Association of Elevated Reward Prediction Error Response With Weight Gain in Adolescent Anorexia Nervosa
Abstract
Objective:
Anorexia nervosa is a psychiatric disorder of unknown etiology. Understanding associations between behavior and neurobiology is important in treatment development. Using a novel monetary reward task during functional magnetic resonance brain imaging, the authors tested how brain reward learning in adolescent anorexia nervosa changes with weight restoration.
Method:
Female adolescents with anorexia nervosa (N=21; mean age, 16.4 years [SD=1.9]) underwent functional MRI (fMRI) before and after treatment; similarly, healthy female control adolescents (N=21; mean age, 15.2 years [SD=2.4]) underwent fMRI on two occasions. Brain function was tested using the reward prediction error construct, a computational model for reward receipt and omission related to motivation and neural dopamine responsiveness.
Results:
Compared with the control group, the anorexia nervosa group exhibited greater brain response 1) for prediction error regression within the caudate, ventral caudate/nucleus accumbens, and anterior and posterior insula, 2) to unexpected reward receipt in the anterior and posterior insula, and 3) to unexpected reward omission in the caudate body. Prediction error and unexpected reward omission response tended to normalize with treatment, while unexpected reward receipt response remained significantly elevated. Greater caudate prediction error response when underweight was associated with lower weight gain during treatment. Punishment sensitivity correlated positively with ventral caudate prediction error response.
Conclusions:
Reward system responsiveness is elevated in adolescent anorexia nervosa when underweight and after weight restoration. Heightened prediction error activity in brain reward regions may represent a phenotype of adolescent anorexia nervosa that does not respond well to treatment. Prediction error response could be a neurobiological marker of illness severity that can indicate individual treatment needs.
Anorexia nervosa is an eating disorder that primarily affects young females and is associated with high mortality (1). The diagnostic criteria include restriction of energy intake that leads to significantly low body weight and an intense fear of gaining weight or becoming fat (2). The etiology of anorexia nervosa is complex, and only recently have we begun to better understand its underlying neurobiology.
Brain imaging studies in anorexia nervosa have implicated central reward circuits that take part in the control of food intake (3–5). For instance, structural and functional differences in the insula have been found between patients with anorexia nervosa and healthy subjects (6, 7). Prefrontal and striatal responses to monetary reward are also altered in adults ill with or recovered from anorexia nervosa (8, 9). In adolescent anorexia nervosa, heightened posterior caudate response to monetary losses was associated with altered reward learning (10). These studies provide evidence for altered reward system function in anorexia nervosa. However, neurotransmitter-based hypotheses, which are key to developing pharmacological interventions, are largely lacking.
Dopamine mediates reward learning (11) and has been implicated in the pathophysiology of anorexia nervosa (3, 12, 13). Midbrain dopaminergic neurons exhibit a phasic burst when an unexpected reward is received (positive prediction error). They will then shift the signal to the onset of a conditioned stimulus that they have learned predicts reward receipt (11). A negative prediction error (a dip in dopamine neuron activity) is evoked when the predicted stimulus association is violated (unexpected reward omission, negative prediction error). The prediction error can be modeled in a temporal difference, or reinforcement learning, algorithm. In previous studies, we found increased ventral striatum, insula, and prefrontal cortex response in adult anorexia nervosa (11, 14, 15).
We applied this model in a novel monetary reward paradigm, modeled after the taste paradigm (14). Specifically, we aimed to study dopamine-related brain responses independent from food stimuli in adolescent anorexia nervosa. Animal studies show that food restriction or weight loss enhances dopamine response to rewards (16), and we expected heightened brain activity in underweight adolescent anorexia nervosa. Using a longitudinal design, we tested whether group differences would normalize with weight restoration. We expected that dopamine-model-related activation would show incomplete normalization with weight restoration. We further expected that greater brain response using the prediction error model would predict poor recovery, indicating a more severe illness and suggesting that dopamine function could be a treatment target in adolescent anorexia nervosa.
Method
Participants
Twenty-one female adolescents diagnosed with anorexia nervosa (age range, 13–20 years) and 21 healthy comparison female adolescents (age range, 11–20 years) participated (Table 1). The anorexia nervosa group was recruited from partial hospitalization programs, where closely supervised meal plans mitigated acute starvation or dehydration effects. Healthy control participants were recruited through local advertisements. All participants in the anorexia nervosa group were diagnosed with restricting type, except for one with binge/purge type. Each participant underwent functional MRI (fMRI) twice: individuals with anorexia nervosa before weight restoration and at discharge to a lower level of care, and healthy control subjects during the early follicular phase, two menstrual cycles apart, to reduce sex hormone effects on brain reward function. Participants with anorexia nervosa were without menstrual cycle. For participants age 18 or older, the Structured Clinical Interview for DSM-5 (2) was administered by a doctoral-level interviewer (four in the anorexia nervosa group, two in the healthy control group). Those under age 18 completed the Mini-International Neuropsychiatric Interview (17). All participants were right-handed and had no history of head trauma, neurological disease, major medical illness, psychosis, or substance use disorders. Two participants in the healthy control group and four in the anorexia nervosa group took oral contraceptives. In the anorexia nervosa group, 10 individuals at scan 1 and 12 at scan 2 took antidepressants, and two at scan 1 and five at scan 2 took atypical antipsychotics. The Colorado Multiple Institutional Review Board approved the study. Participants who were age 18 and older, and the parents of those under age 18, provided written informed consent.
