Early Detection and Preventive Intervention in Schizophrenia: From Fantasy to Reality
Abstract
Scientific progress in understanding human disease can be measured by the effectiveness of its treatment. Antipsychotic drugs have been proven to alleviate acute psychotic symptoms and prevent their recurrence in schizophrenia, but the outcomes of most patients historically have been suboptimal. However, a series of findings in studies of first-episode schizophrenia patients transformed the psychiatric field’s thinking about the pathophysiology, course, and potential for disease-modifying effects of treatment. These include the relationship between the duration of untreated psychotic symptoms and outcome; the superior responses of first-episode patients to antipsychotics compared with patients with chronic illness, and the reduction in brain gray matter volume over the course of the illness. Studies of the effectiveness of early detection and intervention models of care have provided encouraging but inconclusive results in limiting the morbidity and modifying the course of illness. Nevertheless, first-episode psychosis studies have established an evidentiary basis for considering a team-based, coordinated specialty approach as the standard of care for treating early psychosis, which has led to their global proliferation. In contrast, while clinical high-risk research has developed an evidence-based care model for decreasing the burden of attenuated symptoms, no treatment has been shown to reduce risk or prevent the transition to syndromal psychosis. Moreover, the current diagnostic criteria for clinical high risk lack adequate specificity for clinical application. What limits our ability to realize the potential of early detection and intervention models of care are the lack of sensitive and specific diagnostic criteria for pre-syndromal schizophrenia, validated biomarkers, and proven therapeutic strategies. Future research requires methodologically rigorous studies in large patient samples, across multiple sites, that ideally are guided by scientifically credible pathophysiological theories for which there is compelling evidence. These caveats notwithstanding, we can reasonably expect future studies to build on the research of the past four decades to advance our knowledge and enable this game-changing model of care to become a reality.
Scientific progress in understanding human disease can be measured by the effectiveness of its treatment. There are three ways in which it can be measured: alleviation of symptoms without affecting the disease course (e.g., l-dopa for Parkinson’s disease, cholinesterase inhibitors for Alzheimer’s disease, triptans for migraine headaches); prevention of progression, but not curative (e.g., glatiramer and interferon for multiple sclerosis, anticoagulants for cerebrovascular disease, anticonvulsants for seizure disorders); and curative or preventive (e.g., surgical procedures for tumors, antibiotics and vaccines for infectious diseases).
The first effective treatment for schizophrenia and related psychotic disorders was chlorpromazine, the prototypical antipsychotic drug (1). Numerous other compounds were subsequently developed, all of which work by a common mechanism of action: inhibition of dopamine at the postsynaptic D2 receptor. More than four decades of research with antipsychotics has definitively proven their efficacy in alleviating psychotic symptoms and preventing symptom recurrence (2–4). Despite these results, antipsychotics were considered to be a symptomatic treatment, acting like cholinesterase inhibitors for Alzheimer’s disease or l-dopa for Parkinson’s disease, and were not believed to resolve the fundamental pathology or alter the course of the illness (5, 6).
However, beginning in the late 1980s, studies of first-episode psychosis patients (7), in conjunction with seminal reviews by McGlashan (8) and Wyatt (9), revealed two revolutionary findings that challenged that premise: 1) longer durations of untreated psychosis were associated with worse treatment responses and outcomes (10, 11); and 2) a significant portion of first-episode psychosis patients could achieve symptom remission and recovery if they received effective treatment in a timely fashion (12–14). These studies indicated that the timing of treatment in first-episode patients was a critical factor in determining their prognoses. More generally, they also suggested that active psychosis was, in effect, “bad for the brain” in that it reflected an underlying pathophysiologic process that was progressive unless alleviated by treatment.
These findings significantly influenced scientific thinking and clinical approaches to schizophrenia and raised the possibility of treatment being able to limit its morbidity and even prevent its onset—or, in other words, modify the course of the illness. In this article, we review the evidence for this radical reconceptualization of schizophrenia and therapeutic prospects for those affected.
Nosology: the Early Classification of Schizophrenia
Progress in understanding schizophrenia can be said to begin with the description of dementia praecox by Pick and Kraepelin (15, 16) (Table 1). (Before that, psychiatric nosology was chaotic and characterized by a conflicting mosaic of inconsistent systems in which disease categories were based on short-term and cross-sectional observations of patients, from which the putative characteristic signs and symptoms of a given disease concept were derived.) The dominant psychiatric constructs, which gave a semblance of order to this fragmentary nosology, were the theory of degeneration and the concept of “unitary psychosis” (Einheitspsychose) (17). What distinguished what we now call schizophrenia from other conditions whose clinical profiles included psychotic symptoms was the patients’ clinical deterioration over the course of their illness. Hence, Pick and Kraepelin applied the term dementia praecox because of the progressive impairment in cognitive function in young patients in contrast to senile dementia. In addition to establishing diagnosis for a disorder, their conceptualization of the illness hinted at its pathophysiology and defined a potential target for therapeutic intervention.
| Milestone | Authors, Year (Reference) | Significance |
|---|---|---|
| Defined dementia praecox | Pick 1891 (15), Kraepelin 1896 (16) | Established nosology of mental illness |
| Defined schizophrenia | Bleuler 1908 (18) | Redefined dementia praecox as schizophrenia |
| Published paper on prevention of onset | Sullivan 1927 (65) | First proposed idea for prevention |
| Discovery and early use of chlorpromazine | Laborit 1950 (cited in Thuillier 1999 [1]), Lehmann and Hanrahan 1954 (146), Delay and Denniker 1955 (147) | Development of antipsychotics and confirmation of effectiveness |
| Dopamine hypothesis | Snyder et al. 1974 (19), Seeman and Lee 1975 (148), Meltzer and Stahl 1976 (149) | First scientifically credible pathophysiological theory of schizophrenia |
| Early brain imaging studies | Huber 1957 (150), Haug 1982 (151), Johnstone et al. 1976 (152), Weinberger et al. 1979 (153) | First in vivo neuroimaging studies showing structural abnormalities |
| Synaptic pruning hypothesis | Feinberg 1982 (120), Keshavan et al. 1994 (123) | Heuristic pathophysiological theory of schizophrenia |
| Neurodevelopmental hypothesis | Weinberger 1987 (31), Murray et al. 1992 (32), Bloom 1993 (33) | Heuristic pathophysiological theories of schizophrenia |
| Revised dopamine hypothesis | Pycock et al. 1980 (24), Carlsson 1988 (25), Davis et al. 1991 (26), Lieberman et al. 1997 (27), Laruelle and Abi-Dargham 1999 (28), Kapur 2003 (29), Howes and Kapur 2009 (30) | Heuristic pathophysiological theories of schizophrenia |
| Two-syndrome hypothesis | Carpenter et al. 1973 (154), Crow et al. 1982 (34), Andreasen and Olsen 1982 (155) | Heuristic pathophysiological theories, positive and negative symptoms of schizophrenia, two-syndrome typology of schizophrenia, positive and negative syndromes of schizophrenia |
| Genetic high-risk studies | Gottesman and Shields 1966, 1967 (67, 68), Erlenmayer-Kimling and Cornblatt 1987 (69), Mednick et al. 1987 (70), Mirsky 1995 (71), Hodges et al. 1999 (72) | Long-term high-risk cohort studies to identify predictors of schizophrenia |
| First-episode psychosis studies | May et al. 1981 (36), Rabiner et al. 1986 (12), Nuechterlein et al. 1992 (156), Kirch et al 1992 (7), Lieberman et al. 1993 (14), Thara et al. 1994 (13), Melle et al. 2004, 2008 (48, 49), Norman et al. 2011 (157), Nordentoft et al. 2014 (56), Dixon et al. 2015 (50), Kane et al. 2016 (54) | Prospective studies of early stages of schizophrenia demonstrated good treatment response, reduction of duration of untreated psychosis, feasibility and efficacy of specialized treatment programs |
| Neuroprogression hypothesis of schizophrenia | McGlashan 1988 (8), Wyatt 1991 (9), DeLisi et al. 1997 (158), Lieberman 1999 (159) | Influential review of schizophrenia course found deterioration in early stages of illness inspired heuristic pathophysiological theory of schizophrenia and the early detection and intervention initiative |
| Glutamate hypothesis | Javitt and Zukin 1991 (128), Krystal et al. 1994, 2005 (137, 160), Olney and Farber 1995 (161), Goff and Coyle 2001 (162), Moghaddam and Javitt 2012 (129), Schobel et al. 2013 (93) | Heuristic pathophysiological theories of schizophrenia |
| Brain MRI studies of progression | Lieberman et al. 1992, 2001 (163, 164), DeLisi et al. 1997 (158), Gur et al. 1998 (165), Andreasen et al. 2011 (166), Cahn et al. 2002, 2006, 2009 (38–40), Bartzokis et al. 2012 (42), Schobel et al. 2013 (93) | Studies showing progressive brain morphologic changes over the course of schizophrenia |
| Clinical high-risk studies | Falloon 1992 (66), Yung and McGorry 1996 (74), McGlashan et al. 2001 (167), Addington et al. 2007 (77), Klosterkötter et al. 2005 (80), Ruhrmann et al. 2010 (81), Egerton, Howes et al. 2009, 2011, 2013 (98–100), Schobel et al. 2013 (93), McFarlane et al. 2015 (57), Brucato et al. 2017 (82), Koutsouleris et al. 2018 (78), Tognin et al. 2019 (79) | Clinical high-risk studies that demonstrated rates and time course of conversion and phenomenologic criteria for identification and diagnosis |
TABLE 1. Timeline of progress in preventive intervention research for schizophrenia
Soon after that, however, Bleuler shifted the focus to the symptoms of the illness within a given episode and renamed the illness schizophrenia, referring to the schism between thought and affect in patients (18). As a result of the emphasis on cross-sectional symptomatology, the goal of future treatments would be to control symptoms rather than altering the course of the illness.
