Age Differences in the Neural Correlates of Anxiety Disorders: An fMRI Study of Response to Learned Threat
Abstract
Objective:
Although both pediatric and adult patients with anxiety disorders exhibit similar neural responding to threats, age-related differences have been found in some functional MRI (fMRI) studies. To reconcile disparate findings, the authors compared brain function in youths and adults with and without anxiety disorders while rating fear and memory of ambiguous threats.
Methods:
Two hundred medication-free individuals ages 8–50 were assessed, including 93 participants with an anxiety disorder. Participants underwent discriminative threat conditioning and extinction in the clinic. Approximately 3 weeks later, they completed an fMRI paradigm involving extinction recall, in which they rated their levels of fear evoked by, and their explicit memory for, morph stimuli with varying degrees of similarity to the extinguished threat cues.
Results:
Age moderated two sets of anxiety disorder findings. First, as age increased, healthy subjects compared with participants with anxiety disorders exhibited greater amygdala-ventromedial prefrontal cortex (vmPFC) connectivity when processing threat-related cues. Second, age moderated diagnostic differences in activation in ways that varied with attention and brain regions. When rating fear, activation in the vmPFC differed between the anxiety and healthy groups at relatively older ages. In contrast, when rating memory for task stimuli, activation in the inferior temporal cortex differed between the anxiety and healthy groups at relatively younger ages.
Conclusions:
In contrast to previous studies that demonstrated age-related similarities in the biological correlates of anxiety disorders, this study identified age differences. These findings may reflect this study’s focus on relatively late-maturing psychological processes, particularly the appraisal and explicit memory of ambiguous threat, and inform neurodevelopmental perspectives on anxiety.
Pediatric anxiety disorders predict risk for adult anxiety disorders, which may reflect persistent brain dysfunction (1, 2). However, most pediatric anxiety disorders remit by adulthood, and some anxiety disorders only begin after childhood (3). Hence, functional MRI (fMRI) studies that examine late-maturing capacities should reveal age differences. Indeed, preliminary data have shown such discontinuities in paradigms where healthy volunteers and patients with anxiety disorders rate fear evoked by, and explicit memory for, extinguished threat cues (4, 5). In this study, we more clearly explicate such discontinuities. We examined age-related differences in brain function in individuals with and without anxiety disorders as they rated their fear of and memory for ambiguous, extinguished threat cues.
Threat conditioning provides a useful context for this research (6). Threat conditioning occurs when neutral stimuli are paired with aversive unconditioned stimuli, leading the neutral stimulus to acquire the capacity to evoke defensive behavior (7, 8). Developmentally and across species, threat conditioning research shows preadolescent maturation of some amygdala-related functions, including aspects of learning and attention (9–12). In youths with anxiety, previous studies have suggested that perturbation in such circuitry begins early in life and endures (1, 2). In contrast, consistent with the late maturation of the prefrontal cortex (PFC), research has revealed differences between pediatric and adult anxiety disorders for PFC-supported functions, such as the sustained maintenance of extinction (4, 5, 10–13). In addition, late-maturing circuits support functions unique to primates, such as the efficient categorization of extinguished threats and other ambiguous cues (14–16). Thus, distinct amygdala-PFC circuitry dysfunctions may manifest in pediatric and adult anxiety disorders during categorization processes, such as subjective appraisal or declarative memory, triggered by ambiguous threat cues. To evaluate this possibility, fMRI paradigms can parametrically model ambiguity, using stimuli that blend features of extinguished threat and safety cues (4, 17). Such paradigms are well suited (18) for mapping age differences in the neural correlates of anxiety disorders.
To examine age-related variation in the neural correlates of anxiety disorders, we adapted a well-validated paradigm (4, 5, 11, 16, 18). In this paradigm, participants rate their fear of and memory for ambiguous stimuli resembling extinguished threat and safety cues, thereby categorizing ambiguous cues. We adapted the paradigm to include more stimulus replicates and to allow ratings on continuous scales. These adaptations enhanced statistical power when evaluating group differences in stimulus-specific and attention-specific responding, expected to manifest as high-order interactions with task features (16, 19). Relatively large samples are needed to provide appropriate statistical power for testing such multilevel interactions across age and patient groups. We examined 200 medication-free volunteers and treated age as a continuous variable to maximize statistical power, while applying relatively conservative statistical thresholds to limit type I errors.
Based on previous research, we hypothesized that fMRI response profiles would manifest differently in pediatric and adult anxiety disorders in late-maturing, recently evolved circuitry, encompassing the heteromodal association cortex (16). We expected greater engagement of this circuitry in healthy, older individuals compared with similarly aged patients with anxiety disorders, thereby extending findings from previous studies on adult anxiety. We further hypothesized that functional connectivity between the amygdala and ventromedial prefrontal cortex (vmPFC) would differ between healthy adults and adults with anxiety, also extending findings from previous investigations (5, 20, 21). Finally, limited previous data have generated less precise hypotheses for children than for adults, although the available data reveal different patterns in pediatric than adult anxiety (4, 5). Thus, given sparse and inconsistent findings, support appears strongest for an overall expectation of differing patterns in pediatric and adult anxiety disorders, both for heteromodal association cortex activation and amygdala-vmPFC functional connectivity, with unclear expectations regarding diagnostic differences in youths.