| Scan 1 | Scan 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Healthy Control Group (N=21) | Anorexia Nervosa Group (N=21) | Healthy Control Group (N=21) | Anorexia Nervosa Group (N=21) | ||||||||
| Mean | SD | Mean | SD | t | p | Mean | SD | Mean | SD | t | p | |
| Age (years) | 15.20 | 2.37 | 16.39 | 1.95 | 1.78 | <0.083 | 15.36 | 2.34 | 16.51 | 1.96 | 1.74 | <0.090 |
| BMIa | 20.41 | 2.40 | 16.42 | 1.02 | –7.011 | <0.001 | 20.57 | 2.41 | 18.66 | 1.08 | –3.31 | <0.003 |
| Age-adjusted BMI percentile | 51.74 | 24.34 | 4.90 | 6.40 | –8.53 | <0.001 | 53.92 | 24.47 | 24.66 | 13.43 | –4.80 | <0.001 |
| Drive for thinnessb | 1.48 | 2.11 | 19.67 | 5.22 | 14.81 | <0.001 | 1.75 | 3.13 | 17.19 | 9.97 | 8.24 | <0.001 |
| Body dissatisfactionb | 2.29 | 3.12 | 25.10 | 9.93 | 10.04 | <0.001 | 2.70 | 4.50 | 24.76 | 11.52 | 8.15 | <0.001 |
| Punishment sensitivityc | 4.29 | 4.35 | 11.20 | 3.78 | 5.49 | <0.001 | 4.48 | 3.63 | 10.19 | 4.20 | 4.72 | <0.001 |
| Reward sensitivityc | 6.67 | 4.62 | 7.57 | 3.80 | 0.692 | <0.493 | 6.81 | 4.40 | 8.33 | 4.18 | 1.15 | <0.257 |
| State anxietyd | 28.67 | 6.54 | 47.33 | 15.41 | 5.11 | <0.001 | 28.67 | 10.69 | 48.57 | 13.53 | 5.29 | <0.001 |
| Trait anxietyd | 28.86 | 7.57 | 52.05 | 11.71 | 7.62 | <0.001 | 27.71 | 3.57 | 50.57 | 12.36 | 8.14 | <0.001 |
| Harm avoidancee | 11.00 | 5.77 | 19.95 | 6.40 | 4.76 | <0.001 | 10.86 | 5.04 | 19.24 | 6.90 | 4.50 | <0.001 |
| Reward dependencee | 15.86 | 4.22 | 14.76 | 3.28 | –0.938 | <0.354 | 15.52 | 4.18 | 15.00 | 3.35 | –0.45 | <0.656 |
| Breakfast calories | 606.76 | 101.17 | 591.43 | 141.66 | –0.404 | <0.689 | 604.21 | 123.35 | 638.61 | 138.31 | 0.80 | <0.431 |
| Days between scans | 56.95 | 13.10 | 42.29 | 14.90 | –3.39 | <0.002 | ||||||
| N | % | N | % | |||||||||
| Antidepressant use | 10 | 47.6 | 12 | 57.1 | ||||||||
| Antipsychotic use | 2 | 9.5 | 5 | 23.8 | ||||||||
| Mood disorder | 7 | 33.3 | 7 | 33.3 | ||||||||
| Anxiety disorder | 12 | 57.1 | 12 | 57.1 | ||||||||
TABLE 1. Demographic and Behavioral Variables for Participants in a Study of Reward Prediction Error Response With Weight Gain in Adolescent Anorexia Nervosa
Self-Assessments
Participants completed the Eating Disorder Inventory–3 (18), the Revised Sensitivity to Punishment and Reward Questionnaire (19), the State-Trait Anxiety Inventory (20), and the Temperament and Character Inventory (21).
Monetary Reward Task
During fMRI, participants received three monetary unconditioned stimuli (US): win (100 trials, 25 cents each), no-win (100 trials), or neutral (80 trials). Participants learned to associate unique visual conditioned stimuli (CS, geometric shapes) with each US (see Figure S1 in the data supplement that accompanies the online edition of this article) (15). Fixing the first 10 trials as CS-win followed by US-win established an initial association. All subsequent trials were fully randomized and CS was probabilistically associated with its corresponding US: CS-win was followed in 20% of trials by no-win (unexpected reward omission condition), and CS-no win was followed in 20% of trials by win (unexpected reward receipt condition).