Efforts to Understand the Etiology and Pathophysiology of Schizophrenia
The serendipitous discovery of antipsychotics gave rise to the first scientifically credible pathophysiological theory of schizophrenia, the dopamine hypothesis (19). Previous theories (humoral, psychoanalytic, social learning, existential, constitutional, developmental, family system, infectious, immune), with the exception of the genetic theory, which was supported by epidemiologic genetic data, lacked substantive evidentiary bases (20–23). Since its inception five decades ago, the dopamine hypothesis has undergone multiple iterations (24–30). Despite these, while durable and heuristic, the dopamine hypothesis has been limited in its ability to fully explain the clinical pathology and course of schizophrenia and lead to innovative and better treatments.
A decade later, two competing pathophysiological theories emerged that subsequently framed the field’s thinking and research (31–34). The neurodevelopmental hypothesis of schizophrenia postulates that etiologic and pathogenic factors (such as genes and environmental insults), antedating the onset of the illness, disrupt the course of normal brain development, resulting in subtle alterations of specific cells, circuits, and their connectivity (31–33). This developmental disruption confers vulnerability to malfunction and the consequent manifestation of symptoms diagnostic of the illness. However, in contrast to other genetic neurodevelopmental disorders, such as autism, fragile X syndrome, or Down’s syndrome, the phenotype of schizophrenia is expressed after a latency period extending into the second and third decades of life. After its onset and over its course, the illness persists as a static encephalopathy, and the brain abnormalities only progress in the context of the aging process (35).
Although heuristic, the neurodevelopmental hypothesis of schizophrenia did not explain variation in the course of the illness after its onset or in response to treatment, and specifically its progressive nature. In addition, it fostered a therapeutic pessimism in which people with schizophrenia were “doomed from the womb” and had bleak prognoses regardless of treatment.
Because of these limitations and in an effort to accommodate the heterogeneity observed in the clinical presentations and research findings of schizophrenia, Crow proposed a “two-syndrome hypothesis” of schizophrenia (34) (Figure 1). Type I was characterized by acute episodes with mostly positive symptoms, minimal negative and cognitive symptoms, good antipsychotic treatment response, and indices of excessive dopamine activity. Type II was characterized by negative and cognitive symptoms (in addition to positive symptoms), poor treatment response, and evidence of brain structural pathology in vivo and postmortem. A novel feature of this hypothesis was the element of unidirectional progression of the illness from the type I to type II syndrome over time.
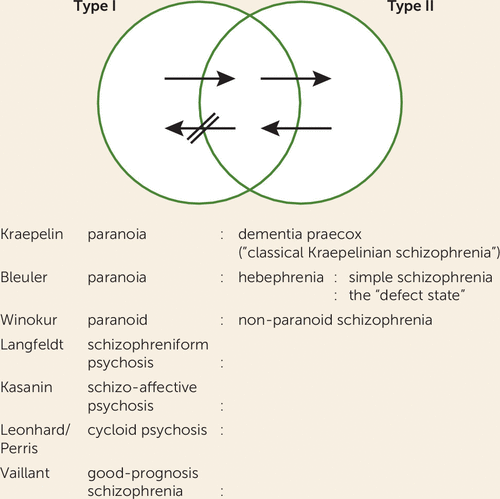
FIGURE 1. Features and relationship of the two forms of schizophrenia and the potential for progression over the course of illnessa
a Some illnesses present with positive symptoms, as in the left segment of the figure. If they remit, they tend to be labeled as “schizophreniform,” “schizoaffective,” or “reactive.” If they persist and no negative symptoms appear, they can be considered as chronic paranoid illnesses (according to the paranoid-nonparanoid dichotomy of Tsuang and Winokur (190). Illnesses in which negative symptoms also appear (or are already present) will tend to be labeled as “nonparanoid,” “true,” “process,” or “classical Kraepelinian” schizophrenia. In some of these illnesses, positive symptoms will remit (as Pfohl and Winokur [191] have documented), leaving the “pure deficit” type II syndrome (right-hand segment of Venn diagram). In some cases, positive symptoms reappear. However, the crossed arrow between the middle and left-hand segments of the diagram is intended to indicate the relative resistance to remission of the components of the type II syndrome, that is, primary negative symptoms and intellectual impairment. (From Crow TJ, The two-syndrome concept: origins and current status, Schizophrenia Bulletin 1985, volume 11, p. 480, by permission of Oxford University Press.)
Shortly after publication of Crow’s two-syndrome hypothesis, McGlashan published a comprehensive review of the natural history of schizophrenia (8) that highlighted the clinical deterioration associated with the illness and specified that this occurred predominantly in the first 5 to 10 years of the illness. Subsequently, Wyatt (9) published an influential review that found that delays in treatment were associated with poorer outcomes. In addition, prospective studies of first-episode schizophrenia patients mapped the progressive nature of the illness and the therapeutic effects of treatment, beginning with the classic treatment study by May et al. (36). Concurrently, longitudinal MRI assessments of first-episode patients found progressive volumetric reduction of gray matter in specific brain regions and enlargement of the lateral ventricles and subarachnoid space (14, 37), in some cases reflecting illness course (38–41) and mitigated by treatment (39–43).
Studies of Natural History and Early Intervention
The consistently replicated correlation between longer duration of untreated psychosis and poorer outcome, along with the increasing reports of morphological changes demonstrated by longitudinal brain imaging studies, resonated with the historical descriptions of clinical deterioration by Kraepelin and subsequent long-term follow-up studies of (Manfred) Bleuler and Ciompi (8). These results prompted researchers to describe the natural history of schizophrenia as progressing through four stages of illness—premorbid, prodromal, onset/progressive, and chronic residual (44, 45). They also prompted researchers to redouble their efforts to develop a strategy to diagnose and treat patients as early in the course of their illness as possible.
The early identification and intervention strategy for schizophrenia offered hope to patients and clinicians alike that treatment could possibly limit the chronic morbidity and disability of schizophrenia (46, 47) (Figure 2). However, two critical challenges had to be addressed to achieve this goal: 1) reducing the time between the patient’s onset of symptoms and their diagnosis and treatment, and 2) engaging patients in treatment to foster recovery and prevent relapses.
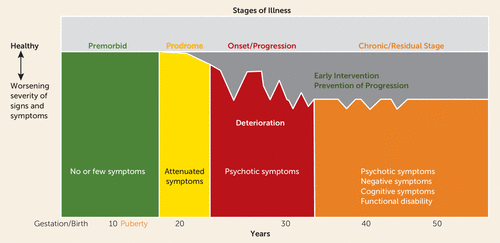
FIGURE 2. Natural history of schizophrenia and the rationale for early detection and interventiona
a Shown are the stages of illness in schizophrenia. Pre-syndromal stages are premorbid and prodromal; syndromal stages include the onset of first episode of psychosis and continue through the progressive stage. The goal of treatment in the early stages of illness, at or prior to the onset of the first episode of psychosis, is to prevent or shorten the duration of psychotic episodes, reduce recurrences, and limit the progressive decline in functioning (deterioration) that occurs in the syndromal stage and leads to the chronic effects of the disease. (From Lieberman JA, First MB, Psychotic disorders, New England Journal of Medicine, volume 379, p. 274. Copyright 2018, Massachusetts Medical Society. Reprinted with permission.)
Duration of Untreated Psychosis and the TIPS Study
First-episode studies revealed that the amount of time from when patients first developed psychotic symptoms until their initial treatment was on average 1 year in the developed countries and twice as long in developing countries (10). Thus, if the duration of untreated psychosis was a critical factor in determining treatment outcome, it would be important to reduce it to optimize outcomes.
To examine whether early detection programs could reduce the duration of untreated psychosis in people seeking care, McGlashan and colleagues in Scandinavia carried out an observational cohort study of 281 individuals with primary psychotic disorders (48, 49). The Early Treatment and Intervention in Psychosis Study (TIPS) was conducted in four health care sectors in Norway and Denmark. A public awareness campaign and an early detection program were implemented in two sectors, and mental health care as usual was provided in the other two sectors. Once patients were diagnosed, they were treated by the same protocol regardless of which sector they were in. The early detection sectors broadcast public service announcements designed to raise awareness in the sector’s population about mental illness. Similar information was provided to primary care providers and other first-line health care personnel. In addition, a telephone number was included as part of the campaign that would allow patients, family, and friends to call the early detection teams as necessary. Usual care involved no outreach and patients seeking care on their own initiative.
Patients were followed for several years. At baseline, individuals in the early detection regions had significantly shorter duration of untreated psychosis (5 weeks) than those in the non–early detection group (16 weeks), indicating that the public outreach worked to encourage people to seek treatment sooner. Individuals in the early detection group were also less symptomatic and had better functioning (49). At 3 months, individuals in the early detection group had less negative and general psychopathology symptoms and better functioning. At 2 years, despite the fact that there were no differences between the two groups in treatment received, the early detection group still had lower levels of negative, cognitive, and depressive symptoms as measured by the Positive and Negative Syndrome Scale (PANSS) (48).
First-Episode Psychosis Studies and the RAISE Initiative
The second challenge was whether first-episode psychosis patients would remain engaged in treatment for sustained periods following their recovery, and whether they could be prevented from relapsing. Previous studies of first-episode patients had shown uniformly high attrition rates for various reasons, including lack of awareness about the potential chronicity of the illness, treatment side effects, and stigma.
To address this question, the National Institute of Mental Health (NIMH) launched the Recovery After an Initial Schizophrenia Episode (RAISE) initiative and funded two projects. RAISE Connection was a demonstration project of the feasibility of team-based multidisciplinary treatment tailored to first-episode patients that was implemented in the public mental health systems of New York and Maryland. The idea was to create a model treatment program to which first-episode patients would be referred and the outcomes of patients measured. The results of the project clearly indicated the feasibility of this model of care and its effectiveness through high rates of patient retention, treatment implementation, and seemingly good outcomes measured in symptom reduction, level of function, and work or school engagement (50–53). However, since it was a demonstration project, there was no comparison group to show whether RAISE Connection care was superior to usual treatment.
