities relevant to emotional disorders. Thus, much like data on cross-species comparisons of brain function, cross-species comparisons of gene-expression profiles reveal novel brain mechanisms that correlate with evolutionary changes in emotional processes.
The research agenda we imagine draws on the evolutionary perspective outlined earlier and targets new forms of memory that emerged during the evolution of anthropoids and humans (5). Its goal is to inform clinical studies across two time scales: 1) for REE, ideas about novel therapeutics relate to changes in brain circuitry that develop over days to weeks during clinical trials; 2) for CES, ideas about improving prediction of developmental change involve cortical ontogeny, which unfolds over months to years. This research agenda could help coordinate studies based on DSM-5 with work guided by the Research Domain Criteria (RDoC). Our conceptual model is relevant to both systems. For DSM, it defines phenomena using DSM criteria and seeks to improve outcomes defined through available measures in clinical studies. For the RDoC, it targets expressions of clinically relevant phenomena across diagnostic boundaries. For both, the objective is to identify biological mediators and moderators of clinically defined outcomes.
Defensive behaviors provide an example to be targeted by this approach. Some functions, such as those related to trauma, influence defensive behavior through effects on conserved homologous circuitry that operates similarly in rodents, nonhuman primates, and humans. Other functions involve primate-unique circuitry and large-scale networks involving cortical areas that emerged during anthropoid evolution and expanded dramatically in humans. Thus, human defensive behavior and associated clinical problems reflect combinations of perturbations in both primate-unique circuitry and circuitry more broadly conserved among mammals. As stated previously (4, 5), our conceptual model presumes that humans reexperience recollected events, construct episodic simulations, and experience their associated emotional responses in a way that is fundamentally different from nonhuman primates. Nevertheless, these functions depend on cortical areas, circuits, and networks that emerged during primate evolution and are homologous among anthropoids. Moreover, cortical areas that are common to all mammals, such as the ACC and the hippocampus, are influenced by primate-specific circuitry in ways that also alter the functions of these pan-mammalian structures. Note that none of this requires empirical knowledge about the subjective mental states of nonhuman anthropoids or other animals, let alone the dozen or more hominin species of the past 6 million years. Instead, our model holds that each species experiences events and plans future behavior in its own way. We do, however, reject the assumption that nonhuman animals have two cognitive traits characteristic of our species: a subjective experience of personal participation in events and a subjective sense of owning one’s knowledge about the physical and social world. By rejecting this assumption, a broad range of clinical and comparative data become both more comprehensible and more translational.
Primate research also shapes clinical thinking by tying information-processing functions and neural representations to tractable anatomical targets of clinical intervention. Neuropsychological research in monkeys contributes to this knowledge via experiments that identify neuroanatomical substrates of specific neural computations and representations. Localization of function is essential because similar-appearing behaviors often arise from distinct neural mechanisms, which are dissociable anatomically. Precision is needed because diverse behavioral effects can arise from circuits and networks separated by millimeters and with interweaving fibers of passage. Earlier, we discussed a core brain network supporting both REE and CES (Figure 2). However, subsystems within this network have differential fMRI activations for remembering events and for imagining events (77). These anatomical distinctions may underlie the difference between REE and CES in terms of the former’s “here-and-now” quality, as well as the different emotional responses that reexperienced and imagined events evoke.
Accordingly, when seeking insights beyond the scope of currently available clinical tools, linked studies in patients and monkeys would serve a unique and specific purpose. By drawing on the concept of phylogenetic disease and the above-elucidated evolutionary perspectives, cross-species studies can identify clinically useful, localizable targets. These targets might be manipulated in an attempt to treat patients’ emotional or behavioral problems, and they might be assessed through imaging in an attempt to predict anticipated developmental changes in symptoms. As just mentioned, neuroimaging research in humans has identified neural targets for differential disruption of REE and CES (77). Consequently, manipulations of their homologues in macaque monkeys can be performed experimentally to generate mechanism-level insights into psychological processes. With this combined knowledge, interventions aimed at mitigating overactive threat-related REE, while leaving reassuring CES relatively intact, could be developed and tested. Concurrently, assessment tools could be created to quantify CES in ways that predict children’s susceptibility to later emotional problems.