fMRI Image Acquisition
Between 7:00 and 8:00 a.m. on the study day, the anorexia nervosa group ate their meal plan breakfast and healthy controls ate a breakfast that was quality- and calorie-matched to the average anorexia nervosa group breakfast (Table 1). Brain imaging was performed between 8:00 and 9:00 a.m. on a 3-T GE scanner, with a three-plane scout scan (16 seconds), sagittally acquired, spoiled gradient sequence T1-weighted (168 slices, thickness=1.2 mm, TI=450 ms, TR=10 ms, TE=3 ms, flip angle=10°, FOV=22 cm, scan matrix=256×256) (see Figure S2 in the online data supplement), and T2*-weighted echo planar imaging scans for blood-oxygen-level-dependent (BOLD) functional activity during task performance (3.4×3.4×4 mm voxels, TR=2.1 seconds, TE=30 ms, flip angle=70°, 28 axial slices, thickness=2.6 mm, gap=1.4 mm).
fMRI Analysis
Image preprocessing and analysis were performed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Participants’ images were realigned to the first volume, normalized to the Montreal Neurological Institute template, and smoothed at 6 mm full width at half maximum Gaussian kernel. Data were modeled with a hemodynamic response function-convolved boxcar function using the general linear model, including temporal and dispersion derivatives, and autoregression. A 128-second high-pass filter was applied for low-frequency BOLD signal fluctuations. Data were preprocessed with slice time correction. Motion parameters were applied as regressors in the first-level analysis. We extracted mean parameter estimates across all voxels within predefined anatomical regions of interest (http://marsbar.sourceforge.net/) in order to avoid problems from small-volume-corrected peak voxel statistics or violation of normal distribution. We explored standard a priori bilateral (22) reward circuitry regions of interest (using the Automated Anatomical Labeling Atlas [23]): the dorsal anterior insula, ventral anterior insula, posterior insula, caudate body, caudate head, ventral caudate/nucleus accumbens, substantia nigra, inferior orbitofrontal cortex, medial orbitofrontal cortex, and middle orbitofrontal cortex (Figure 1).
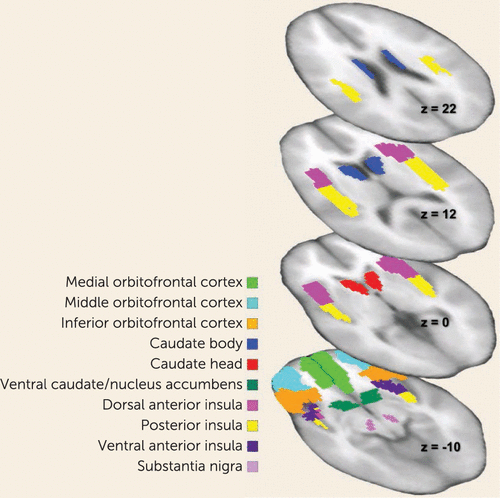
FIGURE 1. Reward Circuit Regions of Interest in a Study of Reward Prediction Error Response With Weight Gain in Adolescent Anorexia Nervosaa
a Horizontal slices depict anatomical regions of interest involved in reward processing explored in this study.
Temporal difference reinforcement learning analysis.
Each participant’s prediction error signal was modeled based on trial sequence to test reinforcement learning model-related brain response and regressed with brain activation across all trials (11, 14, 15). In brief, the predicted reward value (V) at any time (t) within a trial was calculated as a linear product of weights (wi) and the presence of a conditioned visual stimulus (CS) at time t, coded in a stimulus representation vector xi(t). The predicted stimulus value at time t is updated by comparing the predicted value at time t+1 to that actually observed at time t, leading to the prediction error δ(t) (see the online data supplement for a full description).
Group-by-condition analysis.
First-level contrast images were analyzed using general linear models for voxel response as a function of stimulus condition: expected receipt, unexpected receipt, expected omission, unexpected omission, and expected neutral condition. Three contrasts of interest were computed: 1) CS no-win followed by unexpected win, contrasted against CS no-win followed by no win (unexpected receipt); 2) CS win followed by unexpected US no-win, contrasted against CS win followed by expected win (unexpected omission); 3) CS win contrasted against CS no-win (win-expectation) (14).
Statistical Analysis
Behavioral data and extracted brain activation parameter estimates were analyzed with SPSS, version 23 (IBM, Armonk, N.Y.).
Extracted region-of-interest parameter estimates were tested for normality with the Shapiro-Wilk test and rank-transformed. We conducted a mixed analysis of covariance for each condition that included all 20 regions of interest, group, and scan day (including age, antipsychotic and antidepressant use, mood disorders, and anxiety disorders as covariates). We used the tests of within-subject effects to determine significant group-by-scan interactions. The tests for between-subject effects evaluated significant group or scan effects using Bonferroni-corrected pairwise comparisons.
Pearson’s correlation analysis was used to test behavior-brain response relationships for age, body mass index (BMI), treatment duration (number of days between scans), harm avoidance, reward and punishment sensitivity, and state and trait anxiety. Significant correlations were corrected for multiple comparisons (false discovery rate) (24) and verified using bootstrap procedures (1,000 samples, 95% confidence intervals).