Methods
Participants
Participants ages 8–50 attended a psychophysiology visit and an fMRI visit. A total of 327 participants (healthy group, N=172; anxiety group, N=155) started the first visit; of these, 40 were excluded because they discontinued before (healthy group, N=1; anxiety group, N=6) or after (healthy group, N=7; anxiety group, N=16) the initial unconditioned stimulus presentation or because of technical problems (healthy group, N=6; anxiety group, N=4).
After the first visit, 249 participants (76.15%) returned for an fMRI visit (healthy group, N=136; anxiety group, N=113). Data from 49 participants were excluded because of technical problems (healthy group, N=9; anxiety group, N=3), MRI discontinuation (healthy group, N=1; anxiety group, N=2), excessive motion (healthy group, N=2; anxiety group, N=4), poor performance (nonresponse on >25% of trials per condition: healthy group, N=12; anxiety group, N=9), or excessive delay between visits (healthy group, N=5; anxiety group, N=2). This yielded a total of 200 participants (healthy group, N=107; anxiety group, N=93) who completed the analyses.
Diagnosis was determined using semistructured interviews for youths (<18 years old) and adults (≥18 years old). Participants with anxiety (N=93) met DSM-5 criteria for a current primary diagnosis of generalized anxiety disorder, social anxiety disorder, or separation anxiety disorder (and/or panic disorder among adults). All participants were medication free, and healthy volunteers were free of any current psychiatric disorders. For further details, see the online supplement.
Demographic and clinical characteristics of the study participants are presented in Table 1. For hypothesis testing, age was treated as a continuous measure in statistical analyses. However, when significant associations with age were observed in the analyses, we used a post hoc comparison median split (median age, 17.29 years) to illustrate age moderation of patient-comparison differences. For simplicity, the term “adult” indicates participants who were older than the median age, and the term “youths” indicates participants who were younger than the median age. It is noteworthy that overall, associations with an anxiety diagnosis were not significant for age (t=−1.41, df=198, p=0.16) or sex (χ2=2.19, df=1, p=0.14), although in analyses restricted to adults, anxiety was more common in females (χ2=10.83, df=1, p<0.001). The interaction of diagnosis and age was unrelated to IQ or days between study visits (all p values >0.2). Thirty-three participants asked whether they would receive the unconditioned stimulus before the extinction phase (N=3), at the extinction recall phase during fMRI scanning (N=26), or during both phases (N=4) and were instructed that the unconditioned stimulus would not occur. More participants with anxiety disorders (N=23) inquired about unconditioned stimulus occurrence compared with healthy subjects (N=10) (χ2=8.55, df=1, p=0.003). Post hoc analyses repeated the whole-brain analyses controlling for both instructed extinction recall and sex. Written informed consent was obtained from adult participants and from the parents of youths, and assent was obtained from youths. All study procedures were approved by the National Institute of Mental Health Institutional Review Board.
| Anxiety Disorder Group (N=93) | Healthy Group (N=107) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Youths (N=53) | Adults (N=40) | Youths (N=47) | Adults (N=60) | ||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years)b | 12.75 | 2.73 | 28.46 | 8.81 | 13.21 | 2.49 | 27.83 | 6.92 |
| Measurec | ||||||||
| IQ (WASI)d | 114.98 | 13.38 | 113.98 | 14.33 | 111.11 | 11.63 | 115.69 | 13.27 |
| SCARED (parent) | 32.94 | 12.95 | — | — | 4.33 | 4.36 | — | — |
| SCARED (child) | 33.28 | 12.81 | — | — | 9.62 | 7.73 | — | — |
| PARS baselinee | 16.82 | 2.89 | — | — | — | — | — | — |
| PARS postassessmente | 11.54 | 4.04 | — | — | — | — | — | — |
| Days between visits | 18.91 | 8.01 | 18.65 | 6.73 | 21.23 | 6.51 | 21.22 | 7.57 |
| N | % | N | % | N | % | N | % | |
| Female | 29 | 54.72 | 31 | 77.50 | 29 | 61.70 | 29 | 48.33 |
| Instructed statusf | ||||||||
| Extinction | 3 | 5.66 | 0 | 0 | 0 | 0 | 0 | 0 |
| Extinction recall | 15 | 28.30 | 5 | 12.50 | 3 | 6.38 | 3 | 5.00 |
| Both visits | 2 | 3.77 | 2 | 5.00 | 0 | 0 | 0 | 0 |
| Diagnosis | ||||||||
| Generalized anxiety disorder | 41 | 77.36 | 31 | 77.50 | 0 | 0 | 0 | 0 |
| Social anxiety disorder | 33 | 62.26 | 26 | 65.00 | 0 | 0 | 0 | 0 |
| Separation anxiety disorder | 19 | 35.85 | 1 | 2.50 | 0 | 0 | 0 | 0 |
| Panic disorder | 1 | 1.89 | 3 | 7.50 | 0 | 0 | 0 | 0 |
| Specific phobia | 25 | 47.17 | 1 | 2.50 | 0 | 0 | 0 | 0 |
| Major depressive disorder | 0 | 0 | 1 | 2.50 | 0 | 0 | 0 | 0 |
| Attention deficit hyperactivity disorder | 2 | 3.77 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oppositional defiant disorder | 2 | 3.77 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tics or Tourette’s syndrome | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Selective mutism | 1 | 1.89 | 0 | 0 | 0 | 0 | 0 | 0 |
| Elimination disorder | 1 | 1.89 | 0 | 0 | 0 | 0 | 0 | 0 |
TABLE 1. Demographic and clinical characteristics of participants in a study of the neural correlates of anxiety disordersa
Procedures
Visit 1: psychophysiology.