REE and Traumatic Stress Disorders
Not only do patients with posttraumatic stress disorder (PTSD) need therapeutic advances, but so do other patient groups that experience emotionally distressing memories. For instance, among patients with major depressive disorder, those with traumatic exposures present distinct therapeutic challenges compared with other depressed patients. As one example, patients with major depression that occurs in the context of traumatic childhood experiences exhibit poor responses to treatment with antidepressant medications (78, 79).
An RDoC approach to the traumatic reexperiencing of events could prove beneficial for several mental disorders. DSM-5 classifies such memories as instances of intrusive symptoms, one of four PTSD clinical domains. Yet, similar forms of intrusive recollection occur in patients with major depression exposed to trauma (80, 81). As with PTSD patients, when intrusive REE occurs in patients with major depression, they report or act in ways that suggest a “here-and-now” quality to the recollection. Patients in both diagnostic groups act and react emotionally as if the event were actually occurring. Considerable research differentiates REE from the other symptom domains in PTSD (81), so it makes sense to focus on the underlying information processing networks and the unique forms of memory that we described earlier: conjunctive representations of events and one’s self. An understanding of how these networks and their associated forms of memory evolved, based on comparative neuroscience, empowers clinicians to develop concrete ideas about why similar phenomena occur across diagnostic boundaries.
Combined human and monkey research could lead to specific therapies. For example, an fMRI study probed two attributes of episodic memory relevant to REE and CES: vividness and richness of detail (82). As participants engaged in CES, they rated vividness during scans and later conveyed richness of detail verbally. A core network for episodic memory showed significant activation, including several medial areas—the hippocampus, the retrosplenial cortex, and the medial prefrontal cortex (Figure 2A)—along with lateral areas such as the inferior temporal cortex and the angular gyrus (Figure 2B). A contrast of high versus low vividness identified the hippocampus as uniquely sensitive to this aspect of CES, whereas a contrast of high versus low richness of detail yielded the angular gyrus.
These and related findings suggest targets for therapeutic interventions using transcranial or deep brain stimulation. In clinical studies, patients actively recall particular episodes from their past, including instances involving traumatic exposures. It may be possible to disrupt the vividness of REE while leaving the details of memory relatively intact. Neuropsychological experiments in macaques can test this possibility on homologous cortical areas and pathways, based on behavioral designs tailored to the functions that these homologues perform in macaques. Earlier, we mentioned a similar approach aimed at disrupting threat-rich REE while leaving reassuring CES relatively unaffected. In both examples, the evolutionary perspectives elucidated in the first parts of this review explain how research on monkeys can contribute to understanding human-specific cognitive capacities and psychopathologies.
By understanding the functions of structures in nonhuman primates that have homologues in humans, we can gain insight into their specifically human functions. Put another way, if we can develop a clear understanding of how a given structure contributes to the Darwinian fitness of a monkey species, this knowledge should clarify what its homologue does in our species. As a relevant example, amygdala lesions in macaques block the expression of defensive behaviors, and, at the same time, heighten aspects of attentional processing for phobic stimuli, such as snakes and spiders (83). This finding suggests that the intact amygdala mediates defensive behavior that also competes with attentional processing of threats. The article reporting this insight prompted subsequent research on a patient with focal bilateral amygdala lesions. This patient lacked defensive responses to snakes and other threats; although she reported a fear of snakes and spiders, she displayed intense curiosity toward and tended to approach both stimuli (84). Thus, in both macaques and humans, amygdala functions can compete with and lessen certain aspects of sensory processing. In contrast, amygdala functions in other settings might enhance reactions to threat stimuli (85, 86). Parallel studies in humans and nonhuman primates could distinguish scenarios where threat-related processes are enhanced or reduced by amygdala function. Subsequent studies could then link anxiety symptoms in patients with amygdala-dependent attentional biases, which clinical trials could manipulate to determine their causal role in the genesis or maintenance of anxiety.
