Childhood Maltreatment and Psychopathology: A Case for Ecophenotypic Variants as Clinically and Neurobiologically Distinct Subtypes
Abstract
Objective
Childhood maltreatment increases risk for psychopathology. For some highly prevalent disorders (major depression, substance abuse, anxiety disorders, and posttraumatic stress disorder) a substantial subset of individuals have a history of maltreatment and a substantial subset do not. The authors examined the evidence to assess whether those with a history of maltreatment represent a clinically and biologically distinct subtype.
Method
The authors reviewed the literature on maltreatment as a risk factor for these disorders and on the clinical differences between individuals with and without a history of maltreatment who share the same diagnoses. Neurobiological findings in maltreated individuals were reviewed and compared with findings reported for these disorders.
Results
Maltreated individuals with depressive, anxiety, and substance use disorders have an earlier age at onset, greater symptom severity, more comorbidity, a greater risk for suicide, and poorer treatment response than nonmaltreated individuals with the same diagnoses. Imaging findings associated with these disorders, such as reduced hippocampal volume and amygdala hyperreactivity, are more consistently observed in maltreated individuals and may represent a maltreatment-related risk factor. Maltreated individuals also differ from others as a result of epigenetic modifications and genetic polymorphisms that interact with experience to increase risk for psychopathology.
Conclusions
Phenotypic expression of psychopathology may be strongly influenced by exposure to maltreatment, leading to a constellation of ecophenotypes. While these ecophenotypes fit within conventional diagnostic boundaries, they likely represent distinct subtypes. Recognition of this distinction may be essential in determining the biological bases of these disorders. Treatment guidelines and algorithms may be enhanced if maltreated and nonmaltreated individuals with the same diagnostic labels are differentiated.
Maltreated children are more likely to suffer psychiatric disorders over the course of their lifetime. In particular, they are more likely to develop major depression (1–5), bipolar disorder (6), anxiety disorders (2, 3, 7), posttraumatic stress disorder (PTSD) (2, 3), substance abuse (2, 8, 9), personality disorders (10, 11), and psychoses (12). Furthermore, it appears that survivors of early maltreatment differ in critical ways from other individuals with the same psychiatric diagnoses. Disorders emerge earlier in maltreated individuals, with greater severity, more comorbidity, and a less favorable response to treatment (13–15). Maltreated individuals may also have discernible brain abnormalities that are not present in their nonmaltreated counterparts (16, 17). Childhood maltreatment is also linked to a wide array of medical disorders, shortened life expectancy, and reduced telomere length (18, 19). Hence, an understanding of maltreatment as an etiological risk factor is crucial to the development of a science of preventive psychiatry, to the design of effective therapeutic regimens, and to the delineation of an accurate nosology.
Our goal in this review is to advance the thesis (17, 20–23) that affected individuals with childhood maltreatment constitute a critically distinct subtype across depressive, anxiety, and substance use disorders. We also propose that the maltreated subtype may be thought of as a phenotypic specialization (phenocopy) resulting from environmental experience—or more precisely, an ecophenotype.
Why focus on maltreatment? It is maltreatment rather than exposure to other stressors, such as natural disasters, that consistently presents as the antecedent to psychopathology (24, 25). This makes sense. Children are dependent on the adults around them for their survival, and they can endure great hardship if they feel protected and cared for. But when the hardship is the product of their caretakers, and when it is the caretaker who must be protected against, it creates a stressor with far-reaching ramifications.
Epidemiology of Maltreatment Trauma
Maltreatment is characterized by sustained or repeated exposure to events that usually involve a betrayal of trust (20). Active examples include childhood sexual, physical, and various forms of emotional abuse. Passive examples include emotional and physical neglect. (See Figure 1 for proposed assessment criteria and definitions.) As might be expected, parents of maltreated children were often maltreated themselves and show high rates of untreated or undertreated psychopathology (26). Therefore, intergenerational transmission involves some combination of early life stress, deficient parenting skills, genetic or epigenetic risk, and family stressors (27).

