Randomized Controlled Trial Comparing Health Coach-Delivered Smartphone-Guided Self-Help With Standard Care for Adults With Binge Eating
Abstract
Objective:
Cognitive-behavioral therapy (CBT) has shown efficacy in the treatment of eating disorders. The authors conducted a randomized controlled telemedicine trial of CBT-guided self-help (CBT-GSH) assisted with a smartphone app, Noom Monitor, for binge eating with or without purging. They hypothesized that coach-delivered CBT-GSH telemedicine sessions plus Noom Monitor would yield greater reductions in symptoms of binge eating, purging, and eating disorders compared with standard care.
Methods:
Fifty-two-week outcomes for CBT-GSH plus Noom Monitor (N=114) were compared with outcomes for standard care (N=111) among members of an integrated health care system in the Pacific Northwest. Patients in the health system who met inclusion criteria were ≥18 years old, had a body mass index ≥18.5, met criteria for DSM-5 binge eating disorder or bulimia nervosa, had 12 months of continuous health care enrollment in Kaiser Permanente Northwest, and had a personal smartphone. Participants received eight CBT-GSH telemedicine sessions over 12 weeks administered by health coaches, and outcomes were assessed at baseline and at weeks 4, 8, 12, 26, and 52. The use of available treatment offered within the Kaiser Permanente health care system was permitted for participants assigned to standard care.
Results:
Participants who received CBT-GSH plus Noom Monitor reported significant reductions in objective binge-eating days (β=−0.66, 95% CI=−1.06, −0.25; Cohen’s d=−1.46, 95% CI=−4.63, −1.09) and achieved higher rates of remission (56.7% compared with 30%; number needed to treat=3.74) at 52 weeks compared with participants in standard care, none of whom received any eating disorder treatment during the intervention period (baseline and weeks 1–12). Similar patterns emerged for compensatory behaviors (vomiting, use of laxatives, and excessive exercise; 76.3% compared with 56.8%; number needed to treat=5.11), eating disorder symptoms (body shape, weight, eating concerns, and dietary restraint), and clinical impairment (Cohen’s d=−10.07, −2.15).
Conclusions:
These results suggest that CBT-GSH plus Noom Monitor delivered via telemedicine by routine-practice health coaches in a nonacademic health care system yields reductions in symptoms and impairment over 52 weeks compared with standard care.
Psychiatric disorders characterized by binge eating (including binge-eating disorder and bulimia nervosa) and defined by consumption of an objectively large amount of food accompanied by a sense of loss of control (1) affect approximately 2%−4% of the U.S. population (2). Cognitive-behavioral therapy (CBT) has demonstrated efficacy in individual, group, guided self-help (GSH), and pure self-help interventions (3) that have recently migrated into technology-delivered or technology-assisted formats (e.g., Internet, smartphone app, and software) (4). Evidence of efficacy of these technological adaptations is emerging but limited (5, 6). Cost-effectiveness estimates (7) of these interventions favor lower-intensity technology-based interventions over face-to-face methods, despite direct comparisons favoring face-to-face interventions in the short term (8).
Given the limited availability of therapists trained in administering CBT-GSH and, even when available, the potential barrier of attending in-person sessions consistently, digital treatment platforms may offer a preferred and more accessible treatment option. In addition, such platforms allow for scalability, and the more flexible remote delivery may result in better adherence, thereby leading to more robust improvement (6, 9, 10). Therapist-assisted forms of Internet CBT have demonstrated efficacy (11) and yield greater satisfaction over purely digital CBT interventions, even when treatment response is similar (12). Mobile technologies are increasingly available and popular among patients (13) and clinicians (14), and there is evidence that text messaging can help improve adherence to and retention in treatments involving such platforms (15–17). In our previous pilot study, the Noom Monitor, a smartphone app developed to facilitate CBT-GSH, was acceptable to patients, was feasible to deliver, and demonstrated effects similar to those of a traditional paper-and-pencil GSH format over a 6-month follow-up period (18). In addition, CBT-GSH plus Noom Monitor yielded significantly greater reductions in binge episodes and greater adherence to regular eating (meal and snack adherence), as well as a number needed to treat of 4.5 for end-of-treatment differences. These differences did not persist through the 6-month follow-up, because participants in the traditional CBT-GSH group had a significant increase in remission from the 3- to 6-month follow-up assessments, whereas participants in the CBT-GSH plus Noom Monitor group reported a stable rate of reduction in binge episodes between posttreatment and 9-month follow-up assessments.
In the present study, we extended our pilot study by evaluating the robustness of the intervention when delivered by interventionists who were not experts in treatment for eating disorders in an everyday clinical practice setting, comparing the effectiveness of standard care and CBT-GSH plus Noom Monitor delivered using telephone coaching. The study was conducted in the real-world health care delivery setting of Kaiser Permanente Northwest, with health coaches trained in CBT-GSH (19). Our primary hypothesis was that CBT-GSH plus Noom Monitor delivered by telephone would lead to greater reductions in eating disorder symptoms at 12 weeks (end of treatment) and the 9-month follow-up (52 weeks after randomization). Our implementation goal and hypothesized effect were a direct extension of the symptom improvements demonstrated in our pilot study (18) and an effort to determine whether nonspecialist coaches could deliver this intervention in a community health care system via telemedicine.