To this end, three approaches leverage insights from primate research to improve clinical targeting of neural circuits contributing to traumatic REE. Taken together, they provide complementary advantages and disadvantages. One uses cognitive training to alter circuit function through therapeutically relevant exercises. Many such training approaches exist, and they have the advantage of being noninvasive. Of most relevance to traumatic REE, task-based procedures that engage homotypical association areas, including those connected to the medial prefrontal cortex, reduce the clinical impact of REE (87). Training approaches have a clear weakness, however: the indirect relationship between training procedures and levels of engagement of specific areas and pathways. This weakness might be addressed by adapting biofeedback techniques used in PTSD (88). The second approach uses medications, either alone or with psychotherapy. Their utility in PTSD and major depression shows that this approach is beneficial in many cases, as are emerging technical advances for localizing pharmacological effects. Again, research on nonhuman primates will be crucial for validating such advances. However, the lack of anatomical specificity is a weakness: medications affect many neurochemical systems, and it is rarely clear which effect alters symptoms. The third approach, brain stimulation, most directly links research in monkeys to treatments in humans. Stimulation of hippocampal-neocortical networks has robust effects on episodic memory in healthy adults (89), and recent findings in PTSD appear promising (90). Published work in PTSD (90) and major depression (74, 91) typically emphasizes the prefrontal cortex, but such interventions could be adapted to focus on other parts of the core REE/CES network. The potential for adverse effects is the technique’s major weakness.
CES and Development of Emotional Disorders
Like psychopathology involving REE, emotional problems related to CES reflect both ongoing dysfunction of relevant cortical networks and aspects of individuals’ life histories. Some individuals with excessive CES manifest symptoms at many points in life, with clinical features that change with maturation and experience; other individuals acquire capacities that might mitigate these tendencies, as reflected in only temporary symptoms during childhood.
These patterns raise two questions on the developmental nature of emotional problems. First, although most adult emotional problems begin as pediatric anxiety disorders, many children with anxiety disorders mature to become psychiatrically healthy adults. Thus, we need to know which aspects of brain development distinguish forms of pediatric anxiety leading to chronic problems from forms with minimal long-term impact. Second, among affected patients, clinical expressions change during development, often beginning with separation-related problems, followed by fear of particular objects or situations. With adolescence, emotional problems change further to involve abstract concerns, such as consternation about one’s competence or lasting feelings of profound sadness. These patterns parallel broader normative changes seen across cultures in fears, anxieties, and moods (92, 93). Such observations raise questions about developmental trajectories that produce persistent psychopathology, expressed through changing emotional features in anxiety.
Like the trauma-related disorders mentioned above, studies in macaques provide avenues for translational research on developmental features of anxiety. For CES, linked studies in humans and monkeys promise to improve outcome prediction by clarifying the nature of developmental processes contributing to chronic psychopathology in anxious children.
One notable set of findings concerns the stability of individual differences over the lifespan (92, 93). Both acute response to experimental threats and variation in broadly expressed temperaments relate to individual differences in brain function, such as high levels of activity in CES-related circuits. These individual differences generate ideas on predictive biomarkers to be tested experimentally in monkeys. For example, there is continuity between early-childhood anxious temperament and adult outcomes involving internalizing psychopathologies such as depression and anxiety (94). Understanding the underlying biology improves outcome prediction in children with anxious temperament. For example, among children with heightened levels of behavioral inhibition, the subset who also manifest enhanced neural sensitivity to error commission in adolescence, compared with the subset with low neural sensitivity, face higher risk for internalizing psychopathologies in adulthood (94).
Similar continuity of temperament occurs in macaque monkeys (95), which makes parallel studies promising. Research on potential biomarkers that predict long-term stability of temperament in monkeys can support applications useful for predicting clinical outcomes in at-risk children. As explained later and as illustrated in Figure 5, this work could utilize the human intruder paradigm in macaque monkeys, which evokes correlates of pediatric anxiety disorders (96). Studies of choice behavior employing this paradigm in monkeys could include event-related potentials related to error monitoring and their association with functions of medial prefrontal areas such as the ACC. As noted above, children with behavioral inhibition tend to have the linked traits of high error sensitivity in adolescence and internalizing disorders in adulthood. Thus, improvements in the ability to assess functioning of relevant circuitry in adolescence might enhance prediction of clinical outcomes in adulthood. To create such improvements, hypotheses on mechanisms that connect early temperament, error sensitivity, and stable levels of defensive behavior could be tested in monkeys. This research program might involve manipulations of the homologous ACC regions and associated circuitry in juvenile monkeys, using a broader and more invasive array of methods than are available for human research.