The RAISE Navigate study was a 2-year randomized clinical trial of a comprehensive, recovery-based model of care consisting of four core interventions—personalized medication management, family psychoeducation, resilience-focused individual therapy, and supported employment and education—compared with usual community care for 404 individuals with first-episode psychosis. The results indicated that individuals assigned to the experimental intervention continued in treatment longer and received more services. Moreover, the RAISE Navigate group experienced significantly greater improvement on the quality of life measure (Cohen’s d=0.31) and on total PANSS score (d=−0.29), PANSS depressive factor score (Cohen’s d=−0.23), and Calgary Depression Scale for Schizophrenia score (Cohen’s d=−0.18), but not on positive, negative, or disorganization symptoms, nor on the Clinical Global Impressions severity score over the 24-month period (54). Duration of untreated psychosis was found to moderate the treatment effect on outcomes, so that patients with shorter duration of untreated psychosis improved to a greater degree on measures of quality of life and symptom severity compared with those with longer duration of untreated psychosis.
Among the most important measures of effectiveness were whether individuals with first-episode psychosis would need hospitalization and require Social Security disability benefits or not. As first-episode patients were not disabled at their onset of illness, the need for enrollment in disability programs was an objective measure of treatment effectiveness. However, there were no differences between interventions in hospitalization rates (54) or in the number of study subjects who applied for disability benefits (55).
These results were generally consistent with other first-episode psychosis studies, which found superiority of treatment programs, albeit of modest effect sizes, but the superior effectiveness is dependent on continuing treatment (56).
Despite the RAISE Navigate study’s lack of effects on the economically consequential measures of hospitalization and Social Security disability benefits and modest impact on clinical measures, along with the uncontrolled nature of the study, researchers, clinicians, and policy makers remained enthusiastic about the idea of interdicting the illness at its inception and preventing illness progression and disability. Consequently, first-episode psychosis programs have proliferated (57), with a defined program of coordinated specialty services tailored specifically for first-episode patients fast becoming the new standard of care, such as OnTrack-NY and OnTrack-USA (58, 59) in the United States, EPPIC and HEADSPACE in Australia (60, 61), Forward Thinking Birmingham in the United Kingdom (62), Jigsaw in Ireland (63), ACCESS in Canada (64), and OnTrack-Chile.
Pre-Syndromal Detection and Intervention Research
First-episode psychosis studies prompted researchers to extend the strategy of early intervention to people who did not meet criteria for a syndromal psychotic disorder but were deemed to be at clinical high risk for psychosis. (Individuals at clinical high risk are those who are identified as having nonspecific and attenuated psychotic symptoms and are believed to be at increased risk of developing psychotic disorders, while a diagnosis of prodromal symptoms can only be determined retrospectively for those who go on to develop a syndromal psychosis.) However, the knowledge base and methodology developed in clinical high-risk research were neither as extensive nor as well developed as those of the first-episode psychosis research that prompted and informed the RAISE initiative. Nevertheless, researchers pursued what would be psychiatry’s first attempt at prevention of a mental disorder (46).
History
While contemporary researchers may have used what they learned from first-episode psychosis studies to extend their focus to clinical high-risk patients, it was the eminent psychoanalyst Harry Stack Sullivan who first introduced the idea of prevention of schizophrenia. Sullivan claimed that this most serious of mental disorders was the result of maladaptive relationships and experiences, which could be repaired, rather than hereditary or biological factors (65). Over a half century later, Falloon proposed screening individuals in primary care clinics for the prodromal symptoms of schizophrenia and then applying family therapy to prevent onset of the illness (66).
Sullivan and Falloon were trained in the psychoanalytic and family therapy traditions and focused more on treatment than diagnostic or prognostic measures. While their therapeutic efforts were based on causal hypotheses, they were not supported by empirical evidence. Other investigators focused on predictive diagnostic measures by examining individuals at genetic high risk who had not yet reached schizophrenia’s onset age, hoping to identify measures predictive of schizophrenia (67–72), while others focused on nonspecific symptoms, such as cognitive deficits and general abnormalities in perception and thinking, which they referred to as “basic symptoms” (73).
Diagnostic Criteria
What might be considered the modern era of high-risk research was pioneered by Hafner in Germany, McGorry and colleagues in Australia (74), and McGlashan and colleagues (75) in the United States. They were inspired by the first-episode psychosis studies demonstrating the relationship between duration of untreated psychosis and prognosis and Wyatt’s and McGlashan’s influential reviews. (McGlashan coined the term “duration of untreated psychosis,” and McGorry, who organized the International Early Psychosis Association, established an award in Richard Wyatt’s name.) A key difference between theirs and the earlier high-risk research (e.g., Sullivan, Erlenmeyer-Kimling, Mednick) was that it was now motivated by a temporal urgency for effective intervention to avoid the risk of disease onset and progression.
Hafner emphasized gender and age at onset as key features of natural history and developed the Interview for the Retrospective Assessment of the Onset and Course of Schizophrenia and Other Psychoses (IRAOS). The Australian group initially incorporated genetic risk and functional impairment into their criteria for individuals at high risk and then enhanced them with a focus on attenuated positive symptoms. From their work came the Comprehensive Assessment of At-Risk Mental States (CAARMS) (76), an instrument that both assessed psychopathology and identified individuals at high risk for psychosis. Similar work by McGlashan et al. led to the Structured Interview for Prodromal Symptoms/Structured Interview for Psychosis-Risk Syndromes (SIPS) (75).
While their studies were seminal, their results reflected significant methodologic limitations. The case identification criteria produced high false positive rates (>70% of study subjects who met clinical high-risk criteria did not develop a diagnosable psychotic disorder [by DSM or ICD criteria] over the 2- to 5-year follow-up period), which increased as conversion rates to syndromal psychoses decreased in subsequent studies. This led to efforts to ascertain larger sample sizes, broadening the diagnostic criteria, and, with the encouragement of funding agencies, forming consortia, including the North American Prodrome Longitudinal Study (NAPLS) (77), the Early Detection and Intervention for the Prevention of Psychosis Program (EDIPPP) (57), PRONIA (78), PSYSCAN (79), and the European Prediction of Psychosis Study (EPOS) (80).
Studies by these and other groups further characterized the phenomenology of the high-risk state (81–84) and eventually led to the creation of algorithms and risk calculators for predicting development of syndromal psychosis (85–88). While incrementally better, these instruments still lacked adequate specificity and predictive validity. The limitations of the clinical high-risk criteria were dramatically reflected in the fifth revision of the DSM. The DSM-5 Task Force considered a proposal to include attenuated psychosis syndrome as a new diagnosis purported to identify clinical high-risk patients in the prodromal stage of schizophrenia (89). However, because of its limited specificity and predictive validity, it was judged to be premature and was referred for inclusion in an appendix for insufficiently validated diagnoses requiring further research.
Consequently, intensive efforts have been made to develop confirmatory diagnostic biomarkers by applying a range of modalities (neuroimaging, electrophysiologic, neurocognitive, serologic) to the study of this population in an effort to enhance the diagnostic precision and utility of the clinical high risk designation.
Diagnostic Biomarkers
Much of the initial biomarker research with functional and neuroanatomical MRI applications found individuals at high risk to have values intermediate between healthy individuals and individuals with syndromal psychosis (Table 2). Later work focused more on neurochemical imaging with MR spectroscopy and positron emission tomography (PET) and MRI-derived measures of connectivity and function, which found abnormalities similar to those seen in individuals with syndromal psychosis in clinical high-risk patients but of lesser magnitude. Among these were neuroimaging studies that added to past postmortem research (90–92) and highlighted the involvement of medial temporal lobe structures, specifically the hippocampus, as reflecting structural and functional abnormalities in the early stages of, or at high risk for, schizophrenia (93–97).
| Authors, Year (Reference) | Biomarker | Modality | Finding and Significance |
|---|---|---|---|
| Ferrarelli et al. 2007 (168), Manoach et al. 2016 (101) | Sleep spindles | High-density EEG | Sleep spindles are reduced in schizophrenia. They are generated by the thalamic reticular nucleus and dorsal thalamus, then transferred by thalamic relay neurons to the cortex, where spindle oscillations are synchronized. Current pathophysiologic models of schizophrenia highlight the possibility of thalamocortical dysfunctions being related to GABA and NMDA receptor-mediated glutamatergic neurotransmission accounting for core symptoms of illness. |
| Schobel et al. 2009, 2013 (93, 102) | Hippocampal CA1 cerebral blood flow and volume | MRI: structural and gadolinium | Elevated left CA1 cerebral blood volume at baseline predicts transition to syndromal psychosis and hippocampal atrophy. |
| Howes et al. 2009, 2011, Egerton et al. 2013 (98–100) | Dopamine synthesis capacity in striatum | PET: F-DOPA | Individuals at high risk for psychosis have elevated striatal dopamine, particularly in the associative striatum, and this predicts transition to syndromal psychosis. |
| Stone et al. 2009 (169) | Glutamate levels in anterior cingulate cortex and thalamus and gray matter volume | 1H-MR spectroscopy and structural MRI | Higher glutamine levels in anterior cingulate in high-risk individuals compared with control subjects, but lower glutamate and glutamine (Glx) levels in thalamus than controls; less gray matter volume in orbitofrontal cortex and ventral anterior cingulate cortex and more gray matter volume in left cerebellum and left occipital cortex in high-risk individuals than control subjects. |
| de la Fuente-Sandoval 2011, 2013, 2015 (103–105) | Glutamate levels in associative striatum | 1H-MR spectroscopy | Individuals at high risk for psychosis have elevated levels of glutamatergic metabolites in the associative striatum, and this predicts transition to syndromal psychosis. |
| Bodatsch et al. 2011, Perez et al. 2014 (106, 107) | Mismatch negativity | MMN/EEG | Mismatch negativity is decreased in high-risk individuals and predicts transition to syndromal psychosis |
| Walker et al. 2013 (108) | Salivary cortisol | Salivary cortisol | High-risk individuals have higher salivary cortisol than control subjects, higher cortisol correlates with greater psychopathology, and baseline cortisol predicted conversion to psychosis. |
| Bedi et al. 2015 (109) | Automated speech analysis and machine learning | Automated speech analysis and machine learning | Speech features of individuals at high risk for psychosis are able to predict who will transition to a syndromal psychosis. |
| Anticevic et al. 2015 (110) | Thalamic dysconnectivity | Resting-state MRI | Thalamic dysconnectivity is present in individuals at high risk for psychosis and is most pronounced in individuals who progress to syndromal psychosis. |
| Perkins et al. 2015 (111) | Blood-based biomarkers | Blood-based biomarkers | Measures of inflammation, oxidative stress, and the hypothalamic-pituitary axis may enhance risk prediction. |
| Chung et al. 2018 (112) | MRI-based neuroanatomical maturity | Machine learning and MRI | High-risk individuals have a greater deviance in their neuroanatomical maturity than do healthy control subjects, and this predicted transition to syndromal psychosis among younger individuals. |
| Cassidy et al. 2019 (113) | Neuromelanin sensitive MRI | MRI: neuromelanin | NM-MRI signal is positively correlated with severity of positive symptoms in both schizophrenia and in clinical high-risk individuals. |
| Bossong et al. 2019 (170) | Hippocampal glutamate levels | 1H-MR spectroscopy | High-risk individuals who transition to syndromal psychosis have higher hippocampal glutamate levels than high-risk individuals who do not transition. High-risk individuals who have poor functional outcomes have higher hippocampal glutamate levels than high-risk individuals who have good functional outcomes. |
TABLE 2. Candidate biomarkers of clinical high risk and schizophrenia prodrome
Some of the most promising biomarkers were measures of midbrain and striatal dopamine concentrations, metabolic activity in the (predominantly) left CA1 region of the hippocampus, glutamatergic activity in the associative striatum, decreased mismatch negativity, automated speech analysis, thalamic dysconnectivity, reduced number of sleep spindles, salivary cortisol, blood-based biomarkers (oxidative stress, inflammatory, immune analytes), and neuroanatomical maturity (93, 98–113). However, the ability of even these measures to predict which clinical high-risk individuals would progress to syndromal psychosis was insufficiently reliable to be clinically applied.