Methods
Participants
A CONSORT flow diagram for the study is presented in Figure 1. Participants were recruited from Kaiser Permanente Northwest from April 2016 to March 2017 through enrollment-targeted invitations. Electronic medical records were obtained for Kaiser Permanente Northwest members who were between 18 and 55 years old, had a body mass index (BMI) between 18.5 and 40, had 12 months of continuous enrollment in the Kaiser Permanente Northwest plan, and had no diagnosis of bipolar disorder, substance dependence, or psychosis. A study brochure mailed to eligible individuals provided a link to an online eating disorder module of the Patient Health Questionnaire designed to assess the presence of loss-of-control eating episodes. Persons whose online survey responses indicated loss-of-control eating at least one time per month were screened by telephone to determine whether their eating behavior met study eligibility criteria for objective binge episodes, as determined by trained study assessors (T.H. and R.G.), and whether they had enrollment in Kaiser Permanente Northwest for the subsequent 3 months and owned a smartphone. Individuals were excluded if they had prior CBT-GSH, recent changes in psychotropic medication, prior bariatric surgery, or purging or laxative use >14 times per week. At the time of study inclusion, participants were randomly assigned to treatment (CBT-GSH plus Noom Monitor) or the control condition (standard care). Participants assigned to receive CBT-GSH downloaded the Noom Monitor app, were provided with a copy of the CBT-GSH self-help book (19), and were assigned to a health coach who contacted them directly by telephone to set up the first session of CBT-GSH.
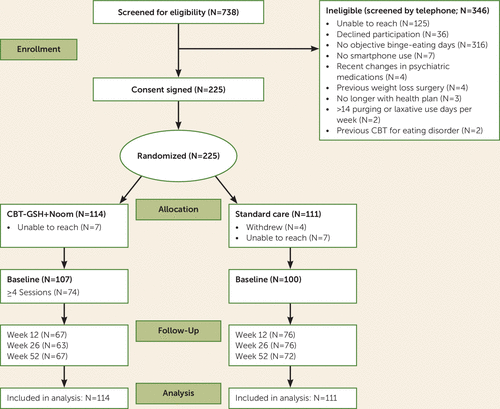
FIGURE 1. CONSORT flow diagram of participants randomly assigned to cognitive-behavioral therapy (CBT) guided self-help (GSH) plus Noom Monitoring or standard care
Assessments
Assessments were sent to participants by e-mail and completed online at baseline and at weeks 4, 8, 12, 26, and 52. Participants completed the Personal Health Questionnaire-8 (20), a brief measure of depressive symptoms; the Clinical Impairment Assessment questionnaire, a 16-item measure of clinical impairment related to eating disorder symptoms (21); the Eating Disorder Examination Questionnaire 6.0 (22), a self-report measure that includes behavioral and attitudinal features of eating disorder psychopathology; and the 16-item Quality of Life Scale. Participants were paid $25 for the baseline assessment, $15 for each follow-up assessment at weeks 4, 8, and 12, and $25 for each assessment at weeks 26 and 52. We randomly sampled 10% of all self-report assessments and administered interviewer-blinded overeating sections of the Eating Disorder Examination (22); participants who completed the interview were paid $25. Interrater reliability ratings between self-report and interview ratings for days on which objective binge episodes were present, objective binge-eating days (kappa=0.89), and compensatory behaviors (kappa=0.84) were acceptable.
Treatment
After completing the baseline assessment, eligible individuals were randomly assigned to receive either CBT-GSH plus Noom Monitor (N=114) or standard care (N=111), stratified by purging and nonpurging type. Randomization was conducted using data-tracking software, and the site personnel conducting assessments were blind to study assignment until after completion of the study.
CBT-GSH plus Noom Monitor.
CBT-GSH treatment involved coaching sessions with a routine-practice health coach employed by Kaiser Permanente Northwest and use of both a CBT-GSH self-help book (19) and the Noom Monitor app, which participants downloaded to a smartphone. All coaching sessions were conducted by telephone and followed the protocol adapted from our pilot trial (18). Briefly, CBT-GSH comprised six sequential steps designed to establish self-monitoring, regular eating (three meals, two snacks), alternative activities to binge eating or purging, problem solving, reduction in dietary restraint, the importance of body shape and weight, and relapse prevention. The intervention gave the coach and the patient the ability to choose between focusing on dietary restraint or the importance of body shape and weight before moving to relapse prevention. The first session lasted 60 minutes, and each subsequent session lasted 20–25 minutes (total coach contact time, approximately 3.5 hours). The first four sessions occurred weekly, and the following four sessions were conducted biweekly. The Noom Monitor app consisted of a customized self-monitoring system designed to track exercise, meals and snacks, compensatory behavior, body checking, craving, and weight. Participants were given a specialized set of instructions on how to use the monitoring app, and coaches were able to check participant monitoring through a virtual dashboard at the time of each self-help session. The dashboard offered summary data for user-entered data (i.e., meals, snacks, binges, purging episodes, urges, body checking, and weight) and was used in-session by coaches via a standard computer workstation.
Therapist training and monitoring.