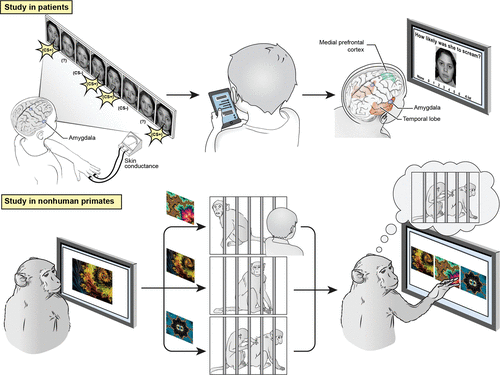
FIGURE 5. Schematic for parallel studies in humans and nonhuman primatesa
a The study in humans is adapted from research on pediatric anxiety disorders. It begins with a threat conditioning exercise, which involves visually presented faces, some of which are associated with an aversive scream (left), followed by a weeks-long period of ecological momentary assessment quantifying emotional experiences and memory (middle). Finally (right), memories linking visual stimuli with emotional responses are probed during fMRI using stimuli adapted from the threat conditioning sessions (left). The study in nonhuman primates is adapted from research on two homotypical association areas: the granular prefrontal cortex and the inferior temporal cortex, both of which are connected with the amygdala (see Figure 3). Macaque monkeys learn to associate each of three novel fractals (left) with one of three events (middle), two of which induce emotional responses (top to bottom): an aversive exposure to a human intruder, a control condition (an innocuous sound, matched in duration to the intruder’s time in the room), or a rewarding exposure to grooming with a socially paired conspecific. With experience, mature macaques can learn a new set of three fractal-event associations within a few minutes. Later (right), the monkey makes a choice among fractals, presumably choosing the one associated with grooming. Parallel studies on juvenile monkeys can reveal how cortical maturation enables adult monkeys to establish memories linking abstract visual stimuli with predicted emotional responses. Such studies also might assess how anxious temperament, quantified using the human intruder paradigm, relates to cortical maturation.
Other findings relevant to outcome prediction concern cortical development, especially the neotenous character of homotypical association areas. Earlier, we cited evidence that anthropoid-unique cortical areas expanded dramatically during evolution (Figure 1A). In monkeys (97), as in humans (15), the most recently evolved and expanded cortical regions are also the last to mature, along with the representations and information-processing functions they support (5). Furthermore, these late-maturing regions exhibit longer windows of plasticity than earlier-maturing regions. Some data suggest clinical relevance. For example, selective serotonin reuptake inhibitor exposure in juvenile monkeys produces particularly large effects in the superior and inferior temporal cortex (98), which are homotypical association areas that expanded during human evolution and exhibit delayed maturation. Delayed and prolonged maturation of anthropoid-unique areas presumably underlies the marked, gradually unfolding adolescent changes in emotional processes.
Figure 5 depicts linked studies based on this idea in patients with anxiety disorders and in monkeys. It exemplifies the kind of coordinated, iterative studies that could improve outcome predictions in patients. For example, when pediatric emotional problems persist into adulthood, their changing clinical features might reflect the maturation of cortical areas. These areas could include regions shown in the figure as engaged during learning, such as temporal areas and the amygdala, which link sensory representations to emotional responses. Such changes might incorporate medial frontal areas, also shown in the figure, which shape such forms of learning through error-monitoring functions. The bottom part of Figure 5 illustrates a study in monkeys aimed at examining these developmental changes, which fMRI experiments can then apply to patients to identify neural correlates of long-term outcomes.
For anxiety disorders, future fMRI studies might target the role of homotypical association areas in the memory of frightening events, which changes in adolescence. Past work informing Figure 5 has established methods for localizing neural correlates of encoding and recall for enduring fears (99). Future studies might employ these methods to quantify individual differences in flexible episodic representations, as assessed with digital techniques (e.g., ecological momentary assessment) over weeks-long time frames separating encoding and recall and which populate imagined events (CES). Such work could test hypotheses relevant to long-term outcomes, such as the idea that persistent (versus transient) emotional problems involve enhanced CES and dysregulation of the association cortex–amygdala circuitry highlighted in Figure 5 (top right).
