Cognitive Change in Schizophrenia and Other Psychoses in the Decade Following the First Episode
Abstract
Objective:
Schizophrenia is associated with a marked cognitive impairment that is widely believed to remain stable after illness onset. Yet, to date, 10-year prospective studies of cognitive functioning following the first episode with good methodology are rare. The authors examined whether schizophrenia patients experience cognitive decline after the first episode, whether this decline is generalized or confined to individual neuropsychological functions, and whether decline is specific to schizophrenia.
Methods:
Participants were from a population-based case-control study of patients with first-episode psychosis who were followed prospectively up to 10 years after first admission. A neuropsychological battery was administered at index presentation and at follow-up to patients with a diagnosis of schizophrenia (N=65) or other psychoses (N=41) as well as to healthy comparison subjects (N=103).
Results:
The schizophrenia group exhibited declines in IQ and in measures of verbal knowledge and of memory, but not processing speed or executive functions. Processing speed and executive function impairments were already present at the first episode and remained stable thereafter. The magnitude of declines ranged between 0.28 and 0.66 standard deviations. Decline in measures of memory was not specific to schizophrenia and was also apparent in the group of patients with other psychoses. Healthy individuals with low IQ showed no evidence of decline, suggesting that a decline is specific to psychosis.
Conclusions:
Patients with schizophrenia and other psychoses experience cognitive decline after illness onset, but the magnitude of decline varies across cognitive functions. Distinct mechanisms consequent to the illness and/or psychosocial factors may underlie impairments across different cognitive functions.
Cognitive impairment is a core feature of schizophrenia (1, 2). Understanding the nature and course of this impairment may have important implications for our understanding of the pathophysiology of the disorder.
Research has shown that individuals diagnosed with schizophrenia experience cognitive decline from the premorbid to the postonset period. There is clear evidence for moderate cognitive deficits in children and adolescents who later develop schizophrenia, with meta-analyses showing an average premorbid deficit equal to 8 IQ points (SD=0.5) (3, 4). Cognitive deficits in adults diagnosed with schizophrenia are more pronounced, with meta-analyses reporting a 14-point IQ deficit (SD=0.90) in first-episode schizophrenia patients (5) and 15- to 21-point IQ deficits (SD=1.0–1.5) in chronic schizophrenia patients (1, 6, 7). In line with cross-sectional evidence, longitudinal studies of cognitive change in schizophrenia from before to after illness onset have shown evidence for cognitive decline (8). Three population-based studies have reported cognitive declines ranging from 6 to 12 IQ points (SD=0.4–0.8) between childhood and adulthood in individuals later diagnosed with schizophrenia (8–10).
Despite evidence for cognitive decline from before to after illness onset, the course of cognitive decline in schizophrenia remains unclear. While it is widely believed that cognitive impairments stabilize after illness onset (11–13), at least until older adult life (12, 14), few longitudinal studies have examined cognitive change from illness onset through to a decade later (see Table S1 in the online supplement), and findings across studies and cognitive domains are mixed. Studies have reported a stabilization of the cognitive deficits, cognitive decline, as well as amelioration of cognitive functioning (see Table S1; see also reference 15).
Previous studies have been unable to comprehensively chart the course of cognitive deficits, for several reasons. First, the majority of studies have used clinical samples, which may not be fully representative of the population of individuals with schizophrenia (8). Second, most studies followed participants for only 1 to 3 years from illness onset (see Table S1). We previously reported a slow, gradual increase in premorbid cognitive deficits, with losses equal to between 0.5 and 1 IQ point per year (16). Studies with short follow-ups, therefore, may be underpowered to capture decline. Third, few studies have included comparison groups, and therefore few have been able to consider the potential impact of normative age-associated changes in cognitive functioning, which is necessary to rigorously test for cognitive change. Since brain maturation continues into the third decade of life (17), previous estimates of the magnitude of cognitive decline may be biased. Finally, few studies have examined the effect of medication on cognitive functioning, and yet recent findings suggest that antipsychotic medications may contribute to the severity of cognitive decline (18).