Preventive Intervention Studies
The ultimate goal of schizophrenia research is to prevent (or delay) the onset of the illness. Suffice it to say, despite efforts to develop treatments for prevention (Table 3), as well as success developing treatments that can decrease the burden of attenuated symptoms, no treatment has yet been proven effective for prevention of transition to syndromal psychosis. Experimental medicine approaches to develop preventive treatments for schizophrenia is a major emphasis of the NIMH Accelerating Medicines Partnership between government, industry, and academic investigators. These studies are intended to test new chemical entities aimed at specific molecular targets with compelling scientific rationales for putative therapeutic efficacy.
| Study | Intervention | Sample Size (N) | Length of Follow-Up | Finding | Notes |
|---|---|---|---|---|---|
| McGorry et al. 2002 (171) | 6 months of risperidone plus CBT versus needs-based intervention | 59 | 12 months from baseline | Early advantages for conversion to syndromal psychosis did not persist | Single-blind |
| Morrison et al. 2004, 2007 (172, 173) | 6 months of cognitive therapy or treatment as usual | 58 | 12 months from baseline | Cognitive therapy was superior for transition to psychosis and positive symptoms | Advantages for transition to psychosis did not persist at 3 years |
| McGlashan et al. 2006 (174) | 12 months olanzapine versus placebo | 60 | 12 months from baseline | Olanzapine protected against conversion but nonsignificantly | Side effects were problematic and led to attrition |
| Amminger et al. 2010 (175) | 12 weeks of omega-3 polyunsaturated fatty acids or placebo | 81 | 40 weeks | Rates of conversion to syndromal psychosis were lower in the intervention group | |
| Yung et al. 2011, McGorry et al. 2013 (176, 177) | 12 months of cognitive therapy plus risperidone versus supportive therapy versus cognitive therapy | 115 | 12 months from baseline | No significant differences in rates of transition to syndromal psychosis | |
| Addington et al. 2011 (178) | 6 months of CBT or supportive therapy | 51 | Up to 18 months from baseline | No difference in transition rates or symptom change | |
| Bechdolf et al. 2012 (179) | 12 months of integrated psychological intervention (CBT, skills training, cognitive remediation, psychoeducation) or supportive counseling | 128 | 12–24 months from baseline | The integrated psychological intervention was superior to supportive counseling for transition to psychosis or subthreshold psychosis | Patients had “basic symptoms” |
| Van der Gaag et al. 2012 (180) | 6 months of CBT or treatment as usual | 201 | 18 months from baseline | CBT was superior for transition to psychosis and symptom reduction | |
| Morrison et al. 2012 (181) | 6 months of cognitive therapy or monitoring of mental status | 288 | 12–24 months from baseline | No difference in transition rates | |
| Woods et al. 2013 (182) | 12 weeks of glycine or placebo in the placebo-controlled trial; 8 weeks of just glycine in the open trial | 8 in placebo-controlled trial; 10 in open-label trial | Up to 24 weeks from baseline | Glycine treatment led to improvements in SIPS score at 8 weeks | Variable levels of follow-up and intervention after 8 and 12 weeks |
| O’Brien et al. 2014 (183) | Family-focused therapy (18 sessions of psychoeducation, communication training, and problem-solving training) or enhanced care treatment, which consisted of three sessions of psychoeducation | 66 | 6 months from baseline | Individuals in the intervention group demonstrated greater improvements on measures of communication and conflict | Intervention performed with high-risk patients and their parents or significant others |
| McFarlane et al. 2015 (57) | 24 months of family-aided assertive community treatment to early first-episode patients and higher-risk patients, and community care to a lower-risk group | 337 | 24 months from baseline | Family-aided assertive community treatment was superior to community care for positive symptoms | Involved both first-episode and high- and low-risk patients, and interventions were assigned based on risk level |
| Piskulic et al. 2015 (184) | 40 hours over 12 weeks of auditory processing cognitive remediation therapy versus computer games | 32 | Up to 9 months from baseline | Within-group cognitive improvements over time (up to 9 months) | |
| Kantrowitz et al. 2015 (185) | 16 weeks of d-serine or placebo | 35 | 16 weeks from baseline | d-Serine had a beneficial effect on total negative symptoms | Included high-risk patients with negative symptoms |
| Loewy et al. 2016 (186) | 40 hours of auditory processing–based exercises designed to target verbal learning and memory operations or computer games | 83 | 2 months from baseline (40 hours of the intervention) | Auditory processing–based exercises improved verbal memory to a greater degree than did computer games | |
| Stain et al. 2016 (187) | 6 months of CBT or nondirective reflective listening | 57 | 12 months from baseline | Individuals in the nondirective reflective listening group experienced greater decreases in distress associated with subclinical psychotic symptoms | |
| McGorry et al. 2017 (188) | 6 months of omega-3 polyunsaturated fatty acids and cognitive-behavioral case management or placebo and cognitive-behavioral case management | 304 | 6 months from baseline | No differences in rates of transition to syndromal psychosis | |
| Bhattacharyya et al. 2018 (189) | One dose of cannabidiol or placebo | 33 | Cannabidiol resulted in intermediate activation in striatal, medial temporal lobe, and midbrain areas of the brain compared with high-risk individuals who received placebo and healthy control subjects | Used BOLD fMRI |
TABLE 3. Treatment studies of clinical high-risk patientsa
The criteria for considering candidate preventive treatments necessarily differ from those that apply to treatments for the full-blown illness. In the latter case, the diagnosis is already established, symptoms are severe, treatments with proven efficacy and known safety exist, and there is some understanding of the treatments’ mechanism of action and relationship to the disease pathophysiology. For the former, there is uncertainty about the goals of treatment (amelioration of the attenuated and nonspecific symptoms versus prevention or delay of progression to syndromal psychosis), limited awareness of the pathophysiology that putative treatments should target, and a lower threshold for safety and risk of iatrogenic harm.
To meet the challenge of developing treatments for prevention in people who are in their prodromal stage of illness and may not be clearly diagnosable, greater methodologic rigor and a higher standard of proof are required. Future research to validate diagnostic or theragnostic biomarkers and treatment strategies for clinical high-risk and prodromal populations ideally should be guided by scientifically credible pathophysiological theories for which there is substantial supporting evidence.
Pathophysiological Theories to Guide Future Research
There are currently three viable theories with various permutations and many areas of overlap to guide research on biomarker and therapeutics development for early detection and preventive intervention in schizophrenia.
Dopamine Theory
The original dopamine hypothesis, in 1974, postulated that overactivity in neurotransmission from dopamine cell bodies located in the ventral tegmental area of the midbrain to their terminal fields in the nucleus accumbens results in the development of psychotic symptoms (19). The next iteration of this hypothesis came with the work of Pycock et al. (24) and was extended to include both frontal-cortical (mesocortical) and subcortical (mesolimbic) components and demonstrated their reciprocal relationship. Deafferentation of frontal cortical neurons has been shown to result in decreased cortical dopamine activity and increased subcortical dopamine activity. The hypodopaminergic state in the frontal cortical terminal fields of the dopamine neurons, whose cell bodies are located in the ventral tegmental area, was hypothesized to be the basis of the negative symptoms of schizophrenia—avolition, apathy, alogia, and asociality—while hyperactivity of dopamine neurons projecting to the nucleus accumbens and limbic cortex were believed to cause the positive (psychotic) symptoms of the disorder (25, 30, 114, 115).
More recently, PET studies have suggested that the rostral caudate (i.e., associative striatum) may be a pathological locus in schizophrenia (98–100, 116, 117). Abi-Dargham and colleagues (118) have proposed that abnormal brain development leads to disruptions in the connectivity of dopamine pathways to the rostral caudate, including altered differentiation of the associative striatum from the sensorimotor striatum, abnormal patterning of dopamine and other inputs to the associative striatum, abnormal interactions between striatal dopamine and other neurotransmitters, and abnormal cortical inputs to the associative striatum.