The parallel studies imagined for macaques could serve to focus this clinical research constructively. For example, mature macaque monkeys can rapidly learn arbitrary associations between abstract visual stimuli and emotional responses. As noted above, embedding such learning with exposure to the human intruder paradigm leverages prior findings. These findings delineate parallels between the neural correlates of anxious temperament in monkeys and anxiety disorders in children (96). Future studies might examine the associative memories linking abstract visual stimuli with exposures to human intruders. As displayed in the bottom half of Figure 5, mature macaques can learn to associate each of three novel, abstract visual cues, such as fractal patterns, with an impending event: entry of a human intruder; a control condition; or access to conspecifics. Studies in mature and juvenile monkeys could delineate mechanisms linking age-related changes in the inferior temporal and granular prefrontal cortex to age-related changes in the ability to establish memories that evoke emotional responses. Similar mechanisms might underlie dynamic features of persistent emotional problems in patients, including transitions from concrete fears to generalized anxiety during maturation.
Conclusions
Although research on nonprimates, such as rodents, can broadly inform an understanding of mental illness, nonhuman primate studies can uniquely target brain structures and functions that distinguish primates from other species. Evolutionary innovations endowed humans with advantageous information-processing functions and new forms of memory. Along with their advantages, however, these phylogenetic developments produced vulnerabilities to emotional problems. Both insufficiencies and excesses in specifically human cognitive capacities can develop over the lifespan, as the underlying cortical networks mature and adapt to modern life. REE and CES are two examples of such clinically relevant capacities unique to humans. Neuroimaging findings in illnesses with prominent emotional features encourage an agenda of linked studies in nonhuman primates and patients to delineate mechanisms that generate these capacities. For REE, these studies promise to generate novel treatments for illnesses that follow trauma. For CES, they could improve outcome prediction by illuminating developmental processes underlying chronic illnesses that begin as early-life anxiety disorders.
1. : Darwin’s mistake: explaining the discontinuity between human and nonhuman minds. Behav Brain Sci 2008; 31:109–130, discussion 130–178Crossref, Medline, Google Scholar
2. : What Is Special About the Human Brain? Oxford, UK, Oxford University Press, 2008Crossref, Google Scholar
3. : The Gap: The Science of What Separates Us From Other Animals. New York, Basic Books, 2013Google Scholar
4. : Using neuroscience to help understand fear and anxiety: a two-system framework. Am J Psychiatry 2016; 173:1083–1093Link, Google Scholar
5. : The Evolution of Memory Systems: Ancestors, Anatomy, and Adaptations. Oxford, UK, Oxford University Press, 2017Google Scholar
6. : Hypothesis: Alzheimer’s disease is a phylogenetic disease. Med Hypotheses 1989; 29:147–150Crossref, Medline, Google Scholar
7. , : Human-specific transcriptional regulation of CNS development genes by FOXP2. Nature 2009; 462:213–217Crossref, Medline, Google Scholar
8. : The evolution of the human brain and disease susceptibility. Curr Opin Genet Dev 2020; 65:91–97Crossref, Medline, Google Scholar
9. : The evolution of endothermy in mammals and birds: from physiology to fossils. Annu Rev Physiol 1995; 57:69–95Crossref, Medline, Google Scholar
10. : The evolution of the human pelvis: changing adaptations to bipedalism, obstetrics, and thermoregulation. Philos Trans R Soc Lond B Biol Sci 2015; 370:20140063Crossref, Medline, Google Scholar
11. , : Australopithecus afarensis endocasts suggest ape-like brain organization and prolonged brain growth. Sci Adv 2020; 6:eaaz4729Crossref, Medline, Google Scholar
12. , : Specializations of the granular prefrontal cortex of primates: implications for cognitive processing. Anat Rec A Discov Mol Cell Evol Biol 2006; 288:26–35Crossref, Medline, Google Scholar