Participants completed the “screaming lady” paradigm (Figure 1A), which has been described elsewhere (4, 11). Self-report and psychophysiological data were recorded during discriminative conditioning to threat and safety conditioned stimuli (CS+ and CS−, respectively), followed by extinction.
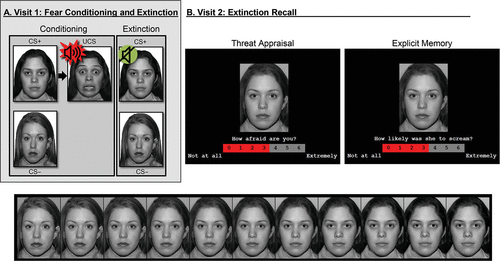
FIGURE 1. Task paradigm of threat conditioning, extinction, and extinction recall among youths and adults with anxiety disorders and healthy subjectsa
a In visit 1 (panel A), participants first underwent threat conditioning, during which one female face (conditioned stimulus [CS+]) was paired with a fearful face coterminating with a loud scream (unconditioned stimulus); the other female face (CS–) was never paired with the unconditioned stimulus. Next, during extinction, the two faces were repeatedly presented without the unconditioned stimulus. In visit 2 (panel B), participants completed extinction recall, during which morphed images continuously varying in similarity from the CS− to CS+ (bottom) were presented. For each image, participants rated (top) their current levels of fear (threat appraisal) or whether the CS screamed in the past (explicit memory).
Visit 2: fMRI.
Approximately 3 weeks after visit 1, participants returned for the extinction recall task adapted from previous work (4). Participants made threat-safety discriminations under two attention conditions: threat appraisal and explicit memory. Specifically, participants rated their current levels of fear evoked by, and memory for, facial morph stimuli falling along a continuum with varying degrees of similarity to the extinguished threat (CS+) and safety (CS−) cues (Figure 1B).
Data Analysis
Data were analyzed using Analysis of Functional NeuroImages (AFNI [22]) (for further details, see the online supplement).
Individual level.
Using the AFNI 3dDeconvolve, three general linear models were generated to estimate blood-oxygenation-level-dependent (BOLD) signal change with amplitude modulation based on reaction time, as well as task-related functional connectivity of the amygdala using generalized psychophysiological interaction (gPPI) methods (23). The first general linear model employed the AFNI amplitude modulation option (AM2) to generate two types of regressors: task-related activation at the mean reaction time and reaction time-modulated BOLD response. The latter type provided a direct measure of the proportionality of BOLD activation to changes in the reaction time amplitude factor. Given their complexity, the results from the reaction time-modulated regressor analyses are presented in the online supplement. The other type of general linear models applied gPPI methods to identify brain regions that differed in their functional connectivity with the amygdala as a function of task conditions, with separate models considering left and right amygdala seeds. This resulted in a total of three general linear models generated at the individual level.
Group level.
Coefficients from the following regressors of interest at the individual level were included in four group-level analysis models: reaction time-modulated task activation (see the online supplement), task activation at average reaction time, gPPI for the left amygdala, and gPPI for the right amygdala. Whole-brain voxel-wise tests were used for all fMRI analyses, and age was modeled as a continuous variable to maximize statistical power. Analyses used linear mixed-effects modeling with 3dLME (24), including age (continuous) and anxiety diagnosis (dichotomous) as between-subject variables, with between-visit interval in days (continuous) as a nuisance variable. Attention condition (dichotomous: threat appraisal or explicit memory) and the linear and quadratic trends of threat resemblance across morphed images were within-subject variables. Linear and quadratic trends were based on the morphed level for each image with weights generated from orthogonal polynomials.
Correction for multiple comparisons.
Statistical maps encompassed gray matter voxels for which data were available for >90% of participants to set whole brain-corrected thresholds. The initial voxel-wise threshold was set at a p value <0.001 (two-sided). The AFNI 3dClustSim tool was used for correction for multiple comparisons. The spatial autocorrelation function parameters of the residual time series from the individual-level models were estimated and averaged across participants (0.548465, 3.91625, 11.2995) using AFNI 3dFWHMx with the acf flag, in accordance with recommendations by Cox et al. (25). To account for 28 experiment-wise interactions across four linear mixed-effects models, the corrected threshold was determined using two-sided thresholding for whole brain F tests with first-nearest neighbor clustering (NN=1), with alpha set to <0.05/28=0.0018. This yielded a cluster threshold of 57 voxels (890.625 mm3), based on Monte Carlo cluster-size simulations. All tests were two-sided, and alpha was set at 0.05.