Results
Demographic and Behavioral Data
The anorexia nervosa group had a significantly lower mean BMI and scored significantly higher on eating pathology and anxiety than the healthy control group (Table 1). Punishment sensitivity was significantly higher in the anorexia nervosa group, but reward sensitivity was not (see Figure S3 in the online data supplement). The healthy control group had no significant difference in BMI across scans but significantly more days between scans than the anorexia nervosa group. The mean BMI in the anorexia nervosa group significantly increased from first to second scan (p<0.001).
Brain-Imaging Results
No significant group-by-scan effects were observed for any of the contrasts (Table 2; see also Table S1 in the data supplement).
| Scan 1 | Scan 2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy Control Group (N=21) | Anorexia Nervosa Group (N=21) | ANCOVAa | Healthy Control Group (N=21) | Anorexia Nervosa Group (N=21) | ANCOVAa | Repeated-Measures Group Effect | ||||||||
| Condition and Region of Interest | Mean | SD | Mean | SD | F | p | Mean | SD | Mean | SD | F | p | F | p |
| Prediction error | ||||||||||||||
| Right caudate body | 19.00 | 12.79 | 24.00 | 11.48 | 6.89 | 0.013 | 19.05 | 13.24 | 23.95 | 10.98 | 3.59 | 0.066 | 6.54 | 0.015 |
| Left caudate body | 19.24 | 12.48 | 23.76 | 11.92 | 5.32 | 0.027 | 19.24 | 12.12 | 23.76 | 12.28 | 3.68 | 0.063 | 7.78 | 0.009 |
| Right caudate head | 18.24 | 12.70 | 24.76 | 11.17 | 5.94 | 0.020 | 19.10 | 13.59 | 23.90 | 10.56 | 0.85 | 0.363 | 4.54 | 0.040 |
| Right dorsal anterior insula | 19.33 | 12.10 | 23.67 | 12.34 | 8.41 | 0.006 | 19.57 | 11.51 | 23.43 | 12.97 | 3.16 | 0.084 | 7.85 | 0.008 |
| Right posterior insula | 19.00 | 12.89 | 24.00 | 11.37 | 5.36 | 0.027 | 19.19 | 13.66 | 23.81 | 10.52 | 2.52 | 0.121 | 4.44 | 0.042 |
| Left posterior insula | 20.19 | 12.43 | 22.81 | 12.27 | 9.02 | 0.005 | 19.38 | 13.66 | 23.62 | 10.61 | 1.85 | 0.183 | 5.08 | 0.031 |
| Right ventral anterior insula | 20.38 | 12.57 | 22.62 | 12.16 | 4.76 | 0.036 | 19.05 | 11.06 | 23.95 | 13.17 | 2.99 | 0.093 | 5.08 | 0.031 |
| Right ventral caudate/nucleus accumbens | 18.10 | 12.72 | 24.90 | 11.06 | 6.94 | 0.012 | 19.57 | 13.07 | 23.43 | 11.40 | 1.26 | 0.270 | 6.63 | 0.014 |
| Expectation | ||||||||||||||
| Right posterior insula | 18.10 | 12.34 | 24.90 | 11.49 | 3.62 | 0.065 | 18.67 | 13.17 | 24.33 | 10.87 | 2.51 | 0.122 | 6.24 | 0.017 |
| Unexpected omission | ||||||||||||||
| Left caudate body | 17.90 | 11.53 | 25.10 | 12.19 | 4.91 | 0.033 | 17.90 | 11.50 | 25.10 | 12.21 | 3.31 | 0.077 | 6.77 | 0.014 |
| Unexpected receipt | ||||||||||||||
| Right dorsal anterior insula | 18.43 | 12.29 | 24.57 | 11.73 | 5.90 | 0.020 | 18.86 | 12.17 | 24.14 | 12.07 | 7.35 | 0.010 | 10.19 | 0.003 |
| Left dorsal anterior insula | 22.33 | 11.01 | 20.67 | 13.64 | 1.35 | 0.253 | 19.10 | 11.36 | 23.90 | 12.93 | 5.67 | 0.023 | 4.48 | 0.042 |
| Right posterior insula | 17.33 | 11.66 | 25.67 | 11.66 | 5.17 | 0.029 | 20.81 | 11.99 | 22.19 | 12.79 | 1.50 | 0.299 | 4.68 | 0.037 |
| Right ventral anterior insula | 20.10 | 12.30 | 22.90 | 12.37 | 1.95 | 0.172 | 18.95 | 11.80 | 24.05 | 12.48 | 4.19 | 0.048 | 4.39 | 0.044 |
TABLE 2. Parameter Estimates by Task Condition Across Scans and Groups in a Study of Reward Prediction Error Response With Weight Gain in Adolescent Anorexia Nervosa
Temporal difference reinforcement learning analysis.