In a previous report on this population-based case-control study, we provided evidence for an IQ deficit as well as varying degrees of impairment across individual cognitive domains following the first psychiatric diagnosis of schizophrenia (19). Study participants have since been followed up and have undergone neuropsychological testing a second time. Using identical neuropsychological measures at first assessment and follow-up, we were able to directly examine change in IQ and in individual cognitive functions after the first episode. To provide an accurate estimate of cognitive change over time, we compared patients to the healthy comparison subjects in the study who were followed during the same period. We tested three hypotheses. First, we examined the IQ decline hypothesis to establish whether schizophrenia patients exhibit a static IQ deficit or IQ decline. Second, we tested the generalized decline hypothesis to determine whether decline occurs across multiple cognitive domains, namely, verbal knowledge, memory, language, processing speed, executive function/working memory, and visuospatial ability. Finally, we tested the specificity hypothesis to establish whether any cognitive decline is specific to schizophrenia or common to other psychoses by examining cognitive change in individuals with psychotic disorders other than schizophrenia.
Methods
AESOP Study
Data were derived from the Aetiology and Ethnicity in Schizophrenia and Other Psychoses (AESOP) study, a population-based case-control study of first-episode psychosis. AESOP was approved by local research ethics committees, and each participant gave written informed consent after receiving a complete description of the study. The study identified all cases of first-episode psychosis (ICD-10 codes F10–F29 and F30–F33) among patients 16–65 years old presenting to specialist mental health services in tightly defined catchment areas of the United Kingdom (southeast London, Nottingham, and Bristol) between September 1997 and August 2000. All potential case subjects who made contact with psychiatric services (including adult community mental health teams, inpatient units, forensic services, learning disability services, adolescent mental health services, and drug and alcohol units) for the first time were screened. Exclusion criteria were previous contact with health services for psychosis, organic causes for psychotic symptoms, transient psychotic symptoms as the result of acute intoxication (as defined by ICD-10), and IQ <50. A random sample of comparison subjects with no past or present psychotic disorder were recruited using a sampling method that matched case and comparison subjects by area of residence. Hereafter, we refer to this phase of the AESOP study as “baseline.”
At baseline, detailed information was collected to allow patients to be traced, recontacted, and reinterviewed approximately 10 years later (“follow-up”). At follow-up, patients currently in contact with mental health services were invited to participate through their clinical teams. Letters of invitation were sent to the last known addresses of those not in contact with services. Nonresponders were sent a second letter 2 to 3 weeks later. If patients were thought to have moved, contact was sought through their primary care physician. Comparison subjects also provided contact details at baseline. Letters of invitation were sent and were followed up with telephone calls if no reply had been received within 2 weeks. If no reply had been received after 4 weeks, or where telephone numbers could not be obtained, in-person visits were made to the person’s address. A detailed overview of the AESOP study design and methods, as well as the follow-up, has been published elsewhere (20, 21).
Analytic Cohort
The derivation of the sample included in the present analysis is summarized in Figure 1. The analytic cohort consisted of healthy comparison subjects and patients who had a consensus ICD-10 diagnosis at last follow-up of schizophrenia (F20), bipolar disorder or mania (F30.2, F31.2, F31.5), depressive psychoses (F32.3, F33.3), or other psychotic disorders, including persistent delusional disorders and psychosis not otherwise specified (F22, F23, F28, F29). Both case and comparison subjects were required to be native English speakers or to have migrated to the United Kingdom by age 11. The latter ensured that all participants had a good command of English, even as a nonnative language, by verifying that participants had completed at least their secondary education in the United Kingdom. Thus, the effect of linguistic or cultural biases on cognitive performance in a multiethnic sample was minimized.
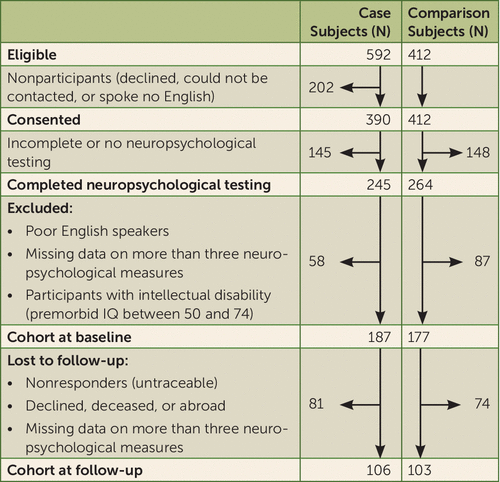
FIGURE 1. Derivation of first-episode psychosis patients and healthy comparison subjects from the Aetiology and Ethnicity in Schizophrenia and Other Psychoses (AESOP) study, baseline and 10-year follow-up cohorts
Neuropsychological Assessment
At baseline and follow-up, participants underwent cognitive testing with a neuropsychological battery, which assessed general intellectual ability (IQ) as well as specific cognitive functions. Administration and scoring followed standard procedures. Full-scale IQ was estimated using the vocabulary, comprehension, digit symbol coding, and block design subtests of the WAIS-R (22). Short forms of the WAIS-R have been shown to produce accurate estimates of full-scale IQ (23, 24). Specific functions were assessed as follows: memory was assessed using the Rey Auditory Verbal Learning Test trials 1 to 7 (learning and immediate and delayed verbal recall) (25) and the visual reproduction subtest of the Wechsler Memory Scale–Revised (WMS-R) (26); verbal knowledge was assessed using the vocabulary and comprehension subtests of the WAIS-R (22); processing speed was assessed using the WAIS-R digit symbol coding subtest and the Trail Making Test, Part A (27); executive function/working memory was assessed using the Trail Making Test, Part B (27) and the letter-number span test (28); language was assessed using category (semantic) and letter fluency (categories: “body parts,” “fruits,” and “animals”; letters: F, A, and S) (29); and visuospatial ability was assessed using the WAIS-R block design subtest.