We focused on findings for diagnosis in omnibus interaction tests, because they most precisely reflect the study design and corresponding hypotheses among age, diagnosis, and task factors. Thus, the AFNI 3dClustSim alpha was set at 0.0018, reflecting the significance level of 0.05, Bonferroni-corrected for 28 F tests. This threshold was based on the four above-described models for two four-way and five three-way interactions (i.e., 4×[2+5]=28), thereby evaluating hypotheses for diagnosis. Results from two-way interactions with diagnosis are reported but not interpreted because of the study’s focus on high-order interactions. To visualize interactions, coefficient values averaged from each cluster were extracted and compared across groups using post hoc analyses. As noted above, age was modeled as a continuous regressor in all analyses. Nevertheless, data are plotted on the basis of a median split (median age, 17.29 years) to illustrate patterns influencing significant interactions. As noted earlier, participants older than the median age were considered to be adults, and those younger than the median age were considered to be youths.
Results
Psychophysiology Visit
Briefly, across participants, skin conductance response data revealed successful conditioning and extinction. Moreover, stimuli evoked greater fear and skin conductance response in participants with anxiety compared with healthy subjects (see Table S1 in the online supplement). There were no other anxiety-related differences.
fMRI Visit
Task performance.
For reaction time, morphed stimuli evoked distinct quadratic trends in each attention condition (see Figure S2 in the online supplement). Thus, appraisal and memory engaged distinct psychological processes. Moreover, for rating data, age differentially moderated anxiety-related differences based on the attention condition (see Figure S3 in the online supplement). For details on behavioral data, see the online supplement.
Task-related activation.
A four-way interaction occurred only in the amplitude-modulation analyses, which is presented in the online supplement. The main task-based analyses at the average reaction time revealed no four-way interactions. However, there were three three-way anxiety-by-age-by-attention clusters: one vmPFC cluster (Talairach coordinates x, y, z: −9, 49, −1; 667 voxels; peak: F=47.63, df=1, 4180, p<0.001 [Figure 2]) and two inferior temporal gyrus clusters (x, y, z: 51, −59, −11; 263 voxels; peak: F=42.05, df=1, 4180, p<0.001; and x, y, z: 31, −54, −19; 65 voxels; peak: F=28.20, df=1, 4180, p<0.001 [Figure 3]). In the vmPFC, age moderated anxiety group differences during appraisal tasks but not memory tasks. During threat appraisal tasks, activation in the vmPFC differed between healthy adults and adults with anxiety (t=3.17, df=98, p=0.002; Cohen’s d=0.65) but not among youths (t=−0.71, df=98, p=0.48; Cohen’s d=0.14 [Figure 2B]). A double dissociation occurred in the larger inferior temporal gyrus cluster (263 voxels), where attention moderated interactions of diagnosis and age. Adults with anxiety, compared with healthy adults, showed greater activation during appraisal tasks (t=2.46, df=98, p=0.016; Cohen’s d=0.49) but not memory tasks (t=1.16, df=98, p=0.25; Cohen’s d=0.23). In youths, however, participants with anxiety compared with healthy subjects showed greater activation during memory tasks (t=3.13, df=98, p=0.002; Cohen’s d=0.64) but not appraisal tasks (t=0.66, df=98, p=0.51; Cohen’s d=0.13). (Full task-related activation results are presented in Table S2 in the online supplement.)
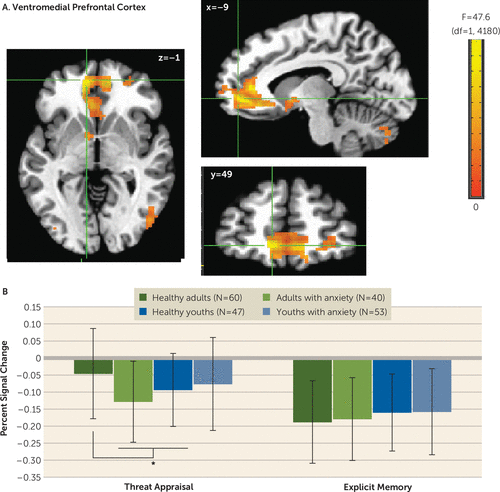
FIGURE 2. Task-related activation in the ventromedial prefrontal cortex among youths and adults with anxiety disorders and healthy subjectsa
a Whole-brain analyses of task-related activation revealed a significant interaction of anxiety diagnosis, age, and attention condition in the ventromedial prefrontal cortex (vmPFC) (panel A). Images are shown in neurological convention (i.e., left is left) and thresholded at F>10.76, df=1, 4180, p<0.001, cluster size >57 voxels (890.625 mm3). To decompose the complex interaction effects, mean extracted values (panel B) for this cluster are plotted separately by attention condition (threat appraisal, explicit memory) and group, based on anxiety diagnosis (healthy, anxiety) and age (median split: adults, youths). In the graph, the y-axis shows extracted vmPFC percent signal change averaged across participants in each group. Error bars indicate standard deviation.