13. , : Quantitative assessment of prefrontal cortex in humans relative to nonhuman primates. Proc Natl Acad Sci USA 2018; 115:E5183–E5192Crossref, Medline, Google Scholar
14. , : Trends and properties of human cerebral cortex: correlations with cortical myelin content. Neuroimage 2014; 93(Pt 2):165–175Crossref, Medline, Google Scholar
15. , : Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci USA 2010; 107:13135–13140Crossref, Medline, Google Scholar
16. , : Reply to Barton and Montgomery: A case for preferential prefrontal cortical expansion. Proc Natl Acad Sci USA 2019; 116:5–6Crossref, Medline, Google Scholar
17. : Human frontal lobes are not relatively large. Proc Natl Acad Sci USA 2013; 110:9001–9006Crossref, Medline, Google Scholar
18. : Is the prefrontal cortex especially enlarged in the human brain allometric relations and remapping factors. Brain Behav Evol 2014; 84:156–166Crossref, Medline, Google Scholar
19. , : Normative brain size variation and brain shape diversity in humans. Science 2018; 360:1222–1227Crossref, Medline, Google Scholar
20. , : High-expanding regions in primate cortical brain evolution support supramodal cognitive flexibility. Cereb Cortex 2019; 29:3891–3901Crossref, Medline, Google Scholar
21. : The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci 2010; 14:172–179Crossref, Medline, Google Scholar
22. , : Human-specific NOTCH2NL genes affect notch signaling and cortical neurogenesis. Cell 2018; 173:1356–1369.e22Crossref, Medline, Google Scholar
23. , : Human-specific NOTCH2NL genes expand cortical neurogenesis through delta/notch regulation. Cell 2018; 173:1370–1384.e16Crossref, Medline, Google Scholar
24. , : Inhibition of SRGAP2 function by its human-specific paralogs induces neoteny during spine maturation. Cell 2012; 149:923–935Crossref, Medline, Google Scholar
25. , : Evolution of human-specific neural SRGAP2 genes by incomplete segmental duplication. Cell 2012; 149:912–922Crossref, Medline, Google Scholar
26. : Human adaptation and evolution by segmental duplication. Curr Opin Genet Dev 2016; 41:44–52Crossref, Medline, Google Scholar
27. , : Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion. Science 2015; 347:1465–1470Crossref, Medline, Google Scholar
28. , : Human-specific ARHGAP11B increases size and folding of primate neocortex in the fetal marmoset. Science 2020; 369:546–550Crossref, Medline, Google Scholar
29. : Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirrhine primate Galago and the anthropoid primate Macaca. J Comp Neurol 1991; 310:429–474Crossref, Medline, Google Scholar
30. : Distinct patterns of hippocampal and neocortical evolution in primates. Brain Behav Evol 2019; 93:171–181Crossref, Medline, Google Scholar
31. : Evolutionary shifts dramatically reorganized the human hippocampal complex. J Comp Neurol 2020; 528:3143–3170Crossref, Medline, Google Scholar
32. : The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci 2014; 1316:29–52Crossref, Medline, Google Scholar
33. , : Remembering and imagining alternative versions of the personal past. Neuropsychologia 2018; 110:170–179Crossref, Medline, Google Scholar
34. : Empirical support for higher-order theories of conscious awareness. Trends Cogn Sci 2011; 15:365–373Crossref, Medline, Google Scholar
35. : Medial frontal cortex: from self-generated action to reflection on one’s own performance. Trends Cogn Sci 2010; 14:16–21Crossref, Medline, Google Scholar
36. : The anterior cingulate gyrus and social cognition: tracking the motivation of others. Neuron 2016; 90:692–707Crossref, Medline, Google Scholar
37. : Neuronal reference frames for social decisions in primate frontal cortex. Nat Neurosci 2013; 16:243–250Crossref, Medline, Google Scholar
38. , : The anterior cingulate cortex is necessary for forming prosocial preferences from vicarious reinforcement in monkeys. PLoS Biol 2020; 18:e3000677Crossref, Medline, Google Scholar
39. : Primate brain size is predicted by diet but not sociality. Nature Ecol Evol 2017; 1:112Crossref, Medline, Google Scholar
40. , : Distinct contributions of frontal areas to emotion and social behaviour in the rat. Eur J Neurosci 2007; 26:2315–2326Crossref, Medline, Google Scholar
41. , : Neuroethology of primate social behavior. Proc Natl Acad Sci USA 2013; 110(suppl 2):10387–10394Crossref, Medline, Google Scholar