Diagnostic Assessment
Clinical data were collected using the Schedules for Clinical Assessment in Neuropsychiatry (SCAN) (30). The SCAN incorporates the Present State Examination, version 10, to elicit symptom-related data at time of presentation. Ratings on the SCAN are based on clinical interview, case note review, and information from informants (e.g., health professionals, close relatives). Researchers were trained on the SCAN with a World Health Organization–approved course, and reliability was established before commencement of the study using independent ratings of videotaped interviews. Rater agreement was evaluated using the kappa statistic, which ranged from 1.0 for psychosis as a category to between 0.6 and 0.8 for individual diagnoses. ICD-10 diagnoses were determined using SCAN data through consensus meetings with one of the principal investigators and other team members. Symptom severity was classified based on the SCAN Symptom Severity Rating Scale, part 2, as 0=absent, 1=mild, 2=moderate, and 3=severe (21).
Covariates and Medication Information
Age was recorded at baseline and follow-up. Sex, ethnicity, and education level were recorded at baseline. Treatment history with first-generation and/or second-generation antipsychotic medication was ascertained for all patients from interview data and record review at follow-up.
Creating Norms for Neuropsychological Tests
A regression-based approach was used to create normative standards for the neuropsychological tests. Age at assessment, sex, ethnicity, and education were regressed on each of the neuropsychological measures in the healthy comparison sample at baseline and follow-up. Next, scores were adjusted on the basis of the regression results, and standard scores (z-scores) were created. The same adjustment and standardization procedures were applied to the patient groups, using the normative standards from the healthy comparison group.
Statistical Analysis
Demographic and clinical characteristics of the baseline and follow-up cohorts were compared using summary statistics. For descriptive purposes, we compared patients with schizophrenia or other psychoses (including bipolar disorder, mania, depressive psychoses, and other psychotic disorders) to the comparison group on normative-adjusted IQ and specific neuropsychological tests at baseline and follow-up using analysis of variance models.
To examine the IQ decline, generalized decline, and specificity hypotheses, we compared the schizophrenia and other psychoses groups to the comparison group on change from baseline to follow-up in normative-adjusted IQ and specific neuropsychological test scores. Change scores were calculated by subtracting follow-up test scores from baseline test scores, so that positive scores indicate cognitive amelioration and negative scores indicate cognitive decline. Analysis of covariance (ANCOVA) models with planned orthogonal comparisons of each psychosis group to the comparison group were used, adjusting for time from baseline assessment and baseline test score. Adjustment for baseline performance is common in studies on cognitive change (31, 32). For the IQ decline hypothesis, the significance threshold was set at a p value of 0.05 (two-sided). For the generalized decline hypothesis, the significance threshold was set at a Bonferroni-corrected level of 0.0038 (0.05/13). All analyses were conducted using SPSS, version 24 (IBM, Armonk, N.Y.).