Another iteration of the dopamine hypothesis draws from the substance abuse literature. Sensitization is defined as a behavioral, physiological, or cellular response to a stimulus that is augmented as a result of repeated prior exposure to that stimulus (119). This definition is derived initially from behavioral models of learning and refers to the progressive increase in the behavioral effects of psychostimulant drugs (which are indirect dopamine agonists) that are elicited by intermittent drug administration (119). Applying this concept to the dopamine hypothesis postulates that dysregulation in presynaptic trafficking of endogenous dopamine in the mesolimbic pathway mimics the effect of exogenous stimulant administration by releasing excess dopamine, which overstimulates postsynaptic receptors and causes neurochemical sensitization (27–29). As this aberration increases, ideas of reference become delusions and illusions become hallucinations.
Kapur elaborated the model of dopamine hyperactivity and neurochemical sensitization by applying it to the cognitive function of salience (29). Salience refers to a process whereby environmental stimuli and internal thoughts command our attention and drive motivation and behavior. The same mesolimbic dopamine system in which hyperactivity produces psychotic symptoms is believed to be a key component in the attribution of salience, as it is in reward and hedonic perception. In Kapur’s innovative conceptualization of salience as a mechanism in the pathogenesis of schizophrenia, dysregulated dopamine leads to aberrant assignment of salience in which normative stimuli are reified to ideas of reference and illusions and ultimately to delusions and hallucinations.
While the earlier versions of the dopamine hypothesis would suggest dopamine antagonists (i.e., antipsychotics) as treatments, the sensitization and salience versions might predict that while antipsychotics could dampen the symptoms, they would not remove their underlying basis. However, novel agents like NMDA antagonists, which have been shown to block the induction of sensitization (although not its expression once established), could be effective (27).
Synaptic Pruning/Immune Theory
In 1982 Feinberg (120), prompted by the work of Huttenlocher (121), proposed another pathophysiologic hypothesis, postulating that the maturational process of synaptic pruning is excessive in persons who develop schizophrenia. Elimination of extraneous synapses normatively occurs in the human brain during adolescence and into the third decade of life. This late phase of cortical maturation is unique to humans and corresponds to the temporal window of symptom expression in schizophrenia. This theory is consistent with the loss of cortical gray matter without cell death, which is the principal finding of postmortem studies in schizophrenia brains (122).
Keshavan et al. subsequently integrated this pathogenic model with the neurodevelopmental hypothesis (123), and, most recently, genetics studies by Stevens and McCarroll and colleagues provided a mechanistic explanation for how excessive synaptic elimination may occur (124, 125). They found an up-regulation of complement genes (specifically C4) in people with schizophrenia and speculated that this gain of function could cause microglial activation, which could phagocytose synapses. This finding leveraged the work of Shatz, who had previously discovered that major histocompatibility class I genes were integral to the molecular mechanisms of synaptic pruning and circuit formation, in addition to their more commonly understood role in conferring cellular immunity (126). They further observed that C4 was expressed in the gray and white matter, with the greatest concentration of C4-positive neurons and synapses in the hippocampus.
This work provided an important link between the immune system—long suspected to play a role in the pathogenesis of schizophrenia—and the trajectory of brain development and maturation (127). The possibility that neuron-microglia interactions via the complement cascade caused excessive synaptic pruning by activating microglia during adolescence offered a potential mechanism to explain the reasons for age at onset, symptom expression, brain volume change, and synapse loss.
The therapeutic implications of this hypothesis are varied, suggesting potential for compounds that would inhibit complement gene expression, block phagocytosis, or target inflammation. There is also the question of timing of the treatment administration to the critical period of development during which this excessive synaptic elimination is thought to occur.
Glutamate and GABA Theory
The glutamate hypothesis of schizophrenia postulated that dysregulation of glutamatergic chemical neurotransmission causes excessive synaptic concentrations of glutamate by pyramidal cells, which leads to the clinical manifestations of the illness and potential excitatory neurotoxicity (128, 129). Javitt and Zukin proposed this hypothesis in 1991 (128) based on their studies of the psychotomimetic effects of phencyclidine. Their initial focus on NMDA receptor hypofunction was expanded to include the surge in presynaptic glutamate release in response to impeded NMDA signal transduction (129).
An important addition to the glutamate hypothesis came from the laboratories of Lewis (130), Benes (131), and Krystal (132), which consistently found reduced numbers of GABA interneurons in the hippocampus and prefrontal cortex. The reduced perisomatic inhibition of interneurons on target pyramidal cells leads to excessive glutamate release, which in turn could result in further loss of GABA interneurons (130).
The work of Hensch and colleagues provides a possible explanation for the deficit in GABAergic regulation of pyramidal cells releasing glutamate involving the erosion of perineural nets surrounding GABA interneurons (133). Perineuronal nets are extracellular matrix chondroitin sulfate proteoglycan–containing structures that surround the soma and dendrites of various mammalian neuronal cell types, including parvalbumin staining basket cells, the class of fast-spiking interneurons that regulate the activity of pyramidal cells. Perineuronal nets are important for the onset and closure of the critical periods for developmental plasticity extending into the rodent equivalent of adolescence and early adulthood (134). These researchers found that when they induced oxidative stress in newborn mice, which disrupted the perineuronal nets that enveloped vulnerable GABA interneurons, they failed to provide normal inhibitory input to target pyramidal cells, leading to excess glutamatergic function. Antioxidant agents and glutamate modulating agents reversed this extended window of vulnerability and restored integrity to the protective perineuronal nets, normalizing GABAergic inhibition and glutamate levels (135).
Finally, the previously described studies of Shatz, Stevens, and McCarroll and colleagues (124–126) provide another mechanistic explanation for the dysregulation of glutamate and GABA. The toxic gain of function in C4 genes that was found to be expressed in neuronal cell bodies, dendrites, and synapses observed in the hippocampus can reduce the inhibitory control of GABA-secreting interneurons on glutamate-releasing pyramidal cells (126). The consequent glutamatergic dysregulation could cause additional GABA cell loss and further disinhibition of pyramidal cells.
In a parallel line of clinical investigation, Small and colleagues (93) demonstrated in humans that neuronal hyperactivity in the CA1 region of the hippocampus is driven by excessive glutamate and constitutes an early locus of pathology in schizophrenia and precedes the development of atrophy and progression to syndromal psychosis in clinical high-risk individuals. The persistence of dysregulated glutamate activity enables the pathophysiology to spread to the subiculum and other subfields of the hippocampus, and externally to the striatum and frontal cortex, eventually producing reduction of cellular matrix, reflected as atrophy (136).
It should be noted that proposals integrating dopamine and glutamate systems have also been described (137). An accelerator-brake model was proposed by Carlsson et al. (138) connecting excess cortical glutamatergic activity to striatal dopamine release. The model predicts that if striatal dopamine release is enhanced, feedback regulation will increase the activity of the brake to counteract dopamine release. If the brake fails because of a (hypothetical) NMDA receptor deficiency in schizophrenia, the release of striatal dopamine will be enhanced—a prediction that was demonstrated with neuroreceptor imaging of dopamine release in humans (139). More recently, a preclinical model connecting hippocampal glutamate hyperactivity to increased striatal dopamine function has been developed by Lodge and Grace (140) that explains the coexisting disturbances in these neurotransmitter systems in individuals with schizophrenia and presumably those at clinical high risk.
A Unified Model
A unified pathophysiological model can be derived from these different but interconnected theories that incorporates the principles of altered neurodevelopment, including the fine tuning of neural circuits through synaptic elimination, and disruption of the excitatory/inhibitory balance between glutamate and GABA systems that in turn leads to symptom expression and disease progression. The key components of this model follow (Figure 3).
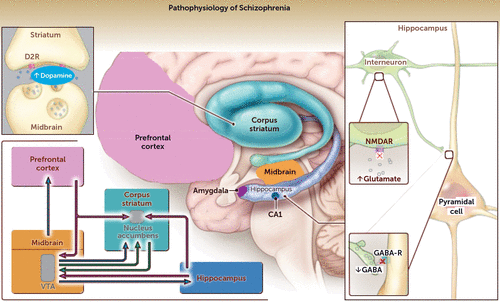
FIGURE 3. Pathophysiological model of psychotic disorders: a schematic diagram illustrating the various permutations of the dopamine and glutamate hypothesesa
a A sagittal view of the brain through the midline depicts the hippocampus, midbrain, corpus striatum, and prefrontal cortex, all regions that are implicated in the pathophysiology of psychotic symptoms in schizophrenia. The neurotransmitters involved include dopamine (blue arrows), glutamate (purple arrows), and γ-aminobutyric acid (GABA) (green arrows). The symptoms of schizophrenia are believed to arise from the dysregulation of pyramidal cells that release glutamate onto cells located in and projecting from the CA1 region of the hippocampus caused by deficits in GABAergic interneurons and hypofunctioning N-methyl-d-aspartate glutamate receptors (NMDARs) (the red X denotes reduced neurotransmitter and receptor hypofunction). Shown in the enlargement of the hippocampus at right is a glutamate-expressing pyramidal cell and a GABA interneuron. There is underactivation of diminished numbers of interneurons because of hypofunctioning NMDARs. Also shown is the interneuron axon forming a synapse with the apical dendrite of the pyramidal cell. Because of its understimulation, due to a hypofunctioning NMDAR, less GABA is released by the interneuron, which in turn disinhibits the pyramidal cell and causes it to release more glutamate from hippocampal projections to the midbrain (ventral tegmental area [VTA]) and the corpus striatum (nucleus accumbens). Hippocampal overactivity augments dopamine release in the striatum either directly (at the level of the nucleus accumbens) or by stimulation of midbrain dopamine neurons, which project to the nucleus accumbens and the prefrontal cortex. Midbrain dopamine neurons further promulgate the dysregulation of dopamine and glutamate through a projection back to the hippocampus. D2R denotes D2 receptor. (From Lieberman JA, First MB, Psychotic disorders, New England Journal of Medicine, volume 379, p. 275. Copyright 2018, Massachusetts Medical Society. Reprinted with permission.)