Differences in definitions make it hard to draw firm conclusions about prevalence. However, retrospective and prospective studies suggest that exposure to one or more forms of childhood maltreatment range from 13.8% in 1-year prevalence rates to about 42% in retrospective estimates covering the full 18 years of childhood (28).
Supporting Methodology
Our conceptualization of ecophenotypes emerged from a systematic review of the English-language literature on the psychiatric and neurobiological consequences of childhood maltreatment. Details on how the review was conducted, as well as tabulated results of sexual abuse as a psychiatric risk factor, are presented in the data supplement that accompanies the online edition of this article. Studies selected for citation are representative. No contradictory studies showing a significant protective effect of maltreatment were encountered. In this review, we excluded disorders for which research suggests that the vast majority of patients were exposed to some type of abuse or neglect, such as borderline personality and dissociative identity disorder (10, 11, 29, 30). We also excluded schizophrenia and bipolar disorder, which are known to be highly heritable. Instead, we focused on moderately inheritable disorders for which major subsets of patients can be distinguished by positive or negative histories of childhood maltreatment. These disorders include major depression, anxiety disorders, posttraumatic stress disorder, and substance abuse. Childhood maltreatment or early adversity accounts for 30%−70% of the population attributable risk fraction for these disorders (1, 3, 9).
Maltreatment and Associated Psychopathology
Major Depressive Disorder
Some of the strongest evidence for an association between exposure to childhood maltreatment and the development of major depression can be found in the Adverse Childhood Experiences study (31), which showed that risk for depression increased in a graded, dose-dependent fashion with the number of maltreatment-related adverse childhood experiences. Exposure to one or more adverse childhood experiences accounted for 54% of the population attributable risk fraction for current episodes of depression (1) and 67% for suicide attempts (32). Having five or more adverse experiences increased the relative risk of receiving a prescription for an antidepressant 2.9-fold (6). Long-term prospective studies also indicate about a twofold greater risk attributable to maltreatment (2, 4, 5) (see Figure 2A). These findings are consistent with results of twin studies showing that heritability plays only a minor role in risk for moderate or even severe depressions (33).
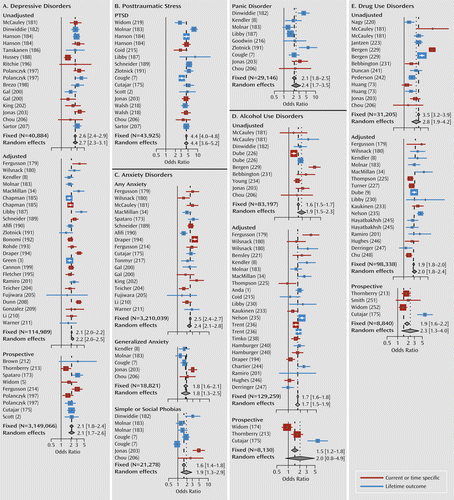
a References not included in the main text are provided in the online data supplement, along with further details of the analysis. The forest plots show odds ratios and 95% confidence intervals. Panels A–E address, respectively, diagnoses or suprathreshold symptoms of major depression; diagnoses of posttraumatic stress disorder; diagnoses or suprathreshold symptoms of anxiety disorders, including generalized anxiety disorder, panic disorder, and simple or social phobias; alcohol-related problems, including heavy episodic drinking, abuse, or dependence; and drug-related problems, including use of illicit drugs, abuse, or dependence. Studies were ordered within each cluster by year of publication. Multiple analyses within studies were pooled to provide assessment for overall risk across severity level and gender.
Maltreatment increases the risk for depression in both males and females, although some studies suggest a greater risk for depression in physically abused females than in physically abused males (34, 35). Hence, the greater female prevalence may be due, at least in part, to greater sensitivity to physical abuse and more frequent exposure to childhood sexual abuse (36).