All coaches were certified health coaches (from Wellcoaches and the Health Sciences Institute) employed by the Department of Health Education and Wellness at Kaiser Permanente Northwest. Coaches completed an 8-hour training course, led by two of the authors (T.H. and R.G.), consisting of an overview of CBT-GSH treatment for bulimia nervosa and binge-eating disorder, execution of skill-specific interventions, role plays of each session, and consideration of psychiatric complexity and special issues in treatment delivery. All sessions were audiotaped and reviewed by the same authors (T.H. and R.G.) to ensure protocol adherence. In addition, coaches participated in weekly group supervision specific to executing CBT-GSH.
This study was reviewed and approved by the institutional review boards of the Icahn School of Medicine at Mount Sinai and Kaiser Permanente Northwest. To assess treatment integrity, 10% of all sessions were randomly sampled and coded for integrity by trained, blind assessors (scale range, 0–8). The mean adherence rating was 7.23 (SD=1.35).
Standard care.
All participants were insured through Kaiser Permanente Northwest and had unrestricted access to clinical resources within the health plan. Although there are no services specifically structured for the treatment of binge-eating disorder within the Kaiser Permanente Northwest health system, there are Kaiser Permanente behavioral health providers whose scope of practice includes treatment for both bulimia nervosa and binge-eating disorder. According to electronic health records data in the system, during the intervention period (baseline through week 12), no participants in the standard care group received eating disorder-related services, and 15 received psychiatric services (mean=2.9, SD=2.3). During the 52-week study period, two participants in the standard care group received eating disorder-related services (mean=2.0, SD=1.4), and 46 received psychiatric services (mean=4.2, SD=5.3).
Statistical Analysis
We used latent growth curve models for within-treatment outcome analysis (baseline and weeks 4, 8, and 12) and posttreatment outcome analysis (6-month follow-up at the 26-week postbaseline assessment, 9-month follow-up at the 52-week postbaseline assessment). Because of the nonlinear change trajectories common to CBT interventions and count distributions of our primary outcome with zero inflation (i.e., a high rate of objective binge episode remission), we fitted negative binomial models with zero inflation. Time was log-transformed for the within-treatment latent growth curve models to account for rapid response and deceleration of change over the treatment period. Linear growth was estimated for the posttreatment latent growth curve models. All models were estimated using robust maximum likelihood estimation, intent to treat, assumption of missing at random, and Monte Carlo integration of random effects. All confidence intervals were bootstrapped using 10,000 draws. We conducted sensitivity analyses estimating completer effects and missing-not-at-random pattern mixture models for objective binge-eating days (23). Exploratory moderator effects were estimated using a composite moderator (i.e., latent variable) of demographic variables, following recommendations by Kraemer (24).
Statistical power.
Monte Carlo simulation studies were conducted using Mplus, version 7.1, and based on published recommendations to determine appropriate sample size for binge eating (25). Power was calculated at 0.80 with a sample size of 160 to test for moderate effect-size differences in objective binge days.
Results
The baseline demographic and clinical characteristics of the study participants are summarized in Table 1. The baseline differences between the two study groups suggest no significant effect on randomization (i.e., randomization was not confounded). The total sample (N=225) was predominantly female (75.1%, N=169) and Caucasian (83.6%, N=188). Five percent (N=11) were Asian, 4.4% (N=10) were African American, and 7.6% (N=17) self-reported as other. Eight percent (N=19) identified as Hispanic or Latino. The majority of participants were high school graduates (32.9%, N=74), and 10.7% (N=24) had less than a high school education. Most participants were married (65.8%, N=148), and 9.3% (N=21) were living with a partner. The remaining participants reported being single, divorced, or widowed. The majority of participants (75.1%, N=169) reported an annual household income >$50,000. The mean BMI was 31.67 (SD=4.58; range, 20.39–39.72), and 33% of participants (N=75) endorsed at least one compensatory behavior (vomiting, use of laxatives, and excessive exercise) in the past month.