There was a significant main effect of group for prediction error regression weights in the left and right caudate body, the right caudate head, the right ventral caudate/nucleus accumbens, the right dorsal anterior, ventral anterior, and posterior insula, and the left posterior insula (Figure 2A). Post hoc tests indicated significantly greater activity in the anorexia nervosa group when underweight compared with the healthy control group. There was a significant left posterior insula scan effect (p<0.020, ηp2=0.15).
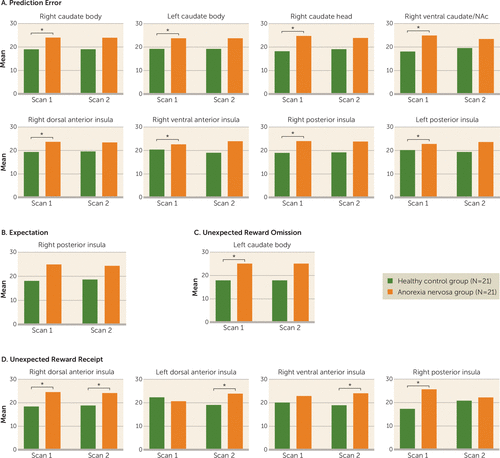
FIGURE 2. Brain Response by Task Condition Across Scans and Groups in a Study of Reward Prediction Error Response With Weight Gain in Adolescent Anorexia Nervosaa
a Panel A depicts computational model results indicating greater prediction error regression in the anorexia nervosa group in the caudate, insula, and striatum. Panels B–D depict greater response in the anorexia nervosa group to expectation, unexpected reward omission, and unexpected reward receipt in insular regions. In all comparisons, there was a significant effect of group in repeated measures. An asterisk indicates a significant (p<0.05) effect of group within scan.
Group-by-condition analysis.
Reward expectation analysis showed a significant main effect of group for the right posterior insula (Figure 2B).
Unexpected reward omission analysis showed a significant group main effect in the left caudate body (Figure 2C), with greater activity in the anorexia nervosa group compared with the control group at scan 1.
Unexpected reward receipt showed a significant group main effect (Figure 2D) in the left and right dorsal anterior insula and the right ventral anterior and posterior insula. Post hoc analysis showed greater posterior insula activity in the anorexia nervosa group when underweight. Left dorsal and right ventral anterior insula activity was greater in the anorexia nervosa group at scan 2. Right dorsal anterior insula activity was significantly greater in the anorexia nervosa group at both scans.
Brain-Imaging Response and Demographic and Behavioral Correlation Results
BMI change in the anorexia nervosa group was significantly negatively correlated with underweight middle orbitofrontal cortex response to reward expectation (Figure 3A). Right caudate head prediction error regression weights were significantly negatively correlated with discharge BMI (Figure 3D). Sensitivity to punishment in the anorexia nervosa group was significantly positively correlated with underweight prediction error regression weights in the left and right ventral caudate/nucleus accumbens (Figure 3B). Harm avoidance in the anorexia nervosa group was significantly positively correlated with left ventral caudate/nucleus accumbens prediction error regression. In an additional partial correlation analysis controlling for harm avoidance, caudate/nucleus accumbens prediction error and punishment sensitivity continued to be significantly related (p<0.020); however, there was no significant correlation between caudate/nucleus accumbens prediction error and harm avoidance when controlling for punishment sensitivity. In the anorexia nervosa group, treatment duration (number of days between scans) was significantly positively correlated with scan 1 prediction error regression weights in the right substantia nigra (Figure 3C). After treatment, the anorexia nervosa group’s sensitivity to reward was significantly positively correlated with left dorsal anterior insula response to reward expectation (r=0.6, R2=0.36, p<0.040).
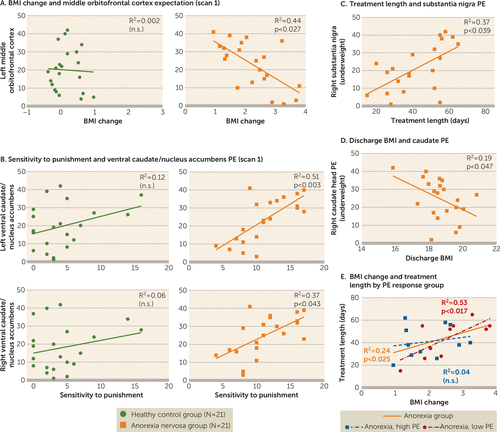
FIGURE 3. Brain-Behavior Correlations in a Study of Reward Prediction Error Response With Weight Gain in Adolescent Anorexia Nervosaa
a Panel A depicts the negative correlation between body mass index (BMI) change and left middle orbitofrontal cortex expectation response in underweight anorexia nervosa. Panel B depicts the positive correlation between sensitivity to punishment and bilateral ventral caudate/nucleus accumbens prediction error (PE) regression in underweight anorexia nervosa. Panel C depicts the positive correlation between treatment duration and right substantia nigra prediction error in underweight anorexia nervosa. Panel D depicts the negative correlation between discharge BMI and right caudate head prediction error in underweight anorexia nervosa. Panel E depicts the positive correlations between change in BMI and treatment duration across the anorexia nervosa group and in those with low prediction error or high prediction error in the right caudate head based on a median split. n.s.=not significant.