Results
The demographic characteristics of the baseline and follow-up cohorts are presented in Table 1. Follow-up neuropsychological assessments were completed for 106 patients (63 of them male) and 103 comparison subjects (40 of them male). Average follow-up duration was 109.3 months (SD=29.5) for patients and 102.9 (SD=34.1) for comparison subjects. Overall, the patients and comparison subjects assessed at follow-up were similar to the respective patients and comparison subjects assessed at baseline on demographic variables, suggesting that the cohort at follow-up was representative of the original cohort.
| Baseline Cohort | Follow-Up Cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Patients (N=187) | Comparison Subjects (N=177) | Patients (N=106) | Comparison Subjects (N=103) | ||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age at baseline (years) | 29.64 | 10.41 | 37.24 | 12.87 | 28.08 | 9.58 | 35.99 | 10.87 |
| N | % | N | % | N | % | N | % | |
| Male | 101 | 54.0 | 77 | 43.8 | 63 | 59.4 | 40 | 38.8 |
| White | 109 | 58.2 | 113 | 64.4 | 62 | 58.5 | 41 | 39.8 |
| Years of education | ||||||||
| 11 (compulsory) | 123 | 65.8 | 76 | 42.9 | 64 | 60.4 | 38 | 36.9 |
| 12–13 (postcompulsory) | 40 | 21.3 | 52 | 29.4 | 21 | 19.8 | 21 | 20.4 |
| 14 or more (college, graduate, and/or postgraduate) | 24 | 12.9 | 49 | 27.7 | 21 | 19.8 | 44 | 42.7 |
TABLE 1. Demographic characteristics of first-episode psychosis patients and healthy comparison subjects from the Aetiology and Ethnicity in Schizophrenia and Other Psychoses (AESOP) studya
Cognitive Impairment in Schizophrenia and Other Psychoses at Baseline and Follow-Up
As we have shown previously in the AESOP study cohort (19), patients with schizophrenia and patients with other psychoses showed deficits in IQ and individual neuropsychological tests at baseline. Figure 2 shows that the schizophrenia patients exhibited widespread, persistent cognitive impairment, performing significantly worse than comparison subjects at both baseline and follow-up on 11 of the 14 measures. Patients with other psychoses also showed widespread impairments, but these were generally of smaller magnitude than among schizophrenia patients. (Table S2 in the online supplement presents the nonadjusted performance in IQ and specific neuropsychological tests at baseline and follow-up.)
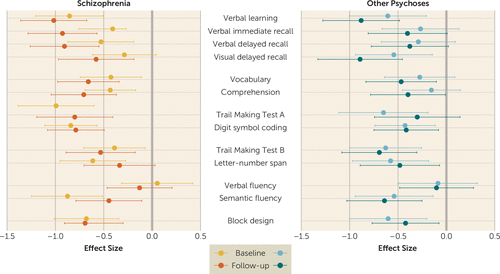
FIGURE 2. Neuropsychological performance among patients with schizophrenia and other psychoses at baseline and follow-upa
a The figure shows effect sizes expressed in standardized (z) scores and 95% confidence intervals of difference from comparison subjects at baseline and follow-up; comparison subjects are set to zero. Effect sizes are adjusted for age, sex, ethnicity, and education level. Confidence intervals that do not include zero indicate a statistical significance level of p<0.05.
Cognitive Change in Schizophrenia and Other Psychoses
Next, we compared cognitive change over time in each of the psychosis groups (schizophrenia and other psychoses) to cognitive change in the comparison group to test the IQ decline, generalized decline, and specificity hypotheses. Figure 3 presents effect sizes of the difference in the within-group change from baseline to follow-up in individual neuropsychological tests between the psychosis groups and comparison subjects. Effect sizes of 0.20, 0.50, and 0.80 reflect small, medium, and large effects, respectively (33).
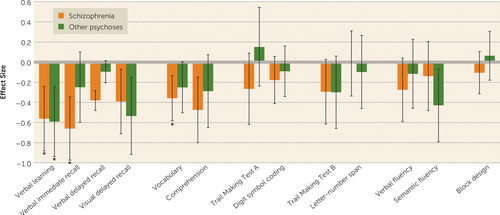
FIGURE 3. Change in neuropsychological performance among patients with schizophrenia and other psychosesa
a The figure shows effect sizes and 95% confidence intervals of difference in change from baseline to follow-up between the diagnostic groups and the comparison group. Confidence intervals that do not include zero indicate a statistical significance level of p<0.05. Effect sizes are adjusted for age, sex, ethnicity, education level, time from baseline assessment, and baseline test score. Asterisks indicate effect sizes that meet the Bonferroni-corrected significance threshold (p≤0.0038).
IQ decline hypothesis.
IQ decline in the schizophrenia group was significantly larger than among comparison subjects, who showed no evidence of IQ decline. The IQ decline in the schizophrenia group relative to the comparison group was of small magnitude (effect size=−0.28, 95% CI=−0.47, −0.09, p=0.003) but was not attenuated when adjusted for education, ethnicity, sex, age at baseline assessment, or duration of follow-up, suggesting that IQ decline could not be attributed to these variables.