| Characteristic | Total Sample (N=225) | CBT-GSH+Noom (N=114) | Standard Care (N=111) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | t | df | Mdiff | 95% CI | |
| Age (years) | 41.19 | 9.91 | 41.54 | 9.75 | 40.85 | 10.10 | 0.52 | 224 | 0.69 | –1.91, 3.30 |
| Body mass index | 31.67 | 4.58 | 31.73 | 4.46 | 31.60 | 4.71 | –0.22 | 224 | 0.61 | –1.34, 1.07 |
| N | % | N | % | N | % | χ2 | df | φ | ||
| Race/ethnicity | ||||||||||
| Nonwhite | 38 | 17.4 | 20 | 17.5 | 18 | 15.3 | 3.2 | 37 | 0.12 | |
| Hispanic or Latino | 19 | 8.4 | 10 | 8.8 | 9 | 8.1 | 3.5 | 18 | 0.00 | |
| Male | 56 | 24.9 | 29 | 25.4 | 27 | 24.3 | 0.04 | 55 | 0.01 | |
| Married | 148 | 65.8 | 76 | 66.7 | 72 | 64.9 | 4.4 | 147 | 0.14 | |
| At least a college degree | 162 | 72.0 | 82 | 71.9 | 80 | 72.1 | 1.2 | 161 | 0.07 | |
| Household income >$50,000 (annual) | 169 | 75.1 | 86 | 77.4 | 83 | 72.8 | 1.2 | 169 | 0.07 | |
| Diagnosis | ||||||||||
| Binge-eating disorder | 150 | 66.6 | 19 | 57.6 | 18 | 54.5 | 1 | 150 | 0.00 | |
| Bulimia nervosa | 75 | 33.3 | 38 | 33.3 | 37 | 33.3 | 1 | 74 | 0.00 | |
TABLE 1. Demographic and clinical characteristics of participants receiving cognitive-behavioral therapy-guided self-help plus Noom Monitoring (CBT-GSH+Noom) or standard carea
Primary Outcomes
Change in objective binge-eating days is presented in Figure 2, and model-estimated treatment effects are summarized in Table 2. Participants in the CBT-GSH plus Noom Monitor group reported significantly greater change in objective binge days (β=–0.66, 95% CI=–1.06, –0.25; Cohen’s d=−1.46, 95% CI=–4.63, –1.09) at the 52-week assessment, as well as higher remission rates (56.7% [38/67] compared with 30% [12/40]; number needed to treat=3.74). If we classify all missing data as treatment failures, the ratios of remission rates are similar (33.3% [33/114] compared with 10.8% [12/111]; number needed to treat=4.1). Raw means and standard deviations, reported in Table S1 in the online supplement, reveal that week-12 (end of intervention period) averages were 3.79 objective binge days per month for the CBT-GSH plus Noom Monitor group and 2.63 days per month for the standard care group. In addition, the CBT-GSH plus Noom group achieved a higher remission rate at the end of the intervention period compared with the standard care group (41.8% [28/67] compared with 16.1% [16/87]). Compensatory behaviors (vomiting, use of laxatives, and excessive exercise) were reduced in the CBT-GSH plus Noom Monitor group (rate of remission, 76.3% [29/38] compared with the standard care group (rate of remission, 56.8% [21/37]; number needed to treat=5.11).
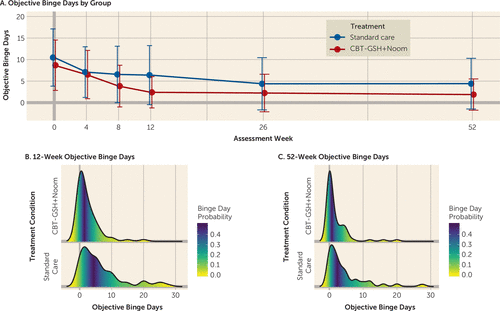
FIGURE 2. Objective binge-eating days among participants receiving cognitive-behavioral therapy-guided self-help plus Noom Monitoring (CBT-GSH+Noom) or standard carea
a Panel A shows the raw means and standard deviations for the change in frequency of objective binge-eating days over the treatment window (12 weeks) and 9-month follow-up (52 weeks). Panel B shows the probability distribution of objective binge days for the 12-week assessment. Panel C shows the probability distribution of objective binge days for the 52-week assessment.
| Variable | Slope | CBT-GSH+Noom | Effect Size | ||||
|---|---|---|---|---|---|---|---|
| µ | 95% CI | β | 95% CI | Cohen’s d | Odds Ratio | 95% CI | |
| Objective binge-eating days | |||||||
| Remission | 6.21 | 0.98, 11.45 | 0.92 | 0.11, 1.72 | 2.41 | 1.11, 5.58 | |
| Count | –0.41 | –1.71, 0.89 | –0.38 | –0.62, –0.14 | –1.46 | –4.63, –1.09 | |
| Compensatory daysa | |||||||
| Remission | –0.09 | –7.73, 7.54 | 1.75 | 0.30, 3.19 | 5.70 | 1.24, 11.13 | |
| Count | –0.10 | –3.85, 3.66 | –0.15 | –0.77, 0.48 | –0.34 | –3.44, 2.15 | |
TABLE 2. Zero inflated latent growth curve model estimates of treatment on 52-week behavioral outcomes for participants receiving cognitive-behavioral therapy-guided self-help plus Noom Monitoring (CBT-GSH+Noom) or standard care
Sensitivity analyses were used to estimate treatment effects for participants with complete data (“completers”) and under the not-missing-at-random assumption. Pattern-mixture modeling of objective binge days revealed a larger treatment effect on objective binge days (β=−0.83, 95% CI=−1.15, −0.32; Cohen’s d=−1.85, 95% CI=−5.28, −1.46) and on remission rates (β=1.87, 95% CI=0.43, 3.30; Cohen’s d=2.66, 95% CI=1.20, 9.24). Completer analysis revealed larger effects of treatment on objective binge days (β=−1.05, 95% CI=−1.79, −0.28; Cohen’s d=−1.80, 95% CI=−6.12, −0.95), but the effect on remission rates was less precise (β=1.10, 95% CI=−0.80, 2.99; Cohen’s d=1.58, 95% CI=−2.24, 8.42). A total of 64.9% (N=74/114) of participants in the CBT-GSH plus Noom Monitor group completed at least four coach-delivered sessions in the first 4 weeks, and 38.6% (N=44/114) completed all eight sessions.