Relationship Between BMI Change and Time in Treatment
As expected, time in anorexia nervosa treatment (in days) was positively correlated with weight gain (r=0.489; p<0.025). We explored whether high or low prediction error signaling at scan 1 differentially predicted rate of weight gain (Figure 3E). The high-low split was conducted in two ways: a low-prediction-error group with N=10 or N=11, and a high-prediction-error group with N=11 or N=10, respectively, to account for the uneven subject number. Correlation results between BMI change and time in treatment for split 1 are presented in Figure 3E; for split 2, R2 was 0.48 (p<0.017) for the low-prediction-error group and 0.02 (n.s) for the high-prediction-error group. BMI at scan 1 was not significantly different between the high (mean=16.4, SD=1.1) and low (mean=16.5, SD=1.0) prediction error response groups (p<0.9), and the high and low prediction error response groups could not have been differentiated by initial BMI. BMI at discharge tended to be lower in the high-prediction-error group (mean=18.3, SD=1.2) than in the low-prediction-error group (mean=19.1, SD=0.8), and BMI change was lower in the high-prediction-error group (mean=1.97, SD=0.8) than in the low-prediction-error group (mean=2.53, SD=0.9). Although differences were not significant (p<0.172 and p<0.126, respectively), medium to large effect sizes (ηp2=0.10 and ηp2=0.12, respectively) support a group difference in a larger sample.
Discussion
This study yielded three main findings. First, brain reward circuit responses were elevated in adolescents with anorexia nervosa in the caudate and insula during a reward learning paradigm that used a computational model for dopamine-related prediction error response. Caudate prediction error response was related to rate of weight gain during treatment and could be a neurobiological marker of illness severity to predict individual treatment needs. Second, after weight restoration, elevated prediction error responses in the striatum and insula tended to normalize but were still elevated. This result aligns with animal models showing that underweight is associated with increased dopamine-related reward system responsiveness that only partially recovers with weight restoration (25). Third, dorsal and ventral anterior insula activations to unexpected monetary receipt were also greater at discharge in the anorexia nervosa group. Those additional results support the insula’s involvement in adolescent anorexia nervosa psychopathology even after weight restoration (26). It remains to be seen whether these patients process positive salient stimuli differently after weeks of treatment, or whether long-term effects of low body weight on insula function during unexpected receipt of salient stimuli becomes exaggerated with weight restoration.
This monetary reinforcement learning model revealed, in adolescent anorexia nervosa, elevated striatal and insular activity (27) comparable to prediction error taste reward results in adults (14). The caudate and nucleus accumbens are known to respond to salient stimuli (28) and encode prediction error signals during stimulus reward learning, which may suggest altered dopamine functioning in anorexia nervosa (29). The insula contains the primary taste cortex and integrates body perception signals, but it also contributes to cognitive control and tracks error (30–32). Insula prediction error signaling has been associated with flexible behavior control, and heightened response could alter reversal learning in adolescent anorexia nervosa. This may be especially relevant when an individual must reverse maintained behavior response to anxiety-provoking food cues. Whether dopaminergic neurons contributed to greater insula signaling in anorexia nervosa is unclear, but it is likely that other neurotransmitter signaling was also involved (32, 33).
Brain response in the middle orbitofrontal cortex during reward expectation before treatment was related to weight change during anorexia nervosa treatment. Higher activation was associated with lower BMI increase, although the monetary task did not reveal group differences in orbitofrontal cortex activation. Activation in this brain region during a food cue task has been associated with a personal sense of lack of control and thoughts of guilt, as well as control over salient stimuli (34, 35). It is possible that high response during reward stimulus expectation triggers thoughts of guilt and strengthens control over or resistance to approach of salient stimuli. Furthermore, in the anorexia nervosa group, the higher the caudate prediction error values were at the beginning of treatment, the lower the BMI was at discharge. This suggests that more severely altered brain function is reflective of more severe illness and worse outcome. A further examination of this relationship indicated that number of days in treatment predicted BMI change only in individuals in the anorexia nervosa group with relatively low prediction error signaling. In other words, individuals in the anorexia nervosa group with prediction error activity closer to that of healthy controls predictably gained weight, about 1 BMI point every 20 days; however, this did not apply to individuals with initially high prediction error brain activation. This suggests that individuals with more severely dysfunctional reward systems do not respond in the same way to the treatment regimen with therapist and family-based meal support. This finding has important implications for developing neurobiological markers of illness severity that can predict treatment needs on an individual basis. It may indicate a need to develop alternative or additional approaches for the high brain response group.