Generalized decline hypothesis.
Relative to the comparison group, the schizophrenia group showed a larger cognitive decline across tests in the memory and verbal knowledge domains (see Figure 3). In the memory domain, the declines in the schizophrenia group on verbal learning (p=0.001), immediate recall (p<0.00006), and delayed recall (p<0.00001) reached the Bonferroni-corrected significance threshold. In the verbal knowledge domain, decline on vocabulary (p=0.003) reached the Bonferroni-corrected threshold. Relative to comparison subjects, the schizophrenia group showed no significant cognitive changes on the digit symbol coding test and the Trail Making Test, Part A, in the processing speed domain; on the block design subtest in the visuospatial domain; on the Trail Making Test, Part B, and on the letter-number span test in the executive functions and working memory domain; and on the letter fluency and category fluency tests in the language domain.
Specificity hypothesis.
There was no evidence for IQ decline in the other psychoses group compared with the comparison subjects (effect size=−0.09, 95% CI=−0.30, 0.11, p=0.37). In terms of cognitive domains, like the schizophrenia group, the other psychoses group showed larger declines than comparison subjects across tests in the memory domain, with verbal learning (p=0.001) reaching the Bonferroni-corrected significance threshold. Like the schizophrenia group, the other psychoses group showed static deficits in tests of processing speed, executive functions and working memory, and visuospatial ability (see Figure 3).
Medication
We examined the potential moderating effect of antipsychotic medication on IQ decline in the schizophrenia group. There was no statistically significant difference in IQ decline (p=0.23) between patients with a history of treatment with first-generation antipsychotics only (45% of the sample) and those with a history of treatment with both first-generation and second-generation antipsychotics (55% of the sample). Duration of antipsychotic medication (mean=323 weeks, SD=192) did not attenuate IQ decline in schizophrenia (F=7.30, p=0.008, compared with F=7.20, p=0.009, for ANCOVA models with and without duration of treatment as a covariate).
Symptom Severity
Since illness severity may influence cognition, we also examined the association between baseline symptom severity and change in cognitive functioning, as well as change in symptom severity between baseline and follow-up and change in cognitive functioning. Schizophrenia patients with severe symptoms at baseline showed statistically significantly greater cognitive decline than patients with mild or moderate symptoms across multiple tests in the memory domain (Figure 4). However, there was no association between change in symptom severity and change in cognitive functioning (see Table S3 and Figure S1 in the online supplement) and no evidence for a dose-response relationship across levels of severity (see Figure 4). In the other psychoses group, there was no evidence for an association between symptom severity, or change in symptom severity, and change in cognitive functioning (see Figure 4; see also Figure S1 in the online supplement).
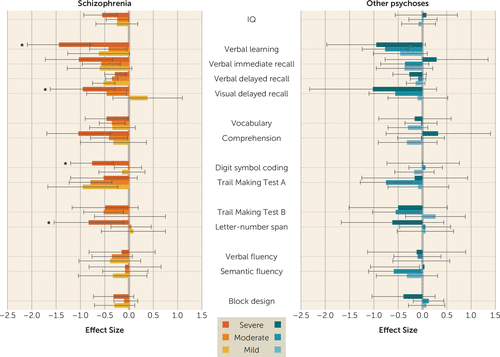
FIGURE 4. Change in neuropsychological performance among patients with schizophrenia and other psychoses in relation to symptom severity at baselinea
a The figure shows effect sizes and 95% confidence intervals of difference in change from baseline to follow-up between the diagnostic groups and the comparison group as a function of symptom severity at baseline. Confidence intervals that do not include zero indicate a statistical significance level of p<0.05. Effect sizes are adjusted for age, sex, ethnicity, education level, time from baseline assessment, and baseline test score. Asterisks indicate a significant difference, at p<0.05, in cognitive change between the severe symptoms group and the moderate or mild symptoms groups.
Sensitivity Analyses
We also examined the potential impact of attrition by applying linear mixed models, which permit varying numbers of measurements per person and time point while adjusting for within-individual (i.e., between measures) variation. Similar results were obtained in models that included only case and control subjects with data from both assessment time points and in models that also included case and control subjects with data from a single assessment, indicating that the results were not biased by attrition.