42. , : A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci 2012; 24:1742–1752Crossref, Medline, Google Scholar
43. , : Large-scale meta-analysis of human medial frontal cortex reveals tripartite functional organization. J Neurosci 2016; 36:6553–6562Crossref, Medline, Google Scholar
44. , : Evolutionary modifications in human brain connectivity associated with schizophrenia. Brain 2019; 142:3991–4002Crossref, Medline, Google Scholar
45. : Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci 2007; 8:657–661Crossref, Medline, Google Scholar
46. : Episodic future thinking: mechanisms and functions. Curr Opin Behav Sci 2017; 17:41–50Crossref, Medline, Google Scholar
47. : Individual differences in the phenomenology of mental time travel: the effect of vivid visual imagery and emotion regulation strategies. Conscious Cogn 2006; 15:342–350Crossref, Medline, Google Scholar
48. , : Positivity bias in past and future episodic thinking: relationship with anxiety, depression, and retrieval-induced forgetting. Q J Exp Psychol (Hove) 2019; 72:508–522Crossref, Medline, Google Scholar
49. : Deconstructing episodic memory with construction. Trends Cogn Sci 2007; 11:299–306Crossref, Medline, Google Scholar
50. : Memory and temporal experience: the effects of episodic memory loss on an amnesic patient’s ability to remember the past and imagine the future. Soc Cogn 2002; 20:353–379Crossref, Google Scholar
51. , : The role of the pre-commissural fornix in episodic autobiographical memory and simulation. Neuropsychologia 2020; 142:107457Crossref, Medline, Google Scholar
52. : Boundaries shape cognitive representations of spaces and events. Trends Cogn Sci 2018; 22:637–650Crossref, Medline, Google Scholar
53. : Self-projection and the brain. Trends Cogn Sci 2007; 11:49–57Crossref, Medline, Google Scholar
54. , : Differential contributions of hippocampus and medial prefrontal cortex to self-projection and self-referential processing. Neuropsychologia 2015; 73:116–126Crossref, Medline, Google Scholar
55. : Neurocircuitry of mood disorders. Neuropsychopharmacology 2010; 35:192–216Crossref, Medline, Google Scholar
56. : Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev 2001; 38:247–289Crossref, Medline, Google Scholar
57. : A description of the amygdalo-hippocampal interconnections in the macaque monkey. Exp Brain Res 1986; 64:515–526Crossref, Medline, Google Scholar
58. : Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat Rev Neurosci 2016; 17:173–182Crossref, Medline, Google Scholar
59. : Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus 1995; 5:511–533Crossref, Medline, Google Scholar
60. , : Complementary patterns of direct amygdala and hippocampal projections to the macaque prefrontal cortex. Cereb Cortex 2015; 25:4351–4373Crossref, Medline, Google Scholar
61. , : fMRI studies of successful emotional memory encoding: a quantitative meta-analysis. Neuropsychologia 2010; 48:3459–3469Crossref, Medline, Google Scholar
62. : Neural correlates of successful emotional episodic encoding and retrieval: an SDM meta-analysis of neuroimaging studies. Neuropsychologia 2020; 143:107495Google Scholar
63. , : Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron 2006; 49:631–638Crossref, Medline, Google Scholar
64. :
65. : Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol Rev 2001; 108:483–522Crossref, Medline, Google Scholar
66. : Amygdala circuitry in attentional and representational processes. Trends Cogn Sci 1999; 3:65–73Crossref, Medline, Google Scholar
67. : From circuits to behaviour in the amygdala. Nature 2015; 517:284–292Crossref, Medline, Google Scholar
68. : The amygdala and reward. Nat Rev Neurosci 2002; 3:563–573Crossref, Medline, Google Scholar
69. , : Amygdala and ventral striatum make distinct contributions to reinforcement learning. Neuron 2016; 92:505–517Crossref, Medline, Google Scholar
70. , : Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience 2005; 132:943–953Crossref, Medline, Google Scholar
71. , : Posterior orbitofrontal and anterior cingulate pathways to the amygdala target inhibitory and excitatory systems with opposite functions. J Neurosci 2017; 37:5051–5064Crossref, Medline, Google Scholar
72. , : The intercalated nuclear complex of the primate amygdala. Neuroscience 2016; 330:267–290Crossref, Medline, Google Scholar