Important clinical differences exist between depressive illnesses with and without childhood maltreatment. Depressions emerge earlier and have a more sustained course (13, 37) in maltreated individuals. These individuals also have more severe mood, neurovegetative, and endogenous symptoms and more comorbidities, particularly substance abuse (13, 22, 37, 38). Psychotic features are also more common, as are suicide attempts and deliberate self-harm (39).
Maltreated patients with depression also differ with respect to treatment response. A recent meta-analysis of depression outcome studies (13) confirmed that childhood maltreatment unequivocally predicts poor treatment outcome. However, it is also possible that maltreated patients with depression respond preferentially to therapies that are less effective for patients with depression who have no history of maltreatment. In a large clinical trial (40), chronically depressed participants received either pharmacotherapy with nefazodone, psychotherapy using the cognitive-behavioral analysis system of psychotherapy, or both treatments in combination. Psychotherapy was clearly superior to antidepressant monotherapy in the subset of participants with childhood trauma, and nefazodone provided little added benefit. In contrast, chronically depressed patients with no history of trauma or loss responded more favorably to nefazodone than to psychotherapy, and they benefited from combination treatment. On the other hand, in another study (41), maltreatment was associated with a poorer response to interpersonal therapy than to cognitive therapy or medication, and with rapid relapse. With hindsight, we can see that factors found over the years to predict treatment resistance in depression (i.e., early onset, comorbid anxiety and substance use disorders, axis II diagnoses, and presence of psychotic features) are the same factors now known to be characteristic of the maltreatment-related ecophenotype.
Neurobiological studies are beginning to provide compelling reasons for considering depression with a maltreatment history as a distinct subtype. Reduced hippocampal size is one of the more prominent neuroimaging findings in major depression. However, Vythilingam et al. (16) reported that reduced hippocampal size was present only in the subset of depressed individuals who had a history of maltreatment. On balance, there is now more consistent evidence for reduced hippocampal size in adults with a history of maltreatment than in adults with major depression. Furthermore, reduced hippocampal volume in maltreated individuals in the absence of depression or any psychiatric history has been observed in recent large-sample studies (42, 43). In short, what has been regarded as a key finding in major depression may instead be a consequence of early stress that serves in turn as a risk factor. Indeed, reduced hippocampal volume can precede and partially mediate risk for depression with early stress (44).
Amygdala activation during exposure to sad or negative faces is another neuroimaging finding linked to major depression (45) that may be limited to depressed individuals with a history of maltreatment (24). Indeed, bilateral amygdala reactivity to emotional expression is enhanced by a history of emotional maltreatment whether or not the individual has depression (46).
Genetic and epigenetic risk factors may also be distinctly different in patients with major depression with and without a history of maltreatment. A comprehensive meta-analysis by Karg et al. (47) found strong support for a gene-by-environment interaction involving the serotonin transporter promoter polymorphism and risk for depression when the environmental experience was childhood maltreatment but only marginal support when the environmental experience involved postchildhood stressful events.
Epigenetic hypermethylation of the Nr3C1 gene results in decreased expression of glucocorticoid receptors and potential hypersecretion of cortisol during stress. Interestingly, Nr3C1 has been found to be hypermethylated in postmortem tissue from suicide victims with a history of maltreatment, but not in suicide victims without maltreatment or in nonsuicide comparison subjects (48).
While some have speculated that individuals without maltreatment who develop depression do so because of a dense family history and a high heritable risk, this supposition is not supported by our unpublished data or by the observation that less severe forms of depression show little evidence of heritability (33). However, nonmaltreated depressed individuals may show an array of noninherited rare copy number variants—short stretches of DNA that are deleted or duplicated between individuals that contribute disproportionately to risk (49).