As a further comparison, we examined IQ change in comparison subjects with lower IQ (IQ <90 at baseline, equal to one standard deviation below the comparison group mean; N=17, 16.5% of sample). These individuals are of interest because, like schizophrenia patients, they also exhibit lower IQ, and yet they did not develop psychosis. In contrast to patients with schizophrenia, individuals with lower IQ did not show evidence of IQ decline, either in absolute terms or relative to comparison subjects without cognitive impairment (mean IQ was 84.9 at baseline and 89.8 at follow-up (F=0.97, p=0.35).
Discussion
Using a population-based case-control sample followed prospectively from the first psychotic episode, we provide evidence for cognitive decline after illness onset in patients with schizophrenia.
These findings advance knowledge in three important ways. First, the results lend support to the IQ decline hypothesis. As a group, schizophrenia patients showed IQ decline between baseline and follow-up assessments, with an effect size of small magnitude (0.28). This finding contrasts with earlier studies reporting stabilization of cognitive deficits after the onset of psychosis (15). However, previous studies had important methodological limitations, including short follow-up periods and lack of a similarly followed-up comparison group. The finding of IQ decline is in line with findings from neuroimaging studies of greater age-associated brain volume loss (34), as well as deviated gyrification trajectories in schizophrenia patients in adulthood (35). Moreover, reduction in cortical volume has been associated with IQ decline in schizophrenia patients (36).
Second, our findings here do not support the generalized decline hypothesis. Decline was not ubiquitous, and it varied across cognitive domains. The schizophrenia group exhibited declines in verbal knowledge and memory. In contrast, processing speed, executive functions, and visuospatial ability did not decline. These contrasts can be generally viewed as reflecting differences between the impact of the illness on crystallized (verbal knowledge) and fluid (processing speed, executive functions, visuospatial) abilities. Our findings of decreasing crystallized abilities and memory scores between baseline and follow-up are in line with previous evidence (37) and suggest that increasing deficits in these domains may reflect actual loss of ability rather than abnormal cognitive development (i.e., “lag”) (16). Alternatively, our findings may reflect difficulties with the maintenance and acquisition of new verbal knowledge as a result of substantial and increasing memory deficits. While most cognitive abilities in the general population start to show stabilization or even decline in early adulthood, crystallized abilities may peak much later (38–40). In our study, measures of fluid abilities already showed a large deficit at the first episode, which remained static thereafter. While previous longitudinal epidemiological studies have shown cognitive decline in schizophrenia from the premorbid period in childhood to the chronic stage in mid-adulthood (8–10), they were unable to determine when this decline occurred. Our findings suggest that most of the decline in fluid abilities occurs before the first episode, while crystallized abilities may continue to decline after onset. Importantly, the decline in IQ after onset is likely to be due to the decline seen in crystallized abilities.
Third, our findings do not support the specificity hypothesis, since patients with schizophrenia as well as those with other psychoses experienced cognitive decline. However, while patients with schizophrenia showed decline in IQ, memory, and verbal knowledge, patients with other psychoses showed decline only in certain memory functions. Moreover, in line with previous reports (16, 41), the other psychoses group showed an overall impairment profile that was qualitatively similar yet quantitatively smaller than in the schizophrenia group. Thus, our findings suggest that cognitive decline is not specific to schizophrenia but is also evident in other psychoses. However, large, widespread cognitive decline may still be specific to schizophrenia, since the other psychoses group showed a smaller and less generalized cognitive decline. Interestingly, there was no evidence of decline in a key comparison group, namely, individuals with lower IQ who did not develop psychosis. This group may in fact experience a different process of regression to the mean.
Our findings in this study should be viewed in the context of certain limitations. First, although we found evidence for cognitive decline after illness onset, we could not fully map the course of deficits, and cognitive functions may vary in the timing of decline after the first episode. Second, group sizes did not allow for an analysis of the heterogeneity of cognitive course and also limited our ability to investigate more specific diagnostic subgroups, such as bipolar disorder and mania. Third, we ruled out two explanations for the observed cognitive decline, namely, type and duration of antipsychotic treatment. Unfortunately, we did not have information to examine other potential moderators of cognitive decline, such as social isolation, smoking, illicit drug abuse, victimization, or physical health problems such as obesity, diabetes, and hypertension. Moreover, despite the fact that we adjusted for education in all our analyses, poor education in the schizophrenia group after the first psychotic episode could still partly explain some of the group differences.