Secondary Outcomes
Treatment effects on secondary outcomes are summarized in Table 3. Across secondary measures, CBT-GSH plus Noom Monitor was superior to standard care, and effect sizes (Cohen’s d) ranged from −1.14 to −2.75, with the largest effect size found for reduction in clinical impairment related to eating pathology. There was no statistically significant difference in quality of life between the two groups at 52 weeks, although there was significantly greater improvement at week 12 (β=4.64, SE=1.64, 95% CI=1.42, 7.86; Cohen’s d=0.82, 95% CI=0.48, 2.71), but this change did not persist into the follow-up period.
| Measure | Standard Care | CBT-GSH+Noom | Effect Size | |||
|---|---|---|---|---|---|---|
| µ | 95% CI | β | 95% CI | Cohen’s d | 95% CI | |
| Eating Disorders Examination Questionnaire | ||||||
| Shape concern subscale | –1.75 | –3.31, –0.19 | –0.80 | –1.11, –0.49 | –1.23 | –3.35, –0.38 |
| Weight concern subscale | –1.11 | –2.58, 0.37 | 0.81 | –1.10, –0.53 | –1.45 | –3.88, –0.49 |
| Eating concern subscale | –0.31 | –1.86, 1.25 | –0.94 | –1.23, –0.65 | –2.07 | –5.30, –0.73 |
| Restraint subscale (spline)a | –0.94 | –2.25, 0.37 | –0.67 | –0.99, –0.36 | –1.14 | –3.29, –1.18 |
| Global scale | –0.89 | –2.17, 0.39 | –0.78 | –1.03, –0.53 | –1.44 | –3.74, –0.50 |
| Clinical Impairment Assessment | –2.60 | –9.31, 4.11 | –3.22 | –4.55,–1.90 | –2.75 | –4.42, –1.85 |
| Personal Health Questionnaire-8 | –6.73 | –13.63, 0.17 | –2.52 | –3.87, –1.17 | –2.23 | –6.70, –0.53 |
| Quality of Life Survey | –1.01 | –18.68, 16.66 | 1.00 | –3.17, 5.16 | 0.22 | –1.34, 2.19 |
TABLE 3. Latent growth curve model estimates of treatment effect at 52 weeks on secondary outcomes for participants receiving cognitive-behavioral therapy-guided self-help plus Noom Monitoring (CBT-GSH+Noom) or standard care
Predictors and Moderators of Outcome
Predictors of change for primary and secondary outcomes are summarized in Table 4. Married participants reported higher rates of remission from binge eating compared with nonmarried participants (39.62% [42/108] compared with 26.67% [16/60]), as well as greater improvement in concerns about body shape, weight, and eating. Higher annual household income was significantly associated with less improvement in clinical impairment and depression. Men reported greater reductions in clinical impairment, but no other significant sex differences were observed. Race and education were not significant predictors of outcomes. General moderators indicated two significant interactions (Table 5), suggesting that CBT-GSH plus Noom Monitor had a significantly greater effect on eating concerns and quality of life among different demographic and clinical profiles of participants. Higher composite score-by-treatment interaction was associated with greater improvements in quality of life among participants receiving CBT-GSH plus Noom Monitor who reported higher income, education, and being married. Improvements regarding eating concerns were greater for individuals who identified as white and non-Hispanic and had a higher BMI. For interpretation of composite profiles, we followed recommendations by Kraemer et al. (24), and factor loadings for the composite variable are presented in Table S2 in the online supplement.
| Measure | Annual Income >50,000 | Married | Education Level | Sex | Body Mass Index | White | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| Objective binge days | ||||||||||||
| Count | 0.02 | –0.13, 0.17 | 0.03 | –0.03, 0.09 | –0.09 | –0.20, 0.03 | –0.10 | –0.34, 0.14 | 0.01 | –0.02, 0.04 | –0.02 | –0.11, 0.07 |
| Remission | –0.32 | –0.85, 0.21 | –0.29 | –0.58, 0.00 | –0.14 | –0.52, 0.23 | 0.11 | –0.80, 1.03 | –0.05 | –0.13, 0.04 | –0.01 | –0.37, 0.35 |
| Compensatory daysb | ||||||||||||
| Count | –0.14 | –0.68, 0.41 | –0.04 | –0.26, 0.17 | 0.06 | –0.22, 0.35 | –0.24 | –0.88, 0.40 | 0.00 | –0.07, 0.07 | –0.03 | –0.81, 0.76 |
| Remission | 0.15 | –0.88, 1.16 | –0.43 | –0.87, 0.01 | –0.06 | –0.79, 0.68 | 0.25 | –1.27, 1.77 | 0.02 | –0.14, 0.18 | –1.80 | –3.88, 0.27 |
| Eating Disorders Examination Questionnaire | ||||||||||||
| Shape concern subscale | 0.19 | –0.01, 0.39 | 0.12 | 0.03, 0.21 | –0.09 | –0.24, 0.07 | –0.33 | –0.68, 0.02 | 0.04 | 0.00, 0.07 | –0.08 | –0.50, 0.35 |
| Weight concern subscale | 0.14 | –0.05, 0.32 | 0.10 | 0.02, 0.18 | –0.11 | –0.25, 0.04 | –0.11 | –0.43, 0.22 | 0.02 | –0.01, 0.05 | –0.19 | –0.58, 0.21 |
| Eating concern subscale | 0.03 | –0.16, 0.21 | 0.09 | 0.01, 0.17 | –0.07 | –0.22, 0.08 | –0.14 | –0.48, 0.19 | –0.01 | –0.05, 0.02 | –0.30 | –0.70, 0.11 |