Finally, depressed patients differ in their risk for autoimmune, metabolic, and cardiovascular disorders based on maltreatment history. This may be related to chronic low-grade inflammation. Longitudinal data show that depression and inflammation are strongly coupled in depressed individuals with maltreatment but not in those without maltreatment (4).
PTSD
Sexual abuse, physical abuse, and witnessing domestic violence are types of maltreatment that may fulfill the DSM-IV A1 criterion for a traumatic event (50), and they are major risk factors for the development of PTSD (Figure 2B). Scott et al. (2) reported an adjusted odds ratio of 4.86 for lifetime diagnosis of posttraumatic stress disorder in a prospective study of adults with a history of maltreatment. Furthermore, individuals who experienced both childhood adversity and adult traumatic events have been found to be more likely to develop PTSD than those who experienced either type of adverse event alone (51).
However, in recent years there has been growing concern about how well the DSM-IV conceptualization of posttraumatic stress, which is based on exposure to acute life-threatening events in soldiers, applies to maltreated children. Youngsters often experience traumatic or highly stressful events during a substantial portion of their life, which may be perpetrated by one or more family members rather than a faceless enemy. This has led to two important observations. First, DSM-IV criteria are not sufficiently developmentally sensitive. Severely maltreated children often do not meet full diagnostic criteria, as they frequently show symptoms in only two of three category clusters, but may be as impaired as children who meet full criteria (52). Furthermore, risk for posttraumatic stress in children appears to be influenced by frequency of exposure and multiplicity of exposure types rather than the degree to which they witnessed actual or threatened death or serious injury or experienced a threat to their physical integrity. Hence, children may be “traumatized” by repeated exposure to types of maltreatment that do not meet the A1 criterion for a traumatic event, such as emotional abuse (50).
Second, as van der Kolk (50) and others have articulated, traumatized children also show a complex array of problems, such as affective dysregulation, disturbed attachment patterns, behavioral regression, somatic symptoms, and altered attributions and expectancies that are not included in the current DSM conceptualizations and often lead to a host of comorbid diagnoses. Developmental trauma disorder has been proposed as a diagnostic category that more faithfully captures the critical events and clinical presentation of posttraumatic sequelae in chronically maltreated children (50).
However, developmental trauma disorder is best restricted to maltreated individuals with features of posttraumatic stress (see the online data supplement for further discussion). As noted above, many maltreated individuals are more accurately characterized as depressed, and timing of exposure may be a critical determinant. Schoedl et al. (53) found that individuals reporting sexual abuse after age 12 had a 10-fold greater risk of severe PTSD in adulthood than individuals reporting sexual abuse before age 12. Conversely, depressive symptoms were more severe in individuals reporting sexual abuse before age 12 than in those reporting it after age 12 (53).
Multiple lines of evidence suggest that maltreated individuals with PTSD continue to differ from their nonmaltreated counterparts in adulthood. They show greater symptom complexity (54), more comorbid mood disorders (55), and more severe dissociation (56, 57) or alexithymia (58), leading to the designation “complex PTSD” (54, 59, 60). There may also be important neurobiological and genetic differences.
A key neuroimaging finding in PTSD, particularly in combat veterans (61), has been reduced hippocampal volume. However, a study of monozygotic twins discordant for combat exposure found reduced hippocampal volume in combat-exposed individuals with posttraumatic stress as well as in their unexposed twins without posttraumatic stress (62). While these results may be confounded by individual drinking history or personality factors common to both twins, it is also possible that reduced hippocampal volume resulted from shared early stress and functioned as a risk factor for posttraumatic stress. As noted above, reduced hippocampal volume has been observed with considerable consistency in adults with a history of maltreatment. While some early studies with small sample sizes found reduced hippocampal size in maltreated adults with PTSD but not those without (63), recent studies with larger samples report reductions that are unrelated to posttraumatic stress (42, 43). Additional neuroimaging findings in PTSD, including amygdala hyperreactivity and reduced medial prefrontal and anterior cingulate response (61), have also been observed in individuals with a history of childhood abuse, including those without PTSD or any psychopathology (43). Studies are clearly needed to ascertain the degree to which these neuroimaging findings are specific to PTSD, are specific to PTSD in the context of a history of maltreatment, or are a more general consequence of exposure to childhood maltreatment.