There is conflicting evidence regarding the relationship between change in symptoms and cognitive functioning (42, 43). In our study, change in severity of psychosis was only minimally associated with cognitive change. These results are consistent with cross-sectional findings of only a weak association between positive symptoms and cognitive impairment (44). Longitudinal evidence also suggests a minimal association between change in positive as well as negative symptoms and change in cognition (42–45). Interestingly, in our study, schizophrenia patients with severe symptoms at baseline showed greater cognitive decline than patients with mild or moderate symptoms. While this subgroup was small (21% of the overall group), the magnitude of decline in the memory domain was large. Thus, this finding points to a potential subgroup of schizophrenia patients who may greatly benefit from being specifically targeted for cognitive remediation.
Our findings have important implications for understanding the nature and course of cognitive impairment in schizophrenia, as well as in other psychoses. Integrating the present findings with those of previous studies (16) suggests that cognitive dysfunction in schizophrenia may result from a complex interplay between an early, static neuropathology (46, 47) and dynamic age-related processes (48, 49). Hence, cognitive functions that develop and peak relatively early in life, such as processing speed and visuospatial abilities (39), may show aberrant development, resulting in slowed growth before the onset of schizophrenia (16), but relative stabilization throughout the illness course. On the other hand, cognitive functions that continue to evolve through adult life, such as language (39), may show further deterioration throughout the course of schizophrenia. Finally, functions sensitive to age-related cognitive decline, such as memory, may begin to decline in middle adulthood, before normative aging becomes apparent (40).
In conclusion, this study demonstrates that while a substantial proportion of the cognitive impairment seen in adult patients with schizophrenia, as well as in other psychoses, is already present at the first episode, these patients continue to experience cognitive decline after illness onset. However, the nature of this decline varies across neuropsychological functions. While large deficits in processing speed are already apparent at the first episode, deficits in verbal knowledge and memory continue to increase. These findings suggest that different pathophysiological mechanisms may underlie individual neuropsychological deficits seen in adult psychosis patients. Future research should determine which of these are consequent to the illness itself, and which to the psychosocial factors patients experience. Finally, these findings highlight the importance of targeting early developmental stages in future studies of the causes of cognitive deficits associated with psychosis, as well as in cognitive remediation efforts.
1 : Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychol Bull 2007; 133:833–858Crossref, Medline, Google Scholar
2 : Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry 2013; 70:1107–1112Crossref, Medline, Google Scholar
3 : Neuropsychological profiles in individuals at clinical high risk for psychosis: relationship to psychosis and intelligence. Schizophr Res 2010; 123:188–198Crossref, Medline, Google Scholar
4 : A quantitative meta-analysis of population-based studies of premorbid intelligence and schizophrenia. Schizophr Res 2011; 132:220–227Crossref, Medline, Google Scholar
5 : Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology 2009; 23:315–336Crossref, Medline, Google Scholar
6 : Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 1998; 12:426–445Crossref, Medline, Google Scholar
7 : A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychol Rev 2005; 15:73–95Crossref, Medline, Google Scholar
8 : Neuropsychological decline in schizophrenia from the premorbid to the postonset period: evidence from a population-representative longitudinal study. Am J Psychiatry 2014; 171:91–101Link, Google Scholar
9 : Intellectual decline in schizophrenia: evidence from a prospective birth cohort 28 year follow-up study. J Clin Exp Neuropsychol 2006; 28:225–242Crossref, Medline, Google Scholar
10 : Cognitive decline in schizophrenia from childhood to midlife: a 33-year longitudinal birth cohort study. Schizophr Res 2010; 118:1–5Crossref, Medline, Google Scholar
11 : Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry 2001; 58:24–32Crossref, Medline, Google Scholar
12 : Neurocognitive impairment across the lifespan in schizophrenia: an update. Schizophr Res 2005; 74:15–26Crossref, Medline, Google Scholar
13 : Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol Med 2011; 41:225–241Crossref, Medline, Google Scholar
14 : Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: a comparison with Alzheimer’s disease and normal aging. Am J Psychiatry 2001; 158:1441–1448Link, Google Scholar