| Restraint subscale | 0.04 | –0.14, 0.23 | 0.07 | –0.01, 0.15 | –0.02 | –0.17, 0.13 | –0.04 | –0.36, 0.28 | 0.01 | –0.03, 0.04 | –0.16 | –0.56, 0.24 |
| Global scale | 0.07 | –0.08, 0.23 | 0.08 | 0.01, 0.15 | –0.67 | –0.19, 0.06 | –1.11 | –0.39, 0.13 | 0.02 | –0.01, 0.04 | –0.12 | –0.47, 0.22 |
| Clinical Impairment Assessment | 1.13 | 0.26, 2.00 | 0.36 | –0.03, 0.74 | –0.19 | –0.88, 0.50 | –1.55 | –3.06, –0.04 | 0.00 | –0.15, 0.15 | –1.35 | –3.26, 0.57 |
| Personal Health Questionnaire-8 | 0.37 | –0.13, 0.88 | 1.21 | 0.33, 2.08 | 0.02 | –0.38, 0.43 | –0.34 | –1.20, 0.53 | –0.03 | –0.12, 0.06 | –0.75 | –1.88, 0.39 |
| Quality of Life Survey | –1.22 | –2.86, 2.62 | –0.53 | –1.82, 0.76 | 0.16 | –1.91, 2.22 | 3.53 | –0.83, 7.89 | 0.10 | –0.29, 0.48 | 0.48 | –5.78, 6.74 |
TABLE 4. Predictors of change in primary and secondary outcome measures for participants receiving cognitive-behavioral therapy-guided self-help plus Noom Monitoring (CBT-GSH+Noom) or standard carea
| Moderator and Measure | Composite Moderator | |
|---|---|---|
| β | 95% CI | |
| Objective binge-eating days | ||
| Count | –0.06 | –0.29, 0.16 |
| Remission | 0.24 | –0.55, 1.03 |
| Compensatory daysb | ||
| Count | 1.23 | –0.64, 3.11 |
| Remission | 1.66 | –1.72, 5.03 |
| Eating Disorders Examination Questionnaire | ||
| Shape concern subscale | –0.07 | –0.38, 0.24 |
| Weight concern subscale | 0.04 | –0.25, 0.33 |
| Eating concern subscale | –0.42* | –0.80, –0.04 |
| Restraint subscale | 0.09 | –0.17, 0.35 |
| Global scale | 0.05 | –0.20, 0.30 |
| Clinical Impairment Assessment | –0.28 | –1.67, 1.11 |
| Personal Health Questionnaire-8 | 0.08 | –0.74, 0.91 |
| Quality of Life Survey | 5.63* | 0.66, 10.61 |
TABLE 5. Composite moderators of primary and secondary outcomes for participants receiving cognitive-behavioral therapy-guided self-help plus Noom Monitoring or standard carea
Discussion
The results of this trial indicate that CBT-GSH plus Noom Monitor, delivered via telemedicine by routine-practice health coaches and embedded within a commercial health care system, was superior to standard care (control arm), which included no eating disorder treatment (treatment as usual) during the intervention period (baseline through week 12), for improvement of primary eating disorder symptoms, related impairment in functioning, and associated depressive symptoms. CBT-GSH plus Noom Monitor generated one additional responder per four patients treated for binge-eating disorder and bulimia nervosa, respectively (number needed to treat=3.74), with approximately 57% of participants reporting remission at week 52 and changes in secondary symptoms reflecting a similar level of improvement. The remission rate increased beyond the intervention period, even though average objective binge-eating days remained stable, suggesting that the effects of the intervention continued to facilitate changes during the posttreatment follow-up period (weeks 12 through 52) that were not observed among participants who received standard care. Although the coaches ended the intervention at week 12, participants had access to the self-help manual and Noom Monitor app beyond the coaching period and were encouraged to continue using the program until they achieved remission. These results are similar to those of our pilot study with Noom Monitor, conducted within a specialty clinic with face-to-face sessions with a therapist experienced in the treatment of eating disorders (18), suggesting that the effects are transferable to telemedicine and into a broader commercial-care setting facilitated by routine-practice health coaches.
In previous studies of CBT-GSH and/or pure self-help conducted in commercial or primary care settings, remission rates of 30%−40% have been reported as well as Cohen’s d values in the range of 0.4–1.2 (26–29). In the present study, participants assigned to CBT-GSH plus Noom Monitor reported a remission rate of 39.62% and symptom changes of one to two standard deviations more than the control condition (Cohen’s d range, −1.07 to −2.15). Digital interventions have proven efficacious (30, 31), with meta-analyses suggesting that technology-driven self-help interventions are similar to other active self-help interventions (32). The adaptation of the guided self-help model to a mobile platform has support for other mental health conditions (33, 34) and is likely to play a significant role in the evolution of mental health services (35). This study extends these findings to adults with broadly defined symptoms of binge eating and compensatory behavior, suggesting that robust changes are possible through this type of intervention.