Similar to findings for depressive illness, a number of genetic polymorphisms appear to modulate risk for PTSD in individuals with a history of maltreatment. The most compelling involves polymorphisms of FKBP5, which regulates cortisol-binding affinity and the nuclear translocation of the glucocorticoid receptor (64, 65). Interestingly, Xie et al. (65) reported that among individuals with the TT genotype of rs9470080, those with no maltreatment history had the lowest risk for PTSD as adults, and those with a maltreatment history had the highest. This suggests that the search for genetic risk factors may be elusive if study subjects are not subtyped by maltreatment history.
Anxiety Disorders
The National Comorbidity Replication Study showed that childhood sexual or physical abuse was associated with a 2.03- to 3.83-fold increase in risk for specific phobias, social anxiety disorder, generalized anxiety disorder, and panic disorder with or without agoraphobia (7) (Figure 2C). Childhood adversity accounted for 32.4% of the population attributable risk fraction for anxiety disorders (3). Moreover, exposure to multiple types of childhood adversity increased the likelihood of receiving a prescription for an anxiolytic by twofold (6).
The impact of exposure to childhood maltreatment on the clinical presentation and treatment of anxiety disorders has been understudied. Patients with an anxiety disorder and a history of maltreatment have significantly higher rates of concurrent major depression (37, 66), more significant impairment in social functioning, higher state and trait anxiety scores (66), greater chronicity (37), greater symptom severity, and poorer quality of life (67). Severity increases with the number of types of maltreatment experienced, and emotional abuse and neglect are especially salient risk factors for social anxiety disorder (67, 68). Lastly, in a clinical trial with paroxetine, social anxiety patients with a history of emotional abuse were the most likely to drop out of treatment (68).
Neuroimaging studies in individuals with anxiety disorders, particularly disorders involving intense fear and panic, such as panic disorder, specific phobias, and social anxiety, report evidence for amygdala hyperreactivity, which may stem from underactivity of the prefrontal cortex and insufficient inhibition of the amygdala (69, 70). Overactivation of the insula, a paralimbic region associated with perception of somatic sensations, has also been observed (70, 71). However, as indicated above, heightened amygdala activation has been observed in fMRI studies of adults without psychopathology if they were exposed to childhood maltreatment (43, 46). Moreover, a recent report (72) found that threatening faces produced overactivity in both the amygdala and the anterior insula in maltreated children with normal levels of anxiety. Hence, amygdala and insula findings are not specific to individuals with anxiety disorders. An alternative hypothesis is that enhanced amygdala and insula response to threat emerges as a consequence of exposure to childhood maltreatment and serves as a risk factor for the later development of anxiety disorders.
Substance Use Disorders
A substantial body of research shows the important role of maltreatment on risk for drug abuse and dependence (8, 9) (Figure 2D–E), although the nature of the association may be complicated by high rates of substance abuse in maltreating parents and by the possibility of prenatal exposure, prenatal malnutrition, and prematurity. A well-controlled epidemiological and co-twin study of women (8) found that nongenital childhood sexual abuse was associated with a 2.9-fold increase in risk for drug dependence and that sexual abuse involving intercourse was associated with a 5.7-fold increase. Risk was related to the number of different types of maltreatment an individual experienced. Compared with individuals with no adverse childhood events, adults with five or more adverse childhood events are seven to 10 times more likely to report illicit drug use problems, addiction to illicit drugs, and injection drug use (9). The population attributable risk fractions for these outcomes were 56%, 64%, and 67%, respectively (9). Results from the National Longitudinal Study of Adolescent Health and the National Youth Survey provide prospective evidence for a causal relationship between physical abuse and early adulthood substance abuse (73, 74).