*p<0.05.
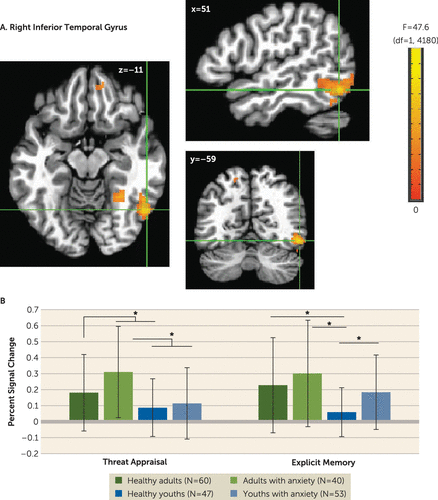
FIGURE 3. Task-related activation in the right inferior temporal gyrus among youths and adults with anxiety disorders and healthy subjectsa
a Whole-brain analyses of task-related activation revealed a significant interaction of anxiety diagnosis, age, and attention condition in two clusters in the right inferior temporal gyrus (ITG) (panel A). Images are shown in neurological convention (i.e., left is left) and thresholded at F>10.76, df=1, 4180, p<0.001, cluster size >57 voxels (890.625 mm3). To decompose the complex interaction effects, mean extracted values (panel B) from the larger ITG cluster (263 voxels) are plotted separately by attention condition (threat appraisal, explicit memory) and group, based on anxiety diagnosis (healthy, anxiety) and age (median split: adults, youths). In the graph, the y-axis shows extracted ITG percent signal change averaged across participants in each group. Error bars indicate standard deviation.
*p<0.05.
The diagnosis-by-attention interaction revealed additional findings that are presented in Table S2 in the online supplement. Overall, a consistent pattern emerged in the vmPFC, angular gyrus, cerebellum, and anterior temporal cortex. Attention state moderated anxiety-related differences, such that significant group differences emerged for threat appraisal (all p values <0.03) but not memory (all p values >0.15).
gPPI.
Whole brain-corrected left amygdala seed analyses revealed one anxiety-by-age-by-linear slope interaction across morphed stimuli in the vmPFC (x, y, z: 9, 59, 1; 58 voxels; peak: F=17.22, df=1, 4180, p<0.001 [Figure 4]). A median split for age was used in post hoc analyses. More positive linear gPPI slope coefficients manifested in healthy adults compared with adults with anxiety (t=4.61, df=98, p=0.00001; Cohen’s d=0.93). Opposite patterns occurred in youths (t=–2.15, df=98, p=0.034; Cohen’s d=0.44). No right amygdala clusters emerged.
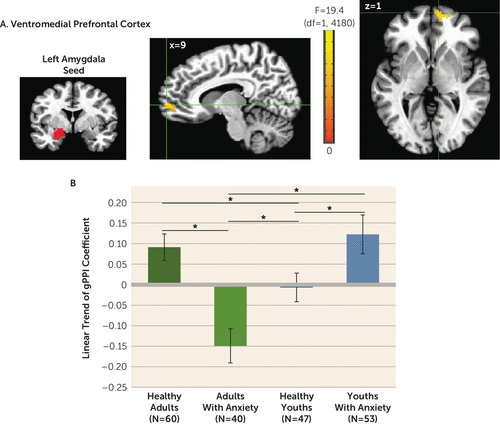
FIGURE 4. Left amygdala connectivity with the ventromedial prefrontal cortex (vmPFC) among youths and adults with anxiety disorders and healthy subjectsa
a Whole-brain generalized psychophysiological interaction (gPPI) analysis revealed a significant interaction of anxiety diagnosis, age, and linear trend in the task-related functional connectivity between the left amygdala seed and the vmPFC (panel A). Images are shown in neurological convention (i.e., left is left) and thresholded at F>10.76, df=1, 4180, p<0.001, cluster size >57 voxels (890.625 mm3). To decompose the complex interaction effects, mean extracted values for the vmPFC cluster are plotted separately by group, based on anxiety diagnosis (healthy, anxiety) and age (median split: adults, youths). In the graph (panel B), the y-axis shows the linear trend of the gPPI coefficient averaged across participants in each group. Error bars indicate standard deviation.
*p<0.05.
These findings remained statistically significant when controlling for sex and instructed extinction recall.
Discussion
Two of our study results could influence neurodevelopmental theory. First, compared with adults with anxiety, healthy adults exhibited more positive amygdala-vmPFC connectivity as stimuli increasingly resembled safety cues, and the opposite pattern was observed in healthy youths compared with youths with anxiety. Second, age moderated anxiety-related differences in activation in ways that varied across attention conditions and brain regions. These implications extend beyond neurodevelopmental theory to highlight methodological complexities in fMRI research as well.
Considerable previous research employed face viewing tasks to examine amygdala and PFC functioning in typical development. In these studies, as rigor increased, notable age differences failed to replicate, including for PFC and valence-specific activation (26, 27). However, findings for amygdala-PFC functional connectivity occurred with some consistency (5, 21), possibly because of stronger reliability data for amygdala-PFC connectivity than valence-specific activation (28, 29). Among healthy volunteers in the present study, adults but not youths exhibited increasingly positive amygdala-PFC coupling as levels of safety information increased in ambiguous face cues.