15 : Longitudinal studies of cognition in first episode psychosis: a systematic review of the literature. Aust N Z J Psychiatry 2011; 45:93–108Crossref, Medline, Google Scholar
16 : Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry 2010; 167:160–169Link, Google Scholar
17 : Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci 2005; 9:104–110Crossref, Medline, Google Scholar
18 : Association of neurocognition with transition to psychosis: baseline functioning in the second phase of the North American Prodrome Longitudinal Study. JAMA Psychiatry 2016; 73:1239–1248Crossref, Medline, Google Scholar
19 : Specific and generalized neuropsychological deficits: a comparison of patients with various first-episode psychosis presentations. Am J Psychiatry 2010; 167:78–85Link, Google Scholar
20 : Heterogeneity in incidence rates of schizophrenia and other psychotic syndromes: findings from the 3-center AESOP study. Arch Gen Psychiatry 2006; 63:250–258Crossref, Medline, Google Scholar
21 : Reappraising the long-term course and outcome of psychotic disorders: the AESOP-10 study. Psychol Med 2014; 44:2713–2726Crossref, Medline, Google Scholar
22 : WAIS-R manual: Wechsler Adult Intelligence Scale-Revised. San Antonio, Tex, Psychological Corp, 1981Google Scholar
23 : Two-and four-subtest short forms of the Wechsler Adult Intelligence Scale–Revised. J Consult Clin Psychol 1982; 50:415Crossref, Google Scholar
24 : Investigation of validity of WAIS-R short forms for patients suspected to have brain impairment. J Consult Clin Psychol 1984; 52:722–723Crossref, Medline, Google Scholar
25 : Rey Auditory Verbal Learning Test: A Handbook. Los Angeles, Western Psychological Services, 1996Google Scholar
26 : Instruction Manual for the Wechsler Memory Scale–Revised. New York, Psychological Corp, 1987Google Scholar
27 : Trail Making Test: Manual for Administration and Scoring. Tempe, Ariz, Reitan Neuropsychology Laboratory, 1992Google Scholar
28 : Auditory working memory and Wisconsin card sorting test performance in schizophrenia. Arch Gen Psychiatry 1997; 54:159–165Crossref, Medline, Google Scholar
29 : A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York, Oxford University Press, 1991Google Scholar
30 : SCAN: Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry 1990; 47:589–593Crossref, Medline, Google Scholar
31 : When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol 2005; 162:267–278Crossref, Medline, Google Scholar
32 : Unreliability of difference scores: a paradox in the measurement of change. Psychol Bull 1975; 82:185–186Crossref, Google Scholar
33 : A power primer. Psychol Bull 1992; 112:155–159Crossref, Medline, Google Scholar
34 : Schizophrenia. Nat Rev Dis Primers 2015; 1:15067Crossref, Medline, Google Scholar
35 : Lifespan gyrification trajectories of human brain in healthy individuals and patients with major psychiatric disorders. Sci Rep 2017; 7:511Crossref, Medline, Google Scholar
36 : Association of IQ changes and progressive brain changes in patients with schizophrenia. JAMA Psychiatry 2015; 72:803–812Crossref, Medline, Google Scholar
37 : Decline in cognitive performance between ages 13 and 18 years and the risk for psychosis in adulthood: a Swedish longitudinal cohort study in males. JAMA Psychiatry 2013; 70:261–270Crossref, Medline, Google Scholar
38 : Factors predicting the use of technology: findings from the Center for Research and Education on Aging and Technology Enhancement (CREATE). Psychol Aging 2006 ; 21:333–352Crossref, Medline, Google Scholar
39 : When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychol Sci 2015; 26:433–443Crossref, Medline, Google Scholar
40 : When does age-related cognitive decline begin? Neurobiol Aging 2009; 30:507–514Crossref, Medline, Google Scholar
41 : Childhood IQ and adult mental disorders: a test of the cognitive reserve hypothesis. Am J Psychiatry 2009; 166:50–57Link, Google Scholar
42 : Longitudinal study of symptoms and cognitive function in chronic schizophrenia. Schizophr Res 2003; 59:137–146Crossref, Medline, Google Scholar
43 : Predictors and longitudinal course of cognitive functioning in schizophrenia spectrum disorders, 10 years after baseline: the OPUS study. Schizophr Res 2016; 175:57–63Crossref, Medline, Google Scholar
44 : Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophr Res 2009; 113:189–199Crossref, Medline, Google Scholar
45 : Ten year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophr Res 2005; 78:27–34Crossref, Medline, Google Scholar
46 : Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Crossref, Medline, Google Scholar
47 : Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed) 1987; 295:681–682Crossref, Medline, Google Scholar
48 : Is schizophrenia a syndrome of accelerated aging? Schizophr Bull 2008; 34:1024–1032Crossref, Medline, Google Scholar
49 : Accelerated brain aging in schizophrenia and beyond: a neuroanatomical marker of psychiatric disorders. Schizophr Bull 2013; 40:1140–1153Crossref, Medline, Google Scholar