Sensitivity to punishment was elevated in adolescent anorexia nervosa, and it was positively correlated with ventral caudate/nucleus accumbens prediction error signaling when underweight, but not at discharge. Bischoff-Grethe et al. (10) found that the posterior striatum was more sensitive to loss during a guessing game in adolescents with anorexia nervosa compared with healthy subjects, which may be in support of our finding. It should be noted, however, that our novel monetary task required participants to learn associations to predict outcomes, rather than make guesses. Elevated prediction error response could reflect high dopamine-neuronal activation in anorexia nervosa, which may drive high punishment sensitivity, especially when underweight. Harm avoidance also is typically elevated in anorexia nervosa. We further hypothesize that harm avoidance could be a functional response to excessively high sensitivity to negative salient stimuli (punishment), and thus reflects an attempt to avoid such negative experiences. In time, harm avoidance may become a learned and self-reinforcing behavior that becomes independent from weight status and high punishment sensitivity.
Interestingly, reward sensitivity scores were higher in the anorexia nervosa group but not significantly different between groups, which is in contrast to some studies but in accord with others (36). This finding might suggest a more flexible sensitivity to reward in adolescent compared with adult anorexia nervosa, but adolescents with anorexia nervosa may nevertheless find negative salient stimuli difficult to tolerate as they navigate recovery. On the other hand, mean values for reward sensitivity in the anorexia nervosa group were comparable to those in our previous study, and the lack of significant group differences could be an effect of sample size (37). We also found a pattern of reward expectation responses in the insula, striatum, and orbitofrontal cortex positively correlating with punishment and reward sensitivity in anorexia nervosa, although it was not significant after multiple comparison corrections. This was primarily the case for punishment sensitivity at scan 1, but with reward sensitivity at scan 2 (see Table S4 in the data supplement). Whether there is a balance between brain reward response and sensitivity to salient stimuli that shifts from punishment to reward during weight restoration is a direction of our ongoing studies.
The results of our study must be interpreted in light of its limitations. fMRI does not directly measure brain dopamine signaling. However, the well-studied behavior of dopaminergic neurons that is modeled in our computational analysis suggests altered dopamine-related reward processing in the brain in adolescent anorexia nervosa. The mechanism for the elevated prediction error response is uncertain and requires further study, but it may occur through up-regulation of dopamine D1 or D2 receptor function (38). However, nondopaminergic neurons also play a role (33). Another potential limitation was the group difference in the time between scans. Variability in anorexia nervosa treatment duration is a challenge in studying this patient population, and treatment duration in the anorexia nervosa group did not exactly match the requirement of two menstrual cycles between scans to study healthy controls at a low estrogen state. However, a significant increase in mean BMI was still achieved in the anorexia nervosa group in this period. Although another limitation is that our patient sample used various medications and had various comorbid disorders, this group reflects a typical clinical sample, and we did account for these variables in our analyses. Our main findings held when the one patient with binge/purge type anorexia nervosa was excluded from the analyses (see Table S2 in the data supplement) and when individuals taking antipsychotics were excluded (see Table S3 in the data supplement).
In summary, this study suggests that reward learning in general, and independently of primary taste reward, is important for our overall understanding of the neurobiology of adolescent anorexia nervosa. Generalized sensitization of brain reward responsiveness could be a result of food restriction and may last long into recovery, consistent with basic research (16, 25). Whether individuals with anorexia nervosa have a genetic predisposition for such a sensitization requires further study. Furthermore, our results suggest that elevated prediction error response in the caudate is a marker for illness severity and predicts weight gain in a highly structured treatment program. The mechanism for such a relationship is unclear. However, starvation-induced altered dopamine receptor expression could cause such a phenomenon (39). Alternatively, metabolic dopamine-related factors could be involved, or high prediction error may characterize a cognitively severe form of adolescent anorexia nervosa that is more “resistant” to treatment. Future studies should aim to elucidate these mechanisms and how elevated dopamine brain response even after weight restoration could be a risk factor for relapse, which is common in the disorder. The answer would have important implications for treatment development. Specifically, reducing high dopamine-related brain response could be a valuable treatment target (13). To explore this relationship, future studies should include longer-term follow-up measures. Behaviorally, sensitivity to punishment could be related to relapse, as anorexia nervosa behaviors are often described as “safe and predictable.” Whether our above-discussed hypothesis that heightened dopamine-related response triggers high sensitivity to punishment, and that high harm avoidance becomes an adaptive behavior that persists even after weight normalization, remains to be further tested. A comprehensive understanding of the role of other neurotransmitters, such as serotonin, in these mechanisms is also needed (40). Despite those limitations and the need for replication, this study provides hope that there are biological markers for adolescent anorexia nervosa that could be used in estimating treatment success as well as in developing pharmacological interventions.