These data extend findings from previous research on amygdala-PFC development. Previous human studies demonstrated age differences in amygdala-PFC connectivity during face emotion viewing, whereas previous rodent studies linked age differences in amygdala-PFC function to age differences in threat learning (12, 13). In the present study, we used conditioning and extinction with face cues depicting neutral expressions, which revealed age differences in amygdala-PFC connectivity during extinction recall. This connects previous face emotion imaging research in humans with threat-learning research in rodents, all revealing age differences in amygdala-PFC functioning.
Functional connectivity results also extend findings from previous research on anxiety disorders, particularly in adults. In this age group, we found lower amygdala-vmPFC connectivity in participants with anxiety compared with healthy subjects, particularly to face cues containing high degrees of safety-related information. These findings were consistent with theory suggesting that anxiety disorders involve deficient safety cue signaling (20, 30). Such theory implied that failure to appropriately maintain safety cue representations in experimental settings also manifested in the everyday lives of individuals with anxiety, where they experience fear when failing to recognize safety information. Notably, such theory highlighted experimental data for adults. In the present study, compared with adults, data for youths demonstrated opposite trends, with greater amygdala-vmPFC connectivity in participants with anxiety compared with healthy subjects.
In disorder subgroups, such as pediatric and adult anxiety disorders, such opposite-appearing trends are termed crossover interactions. Compared with a crossover pattern, interactions more frequently involve disorder-related differences in only one of two subgroups. Relative to our findings on connectivity in youths with anxiety, our data for adults with anxiety more closely replicate findings from previous research. Thus, these new connectivity findings for youths should be viewed with caution and require replication, given our observation of crossover patterns. Recent neurobiological studies have, however, demonstrated crossover interactions in pediatric anxiety with increasing frequency (31, 32), including longitudinal studies showing age-related crossover interaction in neural correlates in pediatric anxiety (32, 33). Finally, it is notable that connectivity profiles in this study differed among younger and older patients and healthy participants. Thus, replication is needed in medication-free youths and adults both with and without acute anxiety.
Although we found age to moderate both connectivity and activation, the patterns differed. Whereas connectivity findings related to cue features, activation findings related to viewing contexts. Moreover, unlike connectivity findings manifesting as a crossover interaction, interactions for activation resembled more typical findings in previous research (i.e., associations in only one age-delimited subgroup).
Differences in vmPFC activation manifested in adults with anxiety but not youths with anxiety. These findings resemble patterns in previous studies of emotional disorders, in which group differences involved mPFC deactivation (20, 34, 35). In such studies, less deactivation or greater activation in healthy adult volunteers compared with adult patients may reflect default-mode dysfunction, which can generate apparent task deactivation through group differences in baseline conditions (36, 37).
In our study, group differences in inferior temporal activation manifested with different patterns in youths with anxiety compared with adults with anxiety, as a function of task instructions. These differences occurred in the context of cue-related activation in all participants, replicating studies of temporal responses to faces. Patterns among youths resembled patterns found in another study, which showed group differences during memory tasks (19), and which extended ample work on developmental differences in face representation within the inferior temporal cortex (26, 27, 38).
Given the novelty of these developmental findings, replication is needed before their significance can be determined. However, these results do provide clues regarding processes underlying anxiety disorder development. For example, the observed mechanisms mediating age differences in brain activation could arise from earlier maturation in circuitry supporting children’s capacity to remember events rather than to appraise their emotional impact. Emerging but immature memory circuit function may create vulnerabilities reflected in our results, including more prominent findings in youths than adults on the threat memory task. Earlier maturation may arise for memory rather than appraisal as a result of greater objectivity in the process of memory encoding. In contrast to subjective fear judgments, the verifiable nature of events encoded in memory may create opportunities for social scaffolding. In this scenario, children’s and adolescents’ discussions of their memory for threatening events with other people witnessing the events could sculpt their threat-related memory circuitry. Such mechanistic neurodevelopmental hypotheses may guide clinical research on anxiety disorders.
For activation, the main analysis compared groups at average reaction time in order to generate reaction time-adjusted findings. To a degree, this accounts for influences of reaction time on activation. Notably, these analyses revealed no four-way interactions, possibly reflecting reduced statistical power in tests of higher-order interactions. Alternatively, previous research suggested that cross-group heterogeneity in brain physiology coupling can obscure findings in higher-order interactions (16, 19). To address this problem and thereby enhance sensitivity to higher-order interactions, one study on adolescent social reticence used subject-specific regressors for skin conductance responses collected during extinction recall (19). Another study on typical adolescent development used ratings during threat conditioning (16). Although we did not measure skin conductance response in our study, analyses presented in the online supplement applied the approach from these previous studies using subject-specific and event-specific reaction time regressors. Future work might measure both reaction time and skin conductance responses while examining higher-order interactions and relations among activation, skin conductance response, and reaction time.