Susceptibility genes (141), including MHC class I genes in the hypervariability region of chromosome 6, instigate aberrations in brain development and cell-circuit connectivity that create vulnerability to neural dysfunction, which is mediated by excessive synaptic elimination and/or erosion of perineuronal nets leading to the loss of interneurons that alters the GABAergic inhibitory control of glutamate neurotransmission. While these genetic factors may affect brain development diffusely, the initial (or an early) site of dysfunction is the CA1 region of the hippocampus. This assumption is based on the principle of regional vulnerability, which stipulates that no matter how complex a disorder’s clinical phenomenology and diffuse its pathology, it must begin somewhere and therefore initially emerges in one brain region (142). In schizophrenia, the region is the hippocampal formation (as also is the case in Alzheimer’s disease), which contains a circuit comprising anatomically discrete but interconnected subregions with distinct cytoarchitecture and connectivity (143). Because the subregions are interconnected, when one is targeted it can spread to other subregions regions within and external to the hippocampus.
Studies have localized early dysfunction in schizophrenia to the CA1 hippocampal subregion (93, 144). This local disturbance in CA1 hyperactivity, caused by increased glutamate, is transmitted through monosynaptic input to the ventral striatum, leading to increased dopamine release (143). This in turn stimulates symptom expression and ultimately leads to CA1 volume reduction and spread of dysfunction to other brain regions (93).
This formulation of extant theories approximates a comprehensive pathophysiological model that includes a neurodevelopmental diathesis and disease progression components. To verify, modify, or disprove this model, it is important to study patients from their earliest stages, ideally in their prodromal stage or even at premorbid stages. Presumably, these theoretical models will be useful in guiding research to validate diagnostic and theragnostic biomarkers and treatments for preventive interventions.
Conclusions
The possibility of disease modification and prevention in schizophrenia is no longer a fantasy but a realistic goal. What stands between us and achieving this milestone in mental health care is sensitive and specific diagnostic criteria for the prodromal stage of schizophrenia, the validation of biomarkers, and proof of effectiveness of therapeutic agents. Imaging (MRI, PET), electrophysiologic (event-related potentials, gamma band, delta wave sleep), and serologic (oxidative stress, inflammatory, immune analytes) measures show promise as diagnostic or theragnostic markers either individually or in combination as part of a profile. These must be validated in studies with large patient samples and rigorous and reliable biomarker methodology robust enough to be applied across multiple sites and, ideally, practical enough to be easily implemented and readily acquired in community settings. Candidate compounds for experimental medication trials must target molecular substrates integral to the aforementioned pathophysiologic models. Despite recent failures, glutamate neurotransmission remains the most promising target among currently viable candidates. In this context, mGluR partial agonists, allosteric NMDA modulators, d-amino acid oxidase inhibitors, and N-acetylcysteine warrant consideration for testing. Other targets of interest include muscarinic cholinergic agonists, trace amine-associated receptor agonists, phosphodiesterase inhibitors, anti-inflammatory agents, immune system inhibitors, and antioxidant neuroprotectants (145). In vetting therapeutic agents for testing in clinical high-risk samples, it is important to carefully select biomarkers that most clearly reflect target engagement in order to accurately determine the dose and confirm the mechanism of action to avoid failed studies and misleading results.
These caveats notwithstanding, we can reasonably expect future studies to build on the research of the past four decades to advance our knowledge and ultimately enable this game-changing model of care to become a reality.
1 : Ten Years That Changed the Face of Mental Illness. London, Martin Dunitz, 1999Google Scholar
2 : Maintenance therapy and the natural course of schizophrenia. J Clin Psychiatry 1985; 46:18–21Medline, Google Scholar
3 : Treatment of schizophrenia. Schizophr Bull 1987; 13:133–156Crossref, Medline, Google Scholar
4 : The history of the psychopharmacology of schizophrenia. Can J Psychiatry 1997; 42:152–162Crossref, Medline, Google Scholar
5 : Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J Intern Med 2014; 275:251–283Crossref, Medline, Google Scholar
6 : Parkinson’s disease: treatment. Pharm J 2000; 264:476–479Google Scholar
7 : First-episode psychosis, part I: editors’ introduction. Schizophr Bull 1992; 18:177–178Crossref, Medline, Google Scholar
8 : A selective review of recent North American long-term follow-up studies of schizophrenia. Schizophr Bull 1988; 14:515–542Crossref, Medline, Google Scholar
9 : Neuroleptics and the natural course of schizophrenia. Schizophr Bull 1991; 17:325–351Crossref, Medline, Google Scholar
10 : Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry 2005; 162:1785–1804Link, Google Scholar
11 : Duration of psychosis and outcome in first-episode schizophrenia. Am J Psychiatry 1992; 149:1183–1188Link, Google Scholar
12 : Outcome study of first-episode psychosis, I: relapse rates after 1 year. Am J Psychiatry 1986; 143:1155–1158Link, Google Scholar
13 : Ten-year course of schizophrenia: the Madras longitudinal study. Acta Psychiatr Scand 1994; 90:329–336Crossref, Medline, Google Scholar
14 : Time course and biologic correlates of treatment response in first-episode schizophrenia. Arch Gen Psychiatry 1993; 50:369–376Crossref, Medline, Google Scholar
15 : Ueber primäre chronische Demenz (so. Dementia praecox) im jugendlichen Alter. Prager medicinische Wochenschrift 1891; 16:312–315Google Scholar
16 : Dementia Praecox and Paraphrenia. Barclay RM, Robertson GM (trans). New York, RE Krieger, 1919Google Scholar
17 : Schizophrenia: clinical section, in A History of Clinical Psychiatry: The Origin and History of Psychiatric Disorders. Edited by Berrios GE, Porter R. London, Athlone Press, 1995Google Scholar
18 : Dementia Praecox, or The Group of Schizophrenias. New York, International Universities Press, 1950Google Scholar
19 : Drugs, neurotransmitters, and schizophrenia. Science 1974; 184:1243–1253Crossref, Medline, Google Scholar
20 : Social predictors of psychotic experiences: specificity and psychological mechanisms. Schizophr Bull 2008; 34:1012–1020Crossref, Medline, Google Scholar
21 : Sanity, Madness, and the Family. London, Tavistock, 1964Google Scholar
22 : Immune system and schizophrenia. Curr Immunol Rev 2010; 6:213–220Crossref, Medline, Google Scholar
23 : Toward a theory of schizophrenia. Behav Sci 1956; 1:251–264Crossref, Google Scholar
24 : Effect of lesion of cortical dopamine terminals on subcortical dopamine receptors in rats. Nature 1980; 286:74–76Crossref, Medline, Google Scholar
25 : The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 1988; 1:179–186Crossref, Medline, Google Scholar
26 : Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 1991; 148:1474–1486Link, Google Scholar
27 : Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology 1997; 17:205–229Crossref, Medline, Google Scholar
28 : Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol 1999; 13:358–371Crossref, Medline, Google Scholar
29 : Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry 2003; 160:13–23Link, Google Scholar
30 : The dopamine hypothesis of schizophrenia: version III: the final common pathway. Schizophr Bull 2009; 35:549–562Crossref, Medline, Google Scholar
31 : Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Crossref, Medline, Google Scholar
32 : A neurodevelopmental approach to the classification of schizophrenia. Schizophr Bull 1992; 18:319–332Crossref, Medline, Google Scholar
33 : Advancing a neurodevelopmental origin for schizophrenia. Arch Gen Psychiatry 1993; 50:224–227Crossref, Medline, Google Scholar
34 : Two syndromes in schizophrenia and their pathogenesis, in Schizophrenia as a Brain Disease. Edited by Henn FA, Nasrallah HA. New York, Oxford University Press, 1982, pp 196–234Google Scholar
35 : The longitudinal stability of cognitive impairment in schizophrenia: Mini-Mental State scores at one- and two-year follow-ups in geriatric in-patients. Br J Psychiatry 1995; 166:630–633Crossref, Medline, Google Scholar
36 : Schizophrenia: a follow-up study of the results of five forms of treatment. Arch Gen Psychiatry 1981; 38:776–784Crossref, Medline, Google Scholar
37 : A prospective follow-up study of brain morphology and cognition in first-episode schizophrenic patients: preliminary findings. Biol Psychiatry 1995; 38:349–360Crossref, Medline, Google Scholar
38 : Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry 2002; 59:1002–1010Crossref, Medline, Google Scholar
39 : Psychosis and brain volume changes during the first five years of schizophrenia. Eur Neuropsychopharmacol 2009; 19:147–151Crossref, Medline, Google Scholar
40 : Brain volume changes in the first year of illness and 5-year outcome of schizophrenia. Br J Psychiatry 2006; 189:381–382Crossref, Medline, Google Scholar
41 : Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 2011; 68:128–137Crossref, Medline, Google Scholar
42 : Impact on intracortical myelination trajectory of long acting injection versus oral risperidone in first-episode schizophrenia. Schizophr Res 2012; 140:122–128Crossref, Medline, Google Scholar
43 : Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry 2005; 62:361–370Crossref, Medline, Google Scholar
44 : [Sex differences in schizophrenia]. Gynakologe 1995; 28:426–433 (German)Medline, Google Scholar
45 : Issues for DSM-V: clinical staging: a heuristic pathway to valid nosology and safer, more effective treatment in psychiatry. Am J Psychiatry 2007; 164:859–860Link, Google Scholar
46 : The arrival of preemptive psychiatry. Early Interv Psychiatry 2007; 1:5–6Crossref, Medline, Google Scholar
47 : Early detection and intervention in schizophrenia: a new therapeutic model. JAMA 2013; 310:689–690Crossref, Medline, Google Scholar
48 : Prevention of negative symptom psychopathologies in first-episode schizophrenia: two-year effects of reducing the duration of untreated psychosis. Arch Gen Psychiatry 2008; 65:634–640Crossref, Medline, Google Scholar