1 : Mortality in eating disorders: results of a large prospective clinical longitudinal study. Int J Eat Disord 2016; 49:391–401Crossref, Medline, Google Scholar
2 : Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5). Washington, DC, American Psychiatric Association, 2013Crossref, Google Scholar
3 : A reward-centred model of anorexia nervosa: a focussed narrative review of the neurological and psychophysiological literature. Neurosci Biobehav Rev 2015; 52:131–152Crossref, Medline, Google Scholar
4 : Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol 2000; 84:3072–3077Crossref, Medline, Google Scholar
5 : Altered brain reward circuits in eating disorders: chicken or egg? Curr Psychiatry Rep 2013; 15:396Crossref, Medline, Google Scholar
6 : Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry 2013; 170:1152–1160Link, Google Scholar
7 : Altered insula response to sweet taste processing after recovery from anorexia and bulimia nervosa. Am J Psychiatry 2013; 170:1143–1151Link, Google Scholar
8 : On weight and waiting: delay discounting in anorexia nervosa pretreatment and posttreatment. Biol Psychiatry 2015; 78:606–614Crossref, Medline, Google Scholar
9 : Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry 2007; 164:1842–1849Link, Google Scholar
10 : Altered brain response to reward and punishment in adolescents with anorexia nervosa. Psychiatry Res 2013; 214:331–340Crossref, Medline, Google Scholar
11 : A neural substrate of prediction and reward. Science 1997; 275:1593–1599Crossref, Medline, Google Scholar
12 : Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biol Psychiatry 2005; 58:908–912Crossref, Medline, Google Scholar
13 : Could dopamine agonists aid in drug development for anorexia nervosa? Front Nutr 2014; 1:19Crossref, Medline, Google Scholar
14 : Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology 2012; 37:2031–2046Crossref, Medline, Google Scholar
15 : Temporal difference models and reward-related learning in the human brain. Neuron 2003; 38:329–337Crossref, Medline, Google Scholar
16 : Chronic food restriction: enhancing effects on drug reward and striatal cell signaling. Physiol Behav 2007; 91:459–472Crossref, Medline, Google Scholar
17 : Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). J Clin Psychiatry 2010; 71:313–326Crossref, Medline, Google Scholar
18 : The Eating Disorder Inventory–3: Professional Manual. Lutz, Fla, Psychological Assessment Resources, 2004Google Scholar
19 : The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Pers Individ Dif 2001; 31:837–862Crossref, Google Scholar
20 : State-Trait Anxiety Inventory for Adults. Palo Alto, Calif, Consulting Psychologists Press, 1983Google Scholar
21 : The Temperament and Character Inventory (TCI): A Guide to Its Development and Use. St Louis, Mo, Washington University, Center for Psychobiology of Personality, 1994Google Scholar
22 : Greater insula white matter fiber connectivity in women recovered from anorexia nervosa. Neuropsychopharmacology 2016; 41:498–507Crossref, Medline, Google Scholar
23 : Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15:273–289Crossref, Medline, Google Scholar
24 : Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995; 57:289–300Google Scholar
25 : Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav 2002; 76:353–364Crossref, Medline, Google Scholar
26 : Anorexia nervosa and the insula. Med Hypotheses 2011; 76:353–357Crossref, Medline, Google Scholar
27 : Prediction error in reinforcement learning: a meta-analysis of neuroimaging studies. Neurosci Biobehav Rev 2013; 37:1297–1310Crossref, Medline, Google Scholar
28 : Human striatal responses to monetary reward depend on saliency. Neuron 2004; 42:509–517Crossref, Medline, Google Scholar
29 : A selective role for dopamine in stimulus-reward learning. Nature 2011; 469:53–57Crossref, Medline, Google Scholar
30 : Reversal learning strategy in adolescence is associated with prefrontal cortex activation. Eur J Neurosci (Epub ahead of print, Sept 15, 2016)Google Scholar
31 : Functional connectivity of the insula in the resting brain. Neuroimage 2011; 55:8–23Crossref, Medline, Google Scholar
32 : Error awareness and the insula: links to neurological and psychiatric diseases. Front Hum Neurosci 2013; 7:14Crossref, Medline, Google Scholar
33 : Neuronal reward and decision signals: from theories to data. Physiol Rev 2015; 95:853–951Crossref, Medline, Google Scholar
34 : The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci 2012; 15:358–366Crossref, Medline, Google Scholar
35 : Neural signature of the Food Craving Questionnaire (FCQ)–Trait. Appetite 2016; 107:303–310Crossref, Medline, Google Scholar
36 : Are extremes of consumption in eating disorders related to an altered balance between reward and inhibition? Front Behav Neurosci 2014; 8:410Crossref, Medline, Google Scholar
37 : Heightened sensitivity to reward and punishment in anorexia nervosa. Int J Eat Disord 2011; 44:317–324Crossref, Medline, Google Scholar
38 : Reward-potentiating effects of D-1 dopamine receptor agonist and AMPAR GluR1 antagonist in nucleus accumbens shell and their modulation by food restriction. Psychopharmacology (Berl) 2009; 202:731–743Crossref, Medline, Google Scholar
39 : Role of prenatal undernutrition in the expression of serotonin, dopamine, and leptin receptors in adult mice: implications of food intake. Mol Med Rep 2014; 9:407–412Crossref, Medline, Google Scholar
40 : A network model of basal ganglia for understanding the roles of dopamine and serotonin in reward-punishment-risk based decision making. Front Comput Neurosci 2015; 9:76Crossref, Medline, Google Scholar