Beyond extending previous fMRI data, our findings carry more general implications. Comparing brain function in youths and adults broadly informs developmental perspectives. Other research on anxiety disorders has shown developmental continuities (39–42), often for processes that basic research localizes to early-maturing circuits with strong cross-species conservation. In contrast, our results suggest that age-related discontinuities exist in the neural correlates of anxiety disorders. Age moderated anxiety-related differences on tasks that engage late-maturing capacities, supported by areas of the heteromodal association cortex unique to primates. Thus, longitudinal work may consider whether developmental changes in the neural architecture of these capacities accounts for age-related discontinuities in anxiety disorders.
Our findings also broadly extend perspectives relating normative and pathological development. Structural imaging has shown maturation throughout adolescence in the heteromodal association cortex (43, 44). Moreover, children’s capacity to appraise their internal state and perform memory tasks matures on similar time scales, consistent with changes in brain regions engaged by tasks engaging these capacities (4, 5, 45, 46). Our study extends this research in healthy youths to youths with anxiety in our observation of age differences in late-maturing cortex on tasks that engage late-maturing psychological capacities.
Longitudinal research could evaluate the clinical significance of our findings by connecting neural and clinical data. Such research may find that youths with anxiety who develop relatively strong appraisal or memory capacities overcome aspects of their anxiety disorder. Conversely, such research may find that healthy youths who do not develop these capacities have a high risk for adult anxiety. If such longitudinal findings do emerge, prospective studies might use the associated insights to locate biomarkers that identify youths with anxiety at risk for persistent emotional problems or healthy youths at risk for new ones.
Beyond such clinical extensions, other studies could examine pathophysiology. Longitudinal genetic research has shown anxiety to involve complex developmental processes (47). Some processes are age invariant, while others are age specific. In tandem with previous imaging research, our data suggest that neural correlates may reflect similarly complex multicomponent processes. Thus, as in genetic studies, prospective imaging studies might seek to differentiate age-invariant from age-specific correlates in pediatric and adult anxiety disorders.
Strengths of this study include a relatively large sample and observation of clinically relevant findings. However, these findings should be considered in the context of four limitations. First, cross-sectional data only provide initial insights on development, as findings may guide longitudinal research. Second, while patients were medication-free and had acute anxiety, there were group differences (i.e., the gender ratio in adults). Only pediatric patients were required to enter a treatment study, and more youths than adults asked whether they would be exposed to the unconditioned stimulus during imaging. Results remained unchanged after statistically accounting for these factors. Third, evoked levels of fear generally were mild, with mean levels in the lower half of a 0–6 rating scale. Future research might use more evocative stimuli. Lastly, although this study was large in the context of clinical imaging, the sample size may still be small, because we examined complex questions with correspondingly complex methods. Statistical thresholds were more conservative than those in other studies, but few effect sizes were large, some interactions reflected unexpected patterns, and larger samples would support more powerful tests and more conservative thresholds. In addition, simpler designs would generate more powerful tests but also could lack sensitivity to multiplex clinical features, such as complex relations among task conditions, diagnosis, and age. Replication is important, given the complexity of interactions. Beyond addressing limitations with cross-sectional data, such replications might examine very large samples to appropriately power conservative statistical tests of complex interactions, with expectations of moderate effect sizes.
In conclusion, discontinuities exist between pediatric and adult anxiety disorders. Our findings suggest that differences in brain function manifest on measures of amygdala-vmPFC functional connectivity and task-related activation in the heteromodal association cortex. Such differences occur when viewing ambiguous, extinguished threat cues and engaging late-maturing psychological capacities. These results could shape refinements in developmental perspectives on anxiety disorders.
1 : Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biol Psychiatry 2013; 74:273–279Crossref, Medline, Google Scholar
2 : Complementary features of attention bias modification therapy and cognitive-behavioral therapy in pediatric anxiety disorders. Am J Psychiatry 2017; 174:775–784Link, Google Scholar
3 : Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatr Clin North Am 2009; 32:483–524Crossref, Medline, Google Scholar
4 : Response to learned threat: an FMRI study in adolescent and adult anxiety. Am J Psychiatry 2013; 170:1195–1204Link, Google Scholar
5 : Amygdala-cortical connectivity: associations with anxiety, development, and threat. Depress Anxiety 2016; 33:917–926Crossref, Medline, Google Scholar
6 : Using neuroscience to help understand fear and anxiety: a two-system framework. Am J Psychiatry 2016; 173:1083–1093Link, Google Scholar
7 : Coming to terms with fear. Proc Natl Acad Sci U S A 2014; 111:2871–2878Crossref, Medline, Google Scholar
8 : The amygdala. Curr Biol 2007; 17:R868–R874Crossref, Medline, Google Scholar