49 : Reducing the duration of untreated first-episode psychosis: effects on clinical presentation. Arch Gen Psychiatry 2004; 61:143–150Crossref, Medline, Google Scholar
50 : Implementing coordinated specialty care for early psychosis: the RAISE Connection program. Psychiatr Serv 2015; 66:691–698Link, Google Scholar
51 : Practical monitoring of treatment fidelity: examples from a team-based intervention for people with early psychosis. Psychiatr Serv 2015; 66:674–676Link, Google Scholar
52 : The RAISE Connection program for early psychosis: secondary outcomes and mediators and moderators of improvement. J Nerv Ment Dis 2015; 203:365–371Crossref, Medline, Google Scholar
53 : The RAISE Connection program: psychopharmacological treatment of people with a first episode of schizophrenia. Psychiatr Serv 2016; 67:1300–1306Link, Google Scholar
54 : Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry 2016; 173:362–372Link, Google Scholar
55 : Incomes and outcomes: social security disability benefits in first-episode psychosis. Am J Psychiatry 2017; 174:886–894Link, Google Scholar
56 : How successful are first episode programs? A review of the evidence for specialized assertive early intervention. Curr Opin Psychiatry 2014; 27:167–172Crossref, Medline, Google Scholar
57 : Clinical and functional outcomes after 2 years in the early detection and intervention for the prevention of psychosis multisite effectiveness trial. Schizophr Bull 2015; 41:30–43Crossref, Medline, Google Scholar
58 : OnTrackNY: the development of a coordinated specialty care program for individuals experiencing early psychosis. Psychiatr Serv 2017; 68:318–320Link, Google Scholar
59 : Results of a coordinated specialty care program for early psychosis and predictors of outcomes. Psychiatr Serv 2018; 69:863–870Link, Google Scholar
60 : Headspace: Australia’s innovation in youth mental health: who are the clients and why are they presenting? Med J Aust 2014; 200:108–111Crossref, Medline, Google Scholar
61 : Early Psychosis Prevention and Intervention Centre long-term follow-up study of first-episode psychosis: methodology and baseline characteristics. Early Interv Psychiatry 2007; 1:49–60Crossref, Medline, Google Scholar
62 : From early intervention in psychosis to youth mental health reform: a review of the evolution and transformation of mental health services for young people. Soc Psychiatry Psychiatr Epidemiol 2016; 51:319–326Crossref, Medline, Google Scholar
63 : Youth engagement with an emerging Irish mental health early intervention programme (Jigsaw): participant characteristics and implications for service delivery. J Ment Health 2015; 24:283–288Crossref, Medline, Google Scholar
64 : Canadian response to need for transformation of youth mental health services: ACCESS Open Minds (Esprits ouverts). Early Interv Psychiatry 2019; 13:697–706Crossref, Medline, Google Scholar
65 : Schizophrenia as a Human Process. New York, WW Norton, 1962Google Scholar
66 : Early intervention for first episodes of schizophrenia: a preliminary exploration. Psychiatry 1992; 55:4–15Crossref, Medline, Google Scholar
67 : Schizophrenia in twins: 16 years’ consecutive admissions to a psychiatric clinic. Br J Psychiatry 1966; 112:809–818Crossref, Medline, Google Scholar
68 : A polygenic theory of schizophrenia. Proc Natl Acad Sci USA 1967; 58:199–205Crossref, Medline, Google Scholar
69 : The New York High-Risk Project: a follow-up report. Schizophr Bull 1987; 13:451–461Crossref, Medline, Google Scholar
70 : The Copenhagen High-Risk Project, 1962–86. Schizophr Bull 1987; 13:485–495Crossref, Medline, Google Scholar
71 : Israeli High Risk Study: editor’s introduction. Schizophr Bull 1995; 21:179–182Crossref, Medline, Google Scholar
72 : People at risk of schizophrenia: sample characteristics of the first 100 cases in the Edinburgh High-Risk Study. Br J Psychiatry 1999; 174:547–553Crossref, Medline, Google Scholar
73 : IRAOS: an instrument for the assessment of onset and early course of schizophrenia. Schizophr Res 1992; 6:209–223Crossref, Medline, Google Scholar
74 : The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull 1996; 22:353–370Crossref, Medline, Google Scholar
75 : Symptom assessment in schizophrenic prodromal states. Psychiatr Q 1999; 70:273–287Crossref, Medline, Google Scholar
76 : Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry 2005; 39:964–971Crossref, Medline, Google Scholar
77 : North American Prodrome Longitudinal Study: a collaborative multisite approach to prodromal schizophrenia research. Schizophr Bull 2007; 33:665–672Crossref, Medline, Google Scholar
78 : Prediction models of functional outcomes for individuals in the clinical high-risk state for psychosis or with recent-onset depression: a multimodal, multisite machine learning analysis. JAMA Psychiatry 2018; 75:1156–1172Crossref, Google Scholar
79 : Towards precision medicine in psychosis: benefits and challenges of multimodal multicenter studies: PSYSCAN: translating neuroimaging findings from research into clinical practice. Schizophr Bull 2019;
80 : The European Prediction of Psychosis Study (EPOS): integrating early recognition and intervention in Europe. World Psychiatry 2005; 4:161–167Medline, Google Scholar
81 : Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European Prediction of Psychosis Study. Arch Gen Psychiatry 2010; 67:241–251Crossref, Medline, Google Scholar
82 : Baseline demographics, clinical features, and predictors of conversion among 200 individuals in a longitudinal prospective psychosis-risk cohort. Psychol Med 2017; 47:1923–1935Crossref, Medline, Google Scholar
83 : North American Prodrome Longitudinal Study (NAPLS 2): the prodromal symptoms. J Nerv Ment Dis 2015; 203:328–335Crossref, Medline, Google Scholar
84 : Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry 2008; 65:28–37Crossref, Medline, Google Scholar
85 : A predictive model for conversion to psychosis in clinical high-risk patients. Psychol Med 2019; 49:1128–1137Crossref, Medline, Google Scholar
86 : Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP project. Am J Psychiatry 2016; 173:989–996Link, Google Scholar
87 : An individualized risk calculator for research in prodromal psychosis. Am J Psychiatry 2016; 173:980–988Link, Google Scholar
88 : Development and validation of a clinically based risk calculator for the transdiagnostic prediction of psychosis. JAMA Psychiatry 2017; 74:493–500Crossref, Medline, Google Scholar
89 : Should attenuated psychosis syndrome be included in DSM-5? Lancet 2012; 379:591–592Crossref, Medline, Google Scholar
90 : Cell loss in the hippocampus of schizophrenics. Eur Arch Psychiatry Neurol Sci 1986; 236:154–161Crossref, Medline, Google Scholar
91 : Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J Neural Transm (Vienna) 1986; 65:303–326Crossref, Medline, Google Scholar
92 : A neurohistological correlate of schizophrenia. Biol Psychiatry 1984; 19:1601–1621Medline, Google Scholar
93 : Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 2013; 78:81–93Crossref, Medline, Google Scholar
94 : Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res 1990; 35:1–13Crossref, Medline, Google Scholar
95 : Hippocampal volume in first-episode psychoses and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Arch Gen Psychiatry 1999; 56:133–141Crossref, Medline, Google Scholar
96 : Temporal lobe volume changes in people at high risk of schizophrenia with psychotic symptoms. Br J Psychiatry 2002; 181:138–143Crossref, Medline, Google Scholar
97 : Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry 1998; 155:1384–1391Link, Google Scholar
98 : Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry 2013; 74:106–112Crossref, Medline, Google Scholar
99 : Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry 2009; 66:13–20Crossref, Medline, Google Scholar
100 : Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry 2011; 168:1311–1317Link, Google Scholar
101 : Reduced Sleep spindles in schizophrenia: a treatable endophenotype that links risk genes to impaired cognition? Biol Psychiatry 2016; 80:599–608Crossref, Medline, Google Scholar
102 : Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry 2009; 66:938–946Crossref, Medline, Google Scholar
103 : Striatal glutamate and the conversion to psychosis: a prospective 1H-MRS imaging study. Int J Neuropsychopharmacol 2013; 16:471–475Crossref, Medline, Google Scholar
104 : Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology 2011; 36:1781–1791Crossref, Medline, Google Scholar
105 : Cortico-striatal GABAergic and glutamatergic dysregulations in subjects at ultra-high risk for psychosis investigated with proton magnetic resonance spectroscopy. Int J Neuropsychopharmacol 2015; 19:
106 : Prediction of psychosis by mismatch negativity. Biol Psychiatry 2011; 69:959–966Crossref, Medline, Google Scholar
107 : Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol Psychiatry 2014; 75:459–469Crossref, Medline, Google Scholar
108 : Cortisol levels and risk for psychosis: initial findings from the North American Prodrome Longitudinal Study. Biol Psychiatry 2013; 74:410–417Crossref, Medline, Google Scholar
109 : Automated analysis of free speech predicts psychosis onset in high-risk youths. NPJ Schizophr 2015; 1:15030Crossref, Medline, Google Scholar
110 : Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry 2015; 72:882–891Crossref, Medline, Google Scholar
111 : Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: preliminary results from the NAPLS project. Schizophr Bull 2015; 41:419–428Crossref, Medline, Google Scholar
112 : Use of machine learning to determine deviance in neuroanatomical maturity associated with future psychosis in youths at clinically high risk. JAMA Psychiatry 2018; 75:960–968Crossref, Medline, Google Scholar
113 : Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proc Natl Acad Sci USA 2019; 116:5108–5117Crossref, Medline, Google Scholar
114 : Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience 1991; 41:1–24Crossref, Medline, Google Scholar
115 : Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci 2016; 17:524–532Crossref, Medline, Google Scholar
116 : Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry 2010; 67:231–239Crossref, Medline, Google Scholar
117 : Stress-induced dopamine response in subjects at clinical high risk for schizophrenia with and without concurrent cannabis use. Neuropsychopharmacology 2014; 39:1479–1489Crossref, Medline, Google Scholar
118 : Pathway-specific dopamine abnormalities in schizophrenia. Biol Psychiatry 2017; 81:31–42Crossref, Medline, Google Scholar
119 Kalivas PW: Development and expression of behavioral sensitization: temporal profile of changes in gene expression, in Molecular Biology of Drug Addiction. Edited by Maldonado R. Totowa, NJ, Humana Press, 2003, pp 161–169Google Scholar