73. : Why we need nonhuman primates to study the role of ventromedial prefrontal cortex in the regulation of threat- and reward-elicited responses. Proc Natl Acad Sci USA 2019; 116:26297–26304Crossref, Google Scholar
74. : From bed to bench side: reverse translation to optimize neuromodulation for mood disorders. Proc Natl Acad Sci USA 2019; 116:26288–26296Crossref, Google Scholar
75. , : A comprehensive transcriptional map of primate brain development. Nature 2016; 535:367–375Crossref, Medline, Google Scholar
76. , : Spatiotemporal transcriptomic divergence across human and macaque brain development. Science 2018; 362:eaat8077Crossref, Medline, Google Scholar
77. , : Constructive episodic simulation of the future and the past: distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia 2009; 47:2222–2238Crossref, Medline, Google Scholar
78. , : Childhood trauma history is linked to abnormal brain connectivity in major depression. Proc Natl Acad Sci USA 2019; 116:8582–8590Crossref, Medline, Google Scholar
79. : The devastating clinical consequences of child abuse and neglect: increased disease vulnerability and poor treatment response in mood disorders. Am J Psychiatry 2020; 177:20–36Link, Google Scholar
80. , : Mental imagery in depression: phenomenology, potential mechanisms, and treatment implications. Annu Rev Clin Psychol 2016; 12:249–280Crossref, Medline, Google Scholar
81. , : Intrusive traumatic reexperiencing: pathognomonic of the psychological response to traumatic stress. Am J Psychiatry 2021; 178:119–122Link, Google Scholar
82. , : Reinstatement of event details during episodic simulation in the hippocampus. Cereb Cortex 2020; 30:2321–2337Crossref, Medline, Google Scholar
83. : Distinct contributions of the amygdala and hippocampus to fear expression. Eur J Neurosci 2009; 30:2327–2337Crossref, Medline, Google Scholar
84. , : The human amygdala and the induction and experience of fear. Curr Biol 2011; 21:34–38Crossref, Medline, Google Scholar
85. , : Cross-species convergence in pupillary response: understanding human anxiety via non-human primate amygdala lesion. Soc Cogn Affect Neurosci 2019; 14:591–599Crossref, Medline, Google Scholar
86. , : Complementary features of attention bias modification therapy and cognitive-behavioral therapy in pediatric anxiety disorders. Am J Psychiatry 2017; 174:775–784Link, Google Scholar
87. , : Preventing intrusive memories after trauma via a brief intervention involving Tetris computer game play in the emergency department: a proof-of-concept randomized controlled trial. Mol Psychiatry 2018; 23:674–682Crossref, Medline, Google Scholar
88. , : Electrical fingerprint of the amygdala guides neurofeedback training for stress resilience. Nat Hum Behav 2019; 3:63–73Crossref, Medline, Google Scholar
89. , : Network-targeted stimulation engages neurobehavioral hallmarks of age-related memory decline. Neurology 2019; 92:e2349–e2354Crossref, Medline, Google Scholar
90. , : Theta-burst transcranial magnetic stimulation for posttraumatic stress disorder. Am J Psychiatry 2019; 176:939–948Link, Google Scholar
91. : Deep brain stimulation for intractable psychiatric disorders. Annu Rev Med 2012; 63:511–524Crossref, Medline, Google Scholar
92. : Childhood antecedents and risk for adult mental disorders. Annu Rev Psychol 2015; 66:459–485Crossref, Medline, Google Scholar
93. :
94. , : Infant behavioral inhibition predicts personality and social outcomes three decades later. Proc Natl Acad Sci USA 2020; 117:9800–9807Crossref, Medline, Google Scholar
95. : A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. Am J Psychiatry 2014; 171:1162–1173Link, Google Scholar
96. , : Evolutionarily conserved prefrontal-amygdalar dysfunction in early-life anxiety. Mol Psychiatry 2014; 19:915–922Crossref, Medline, Google Scholar
97. , : Effects of neonatal inferior prefrontal and medial temporal lesions on learning the rule for delayed nonmatching-to-sample. Dev Neuropsychol 2000; 18:399–421Crossref, Medline, Google Scholar
98. , : Fluoxetine administered to juvenile monkeys: effects on the serotonin transporter and behavior. Am J Psychiatry 2014; 171:323–331Link, Google Scholar
99. , : Age differences in the neural correlates of anxiety disorders: an fMRI study of response to learned threat. Am J Psychiatry 2020; 177:454–463Link, Google Scholar