9 : Prefrontal-amygdala connectivity and state anxiety during fear extinction recall in adolescents. Front Hum Neurosci 2017; 11:587Crossref, Medline, Google Scholar
10 : Developmental rodent models of fear and anxiety: from neurobiology to pharmacology. Br J Pharmacol 2014; 171:4556–4574Crossref, Medline, Google Scholar
11 : Fear conditioning and extinction in anxious and nonanxious youth and adults: examining a novel developmentally appropriate fear-conditioning task. Depress Anxiety 2015; 32:277–288Crossref, Medline, Google Scholar
12 : Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat Commun 2016; 7:11475Crossref, Medline, Google Scholar
13 : Individual differences in frontolimbic circuitry and anxiety emerge with adolescent changes in endocannabinoid signaling across species. Proc Natl Acad Sci USA 2016; 113:4500–4505Crossref, Medline, Google Scholar
14 : The Evolution of Memory Systems. Oxford, United Kingdom, Oxford University Press, 2017Google Scholar
15 : An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001; 24:167–202Crossref, Medline, Google Scholar
16 : Distinct neural signatures of threat learning in adolescents and adults. Proc Natl Acad Sci USA 2011; 108:4500–4505Crossref, Medline, Google Scholar
17 : Neural substrates of overgeneralized conditioned fear in PTSD. Am J Psychiatry 2017; 174:125–134Link, Google Scholar
18 : The need for standards in the design of differential fear conditioning and extinction experiments in youth: a systematic review and recommendations for research on anxiety. Behav Res Ther 2019; 112:42–62Crossref, Medline, Google Scholar
19 : Early-childhood social reticence predicts SCR-BOLD coupling during fear extinction recall in preadolescent youth. Dev Cogn Neurosci 2019; 36:100605Crossref, Medline, Google Scholar
20 : Circuit-wide structural and functional measures predict ventromedial prefrontal cortex fear generalization: implications for generalized anxiety disorder. J Neurosci 2014; 34:4043–4053Crossref, Medline, Google Scholar
21 : A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci 2013; 33:4584–4593Crossref, Medline, Google Scholar
22 : AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162–173Crossref, Medline, Google Scholar
23 : A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage 2012; 61:1277–1286Crossref, Medline, Google Scholar
24 : Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage 2013; 73:176–190Crossref, Medline, Google Scholar
25 : fMRI clustering in AFNI: false-positive rates redux. Brain Connect 2017; 7:152–171Crossref, Medline, Google Scholar
26 : A developmental examination of amygdala response to facial expressions. J Cogn Neurosci 2008; 20:1565–1582Crossref, Medline, Google Scholar
27 : Functional brain activation to emotional and nonemotional faces in healthy children: evidence for developmentally undifferentiated amygdala function during the school-age period. Cogn Affect Behav Neurosci 2013; 13:771–789Crossref, Medline, Google Scholar
28 : Reliability of neural activation and connectivity during implicit face emotion processing in youth. Dev Cogn Neurosci 2018; 31:67–73Crossref, Medline, Google Scholar
29 : Behavioral and neural stability of attention bias to threat in healthy adolescents. Neuroimage 2016; 136:84–93Crossref, Medline, Google Scholar
30 : State-of-the-art and future directions for extinction as a translational model for fear and anxiety. Philos Trans R Soc Lond B Biol Sci 2018; 373:20170025Google Scholar
31 : Prefrontal-amygdala dysregulation to threat in pediatric posttraumatic stress disorder. Neuropsychopharmacology 2016; 41:822–831Crossref, Medline, Google Scholar
32 : Early temperamental fearfulness and the developmental trajectory of error-related brain activity. Dev Psychobiol 2018; 60:224–231Crossref, Medline, Google Scholar
33 : Shyness and trajectories of functional network connectivity over early adolescence. Child Dev 2018; 89:734–745Crossref, Medline, Google Scholar
34 : Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biol Psychiatry 2012; 72:476–482Crossref, Medline, Google Scholar
35 : The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA 2009; 106:1942–1947Crossref, Medline, Google Scholar
36 : The default mode network’s role in discrete emotion. Trends Cogn Sci 2019; 23:851–864Crossref, Medline, Google Scholar
37 : Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007; 8:700–711Crossref, Medline, Google Scholar
38 : Microstructural proliferation in human cortex is coupled with the development of face processing. Science 2017; 355:68–71Crossref, Medline, Google Scholar
39 : Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164:1476–1488Link, Google Scholar
40 : Fear of the unknown: uncertain anticipation reveals amygdala alterations in childhood anxiety disorders. Neuropsychopharmacology 2015; 40:1428–1435Crossref, Medline, Google Scholar
41 : Neural substrates of childhood anxiety disorders: a review of neuroimaging findings. Child Adolesc Psychiatr Clin N Am 2012; 21:501–525Crossref, Medline, Google Scholar
42 : Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry 2009; 166:302–310Link, Google Scholar
43 : Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J Neurosci 2017; 37:3402–3412Crossref, Medline, Google Scholar
44 : Normative brain size variation and brain shape diversity in humans. Science 2018; 360:1222–1227Crossref, Medline, Google Scholar
45 : Changes in the relation of self-efficacy beliefs and behaviors across development. Child Dev 2008; 79:1257–1269Crossref, Medline, Google Scholar
46 : Neurodevelopmental aspects of spatial navigation: a virtual reality fMRI study. Neuroimage 2002; 15:396–406Crossref, Medline, Google Scholar
47 : A longitudinal twin study of fears from middle childhood to early adulthood: evidence for a developmentally dynamic genome. Arch Gen Psychiatry 2008; 65:421–429Crossref, Medline, Google Scholar