120 : Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res 1982–1983; 17:319–334Crossref, Medline, Google Scholar
121 : Synaptic density in human frontal cortex: developmental changes and effects of aging. Brain Res 1979; 163:195–205Crossref, Medline, Google Scholar
122 : The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 1999; 45:17–25Crossref, Medline, Google Scholar
123 : Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res 1994; 28:239–265Crossref, Medline, Google Scholar
124 : Schizophrenia risk from complex variation of complement component 4. Nature 2016; 530:177–183Crossref, Medline, Google Scholar
125 : Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012; 74:691–705Crossref, Medline, Google Scholar
126 : MHC class I: an unexpected role in neuronal plasticity. Neuron 2009; 64:40–45Crossref, Medline, Google Scholar
127 : Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 2004; 101:8174–8179Crossref, Medline, Google Scholar
128 : Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 1991; 148:1301–1308Link, Google Scholar
129 : From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 2012; 37:4–15Crossref, Medline, Google Scholar
130 : Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 2005; 6:312–324Crossref, Medline, Google Scholar
131 : GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 2001; 25:1–27Crossref, Medline, Google Scholar
132 : Impaired tuning of neural ensembles and the pathophysiology of schizophrenia: a translational and computational neuroscience perspective. Biol Psychiatry 2017; 81:874–885Crossref, Medline, Google Scholar
133 : Critical period plasticity in local cortical circuits. Nat Rev Neurosci 2005; 6:877–888Crossref, Medline, Google Scholar
134 : The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci 2019; 20:451–465Crossref, Medline, Google Scholar
135 : Targeting oxidative stress and aberrant critical period plasticity in the developmental trajectory to schizophrenia. Schizophr Bull 2015; 41:835–846Crossref, Medline, Google Scholar
136 : Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry 2018; 23:1764–1772Crossref, Medline, Google Scholar
137 : Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry 2005; 62:985–994Crossref, Medline, Google Scholar
138 : Neurotransmitter interactions in schizophrenia: therapeutic implications. Biol Psychiatry 1999; 46:1388–1395Crossref, Medline, Google Scholar
139 : Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry 2000; 48:627–640Crossref, Medline, Google Scholar
140 : Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci 2011; 32:507–513Crossref, Medline, Google Scholar
141 : Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511:421–427Crossref, Medline, Google Scholar
142 : Isolating pathogenic mechanisms embedded within the hippocampal circuit through regional vulnerability. Neuron 2014; 84:32–39Crossref, Medline, Google Scholar
143 : A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 2011; 12:585–601Crossref, Medline, Google Scholar
144 : Progression from selective to general involvement of hippocampal subfields in schizophrenia. Mol Psychiatry 2017; 22:142–152Crossref, Medline, Google Scholar
145 National Collaborating Centre for Mental Health (UK): Section 5: Preventing psychosis and schizophrenia: treatment of at risk mental states, in Psychosis and Schizophrenia in Adults: Treatment and Management, Updated Edition (NICE Clinical Guidelines, No 178). London, National Collaborating Centre for Mental Health, 2014Google Scholar
146 : Chlorpromazine: new inhibiting agent for psychomotor excitement and manic states. AMA Arch Neurol Psychiatry 1954; 71:227–237Crossref, Medline, Google Scholar
147 : Neuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. J Clin Exp Psychopathol 1955; 16:104–112Medline, Google Scholar
148 : Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science 1975; 188:1217–1219Crossref, Medline, Google Scholar
149 : The dopamine hypothesis of schizophrenia: a review. Schizophr Bull 1976; 2:19–76Crossref, Medline, Google Scholar
150 : Pneumoencephalographische und psychopathologische bilder bei endogen psychosen. Berlin, Springer, 1957Crossref, Google Scholar
151 : Pneumoencephalographic evidence of brain atrophy in acute and chronic schizophrenic patients. Acta Psychiatr Scand 1982; 66:374–383Crossref, Medline, Google Scholar
152 : Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet 1976; 2:924–926Crossref, Medline, Google Scholar
153 : Lateral cerebral ventricular enlargement in chronic schizophrenia. Arch Gen Psychiatry 1979; 36:735–739Crossref, Medline, Google Scholar
154 : Flexible system for the diagnosis of schizophrenia: report from the WHO International Pilot Study of Schizophrenia. Science 1973; 182:1275–1278Crossref, Medline, Google Scholar
155 : Negative v positive schizophrenia: definition and validation. Arch Gen Psychiatry 1982; 39:789–794Crossref, Medline, Google Scholar
156 : Developmental processes in schizophrenic disorders: longitudinal studies of vulnerability and stress. Schizophr Bull 1992; 18:387–425Crossref, Medline, Google Scholar
157 : Symptom and functional outcomes for a 5 year early intervention program for psychoses. Schizophr Res 2011; 129:111–115Crossref, Medline, Google Scholar
158 : Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res 1997; 74:129–140Crossref, Medline, Google Scholar
159 : Is schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspective. Biol Psychiatry 1999; 46:729–739Crossref, Medline, Google Scholar
160 : Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 1994; 51:199–214Crossref, Medline, Google Scholar
161 : Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 1995; 52:998–1007Crossref, Medline, Google Scholar
162 : The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry 2001; 158:1367–1377Link, Google Scholar
163 : Qualitative assessment of brain morphology in acute and chronic schizophrenia. Am J Psychiatry 1992; 149:784–794Link, Google Scholar
164 : Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry 2001; 49:487–499Crossref, Medline, Google Scholar
165 : A follow-up magnetic resonance imaging study of schizophrenia: relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry 1998; 55:145–152Crossref, Medline, Google Scholar
166 : Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry 2011; 70:672–679Crossref, Medline, Google Scholar
167 : Pre-onset detection and intervention research in schizophrenia psychoses: current estimates of benefit and risk. Schizophr Bull 2001; 27:563–570Crossref, Medline, Google Scholar
168 : Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry 2007; 164:483–492Link, Google Scholar
169 : Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol Psychiatry 2009; 66:533–539Crossref, Medline, Google Scholar
170 : Association of hippocampal glutamate levels with adverse outcomes in individuals at clinical high risk for psychosis. JAMA Psychiatry 2019; 76:199–207Crossref, Medline, Google Scholar
171 : Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry 2002; 59:921–928Crossref, Medline, Google Scholar
172 : Three-year follow-up of a randomized controlled trial of cognitive therapy for the prevention of psychosis in people at ultrahigh risk. Schizophr Bull 2007; 33:682–687Crossref, Medline, Google Scholar
173 : Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. Br J Psychiatry 2004; 185:291–297Crossref, Medline, Google Scholar
174 : Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry 2006; 163:790–799Link, Google Scholar
175 : Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry 2010; 67:146–154Crossref, Medline, Google Scholar
176 : Randomized controlled trial of interventions for young people at ultra-high risk of psychosis: twelve-month outcome. J Clin Psychiatry 2013; 74:349–356Crossref, Medline, Google Scholar
177 : Randomized controlled trial of interventions for young people at ultra high risk for psychosis: 6-month analysis. J Clin Psychiatry 2011; 72:430–440Crossref, Medline, Google Scholar
178 : A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophr Res 2011; 125:54–61Crossref, Medline, Google Scholar
179 : Preventing progression to first-episode psychosis in early initial prodromal states. Br J Psychiatry 2012; 200:22–29Crossref, Medline, Google Scholar
180 : Cognitive behavioral therapy for subjects at ultrahigh risk for developing psychosis: a randomized controlled clinical trial. Schizophr Bull 2012; 38:1180–1188Crossref, Medline, Google Scholar
181 : Early detection and intervention evaluation for people at risk of psychosis: multisite randomised controlled trial. BMJ 2012; 344:
182 : Glycine treatment of the risk syndrome for psychosis: report of two pilot studies. Eur Neuropsychopharmacol 2013; 23:931–940Crossref, Medline, Google Scholar
183 : A randomized trial of family focused therapy with populations at clinical high risk for psychosis: effects on interactional behavior. J Consult Clin Psychol 2014; 82:90–101Crossref, Medline, Google Scholar
184 : Pilot study of cognitive remediation therapy on cognition in young people at clinical high risk of psychosis. Psychiatry Res 2015; 225:93–98Crossref, Medline, Google Scholar
185 : D-serine for the treatment of negative symptoms in individuals at clinical high risk of schizophrenia: a pilot, double-blind, placebo-controlled, randomised parallel group mechanistic proof-of-concept trial. Lancet Psychiatry 2015; 2:403–412Crossref, Medline, Google Scholar
186 : Intensive auditory cognitive training improves verbal memory in adolescents and young adults at clinical high risk for psychosis. Schizophr Bull 2016; 42(suppl 1):S118–S126Crossref, Medline, Google Scholar
187 : A randomised controlled trial of cognitive behaviour therapy versus non-directive reflective listening for young people at ultra high risk of developing psychosis: the detection and evaluation of psychological therapy (DEPTh) trial. Schizophr Res 2016; 176:212–219Crossref, Medline, Google Scholar
188 : Effect of ω-3 polyunsaturated fatty acids in young people at ultrahigh risk for psychotic disorders: the NEURAPRO randomized clinical trial. JAMA Psychiatry 2017; 74:19–27Crossref, Medline, Google Scholar
189 : Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry 2018; 75:1107–1117Crossref, Medline, Google Scholar
190 : Criteria for subtyping schizophrenia: clinical differentiation of hebephrenic and paranoid schizophrenia. Arch Gen Psychiatry 1974; 31:43–47Crossref, Medline, Google Scholar
191 : The evolution of symptoms in institutionalized hebephrenic/catatonic schizophrenics. Br J Psychiatry 1982; 141:567–572Crossref, Medline, Google Scholar