Several predictors of treatment outcome were identified in our study. The presence of a marital partner resulted in better outcomes, which is consistent with promising results from couples-focused interventions for binge eating (36). The greater reduction in clinical impairment among men is difficult to interpret, because their symptom reductions were similar to those observed for women. Participants with lower household income experienced greater reduction in depressive symptoms and clinical impairment, which suggests that CBT-GSH may have benefits for individuals with greater obstacles to expert care (i.e., transportation, childcare, etc.).
There were two significant composite moderator effects of treatment response, but no single moderator, which is consistent with findings for CBT more broadly for binge eating (37). Examination of the composite profiles suggested that participants who had higher income and education and who were married experienced greater quality-of-life improvements. Participants who identified as white non-Hispanic and had a higher BMI reported greater improvements in eating concerns. Eating concerns, as measured by the Eating Disorder Examination Questionnaire, relate to guilt and social anxiety about eating. These findings should be interpreted with caution, because the study was not primarily powered to examine moderators, and the findings could reflect type I errors.
The effects of CBT-GSH have clear evidence of cost savings (38), and adaptation to smartphone technology offers an important tool to mitigate some limitations to scalability by increasing opportunities for better adherence and usability and reducing dropout. Providers and patients appear to prefer mobile technologies to standard interventions (39), and evidence that coach-led interventions can significantly improve outcomes relative to standard care with no eating disorder intervention suggests a potentially cost-effective and scalable option for health care systems. Although face-to-face interventions may have some modest clinical advantage, the evidence for cost savings for digital interventions is clear (7). The results of this study support implementation of other ongoing efforts to scale CBT-GSH via mobile health platforms (10).
One limitation of this study is the rate of dropout (38.2% total, 42.1% and 35.1% for CBT-GSH plus Noom Monitor and standard care groups, respectively), which is generally high among self-help and digital interventions for binge eating (40). Sensitivity analyses suggested that the estimated effects were conservative relative to the missing-not-at-random and completer analyses. This finding mitigates some concerns about our primary analyses overestimating treatment effects; however, moderate amounts of missing data can affect the reliability of these estimates. Although we believe that the routine-practice health coaches (who were not behavioral specialists and did not have prior experience with supporting eating disorder treatment) recruited to deliver the intervention is a strength of this trial, we caution that consistent, weekly supervision was provided to support their work and that inferences of these effects to health coaching broadly and outside the context of expert supervision should not be made. Finally, our outcome measures were administered by self-report, which may be less reliable than interview assessments of binge eating (41). However, we found high agreement between interview and self-report measures, suggesting some mitigation of these measurement-related concerns.
Conclusions
Scaling and implementing empirically supported interventions has become an important priority across mental health conditions (42). The results of this study suggest that the Noom Monitor is an efficacious platform for delivering CBT-GSH via telemedicine, and the use of routine-practice health coaches provides some confidence that this intervention can be scaled outside of specialty clinical programs. Much of this success, however, depends on learning the most effective methods for training coaches and maintaining their success in protocol delivery (43).
1 : Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Association, 2013Google Scholar
2 : Epidemiology, course, and outcome of eating disorders. Curr Opin Psychiatry 2013; 26:543–548Crossref, Medline, Google Scholar
3 : Psychological treatments for bulimia nervosa and binging. Cochrane Database Syst Rev 2009; (4):
4 : Evolution of cognitive-behavioral therapy for eating disorders. Behav Res Ther 2017; 88:26–36Crossref, Medline, Google Scholar
5 : Review of smartphone applications for the treatment of eating disorders. Eur Eat Disord Rev 2015; 23:1–11Crossref, Medline, Google Scholar
6 : Apps and eating disorders: a systematic clinical appraisal. Int J Eat Disord 2015; 48:1038–1046Crossref, Medline, Google Scholar
7 : Economic evaluation of cognitive behavioral therapy and Internet-based guided self-help for binge-eating disorder. Int J Eat Disord 2018; 51:155–164Crossref, Medline, Google Scholar
8 : Effect of internet-based guided self-help vs individual face-to-face treatment on full or subsyndromal binge eating disorder in overweight or obese patients: The INTERBED Randomized Clinical Trial. JAMA Psychiatry 2017; 74:987–995Crossref, Medline, Google Scholar
9 : The potential of technology-based psychological interventions for anorexia and bulimia nervosa: a systematic review and recommendations for future research. J Med Internet Res 2015; 17:
10 : Cognitive-behavioral guided self-help for eating disorders: effectiveness and scalability. Clin Psychol Rev 2012; 32:343–357Crossref, Medline, Google Scholar
11 : Web-based cognitive behavioral therapy for female patients with eating disorders: randomized controlled trial. J Med Internet Res 2015; 17:
12 : Web-based fully automated self-help with different levels of therapist support for individuals with eating disorder symptoms: a randomized controlled trial. J Med Internet Res 2016; 18:
13 : Patient experiences using a self-monitoring app in eating disorder treatment: qualitative study. JMIR Mhealth Uhealth 2018; 6:
14 : Clinicians’ perspective on an app for patient self-monitoring in eating disorder treatment. Int J Eat Disord 2018; 51:314–321Crossref, Medline, Google Scholar
15 : Mobile therapy: use of text-messaging in the treatment of bulimia nervosa. Int J Eat Disord 2010; 43:513–519Crossref, Medline, Google Scholar
16 : Technology-enhanced maintenance of treatment gains in eating disorders: efficacy of an intervention delivered via text messaging. J Consult Clin Psychol 2012; 80:700–706Crossref, Medline, Google Scholar
17 : Aftercare intervention through text messaging in the treatment of bulimia nervosa--feasibility pilot. Int J Eat Disord 2006; 39:633–638Crossref, Medline, Google Scholar
18 : Randomized controlled trial comparing smartphone assisted versus traditional guided self-help for adults with binge eating. Int J Eat Disord 2017; 50:1313–1322Crossref, Medline, Google Scholar
19 : Overcoming Binge Eating, 2nd ed. New York, Guilford Press, 2013Google Scholar
20 : The Personal Health Questionnaire: a new screening instrument for detection of ICD-10 depressive disorders in primary care. Psychol Med 2000; 30:831–840Crossref, Medline, Google Scholar
21 : Clinical impairment questionnaire (CIA 3.0), in Cognitive Behavior Therapy and Eating Disorders. Edited by Fairburn CG. New York, Guilford Press, 2008, pp 315–317Google Scholar
22 : Cognitive Behavior Therapy and Eating Disorders. New York, Guilford Press, 2008Google Scholar
23 : Growth modeling with nonignorable dropout: alternative analyses of the STAR*D antidepressant trial. Psychol Methods 2011; 16:17–33Crossref, Medline, Google Scholar
24 : Discovering, comparing, and combining moderators of treatment on outcome after randomized clinical trials: a parametric approach. Stat Med 2013; 32:1964–1973Crossref, Medline, Google Scholar
25 : The benefits of using semi-continuous and continuous models to analyze binge eating data: a Monte Carlo investigation. Int J Eat Disord 2015; 48:746–758Crossref, Medline, Google Scholar
26 : Guided self-help treatment for recurrent binge eating: replication and extension. Psychiatr Serv 2011; 62:367–373Link, Google Scholar
27 : Treatment of bulimia nervosa in a primary care setting. Am J Psychiatry 2004; 161:556–561Link, Google Scholar
28 : Self-help for binge eating disorder in primary care: a randomized controlled trial with ethnically and racially diverse obese patients. Behav Res Ther 2013; 51:855–861Crossref, Medline, Google Scholar
29 : Treatment of binge eating disorder in racially and ethnically diverse obese patients in primary care: randomized placebo-controlled clinical trial of self-help and medication. Behav Res Ther 2014; 58:1–9Crossref, Medline, Google Scholar
30 : Randomised controlled trial of a guided self-help treatment on the Internet for binge eating disorder. Behav Res Ther 2011; 49:482–491Crossref, Medline, Google Scholar
31 : Internet-delivered cognitive-behavioural therapy v. conventional guided self-help for bulimia nervosa: long-term evaluation of a randomised controlled trial. Br J Psychiatry 2013; 202:135–141Crossref, Medline, Google Scholar
32 : Guided self-help for eating disorders: a systematic review and metaregression. Eur Eat Disord Rev 2017; 25:148–164Crossref, Medline, Google Scholar
33 : A randomized controlled trial on a smartphone self-help application (Be Good to Yourself) to reduce depressive symptoms. Psychiatry Res 2018; 269:753–762Crossref, Medline, Google Scholar
34 : A smartphone application to support recovery from alcoholism: a randomized clinical trial. JAMA Psychiatry 2014; 71:566–572Crossref, Medline, Google Scholar
35 : The impact of digital technology on psychological treatments and their dissemination. Behav Res Ther 2017; 88:19–25Crossref, Medline, Google Scholar
36 : A pilot open trial of UNITE-BED: a couple-based intervention for binge-eating disorder. Int J Eat Disord 2018; 51:1107–1112Crossref, Medline, Google Scholar
37 : Predictors, moderators, and mediators of treatment outcome following manualised cognitive-behavioural therapy for eating disorders: a systematic review. Eur Eat Disord Rev 2017; 25:3–12Crossref, Medline, Google Scholar
38 : Cost-effectiveness of guided self-help treatment for recurrent binge eating. J Consult Clin Psychol 2010; 78:322–333Crossref, Medline, Google Scholar
39 : Perceptions of the feasibility and acceptability of a smartphone application for the treatment of binge eating disorders: qualitative feedback from a user population and clinicians. Int J Med Inform 2015; 84:808–816Crossref, Medline, Google Scholar
40 : Participation and outcome in manualized self-help for bulimia nervosa and binge eating disorder: a systematic review and metaregression analysis. Clin Psychol Rev 2014; 34:158–176Crossref, Medline, Google Scholar
41 : A comparison of different methods for assessing the features of eating disorders in patients with binge eating disorder. J Consult Clin Psychol 2001; 69:317–322Crossref, Medline, Google Scholar
42 : group ObotDCPMNSa: Global priorities for addressing the burden of mental, neurological, and substance use disorders. in Mental, Neurological, and Substance Use Disorders: Disease Control Priorities, 3rd ed, vol 4. Edited by . Washington, DC, The World Bank, 2016.Crossref, Google Scholar
43 : Therapist drift redux: why well-meaning clinicians fail to deliver evidence-based therapy, and how to get back on track. Behav Res Ther 2016; 77:129–137Crossref, Medline, Google Scholar