A moderate number of studies have reported differences between substance-abusing individuals with and without a history of maltreatment. The maltreated ecophenotype is associated with an earlier age at initiation, a greater likelihood of engagement in risky sexual behaviors (75), a greater risk for recent incarceration (76), greater ratings of psychological distress (77), and a greater risk for comorbid personality disorders (78). Physical maltreatment appears to be a particularly salient risk factor for the development of substance abuse (35) and progression to injection drug use (79).
Substance abusers with a history of maltreatment respond more poorly to treatment, with greater use of substances during treatment and more persistence of substance-related problems after discharge (80–82). Integrative therapies have been developed to address the combined impact of substance abuse and trauma-related psychopathology (83).
Key neuroimaging findings in substance abusers suggest the possibility of a “dopamine deficiency” that may manifest as reduced activation of the ventral striatum (nucleus accumbens) during rewarding or pleasurable tasks (84, 85). Furthermore, deficits in brain regions implicated in salience attribution (the orbitofrontal cortex) and inhibitory control (the anterior cingulate gyrus) may underlie the patterns of compulsive and impulsive behaviors that characterize addiction (86). Although these factors have not been well studied in maltreated individuals, the few relevant studies report reduced sensitivity to reward and decreased basal ganglia response (87), as well as structural and resting blood flow deficits in the ventral striatum, the anterior cingulate, and the orbitofrontal cortex (43, 88, 89). Further research is needed to ascertain whether these deficits are common to substance abusers in general or more specific to the subset with a history of childhood maltreatment.
How Does Maltreatment Increase the Likelihood of Developing So Many Different Psychiatric Disorders?
Could maltreatment be a nonspecific amplifying factor that “tips the balance” so that individuals at hereditary risk for one disorder or another become more likely to express it? In essence, then, could maltreatment act to enhance the “penetrance” of inherited genetic susceptibilities? This could provide an explanation for the elevations in both prevalence and associated comorbidities.
A richer and more compelling alternative is that the myriad possible outcomes of exposure to childhood maltreatment depend on the timing, type, and severity of exposure, plus a host of genetic factors that influence susceptibility and resilience, and an array of protective factors that attenuate risk. Epigenetic modifications in stress-response systems and neurotrophic factors regulating trajectories of brain development may be the driving force producing the various ecophenotypes. We believe that this explanation best accounts for the available data and suggest that psychiatric disorders presenting in individuals with a substantial history of childhood maltreatment be thought of as ecophenotypic variants or ecophenocopies (see the data supplement for strategies for capturing this in our nosology).
Neurobiological Correlates of Childhood Maltreatment
As indicated above, there is a growing body of reproducible findings linking childhood maltreatment to structural and functional brain differences. The most consistent finding is that of alterations in the corpus callosum, characterized by reduced midsagittal area (90–94) or decreased fractional anisotropy (diminished integrity) on diffusion tensor scanning (95, 96) (Table 1). Another reasonably consistent finding is reduction in hippocampal volume in adults (16, 93, 97–105) but not younger children (91, 92, 106, 107) with a history of maltreatment (Table 2). The hippocampus is likely the most stress-sensitive structure in the brain, and translational studies show that stress or glucocorticoids act on the hippocampus to suppress neurogenesis in the dentate gyrus and provoke remodeling of pyramidal cells in portions of the cornu ammonis, particularly CA3. A recent study (105) found that childhood maltreatment was associated with volume reductions in the same subfields in a relatively large population of young adults, suggesting that the same mechanisms may be at work.
| Number of Subjects | Age (Years) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| First Author (Reference) | Types of Maltreatment | Diagnostic Requirement | Exposed | Comparison | Mean | SD | Gender | Medication | Main Corpus Callosum Findingsb |
| Teicher (90) | Sexual, physical, or neglect | Inpatients with versus without abuse | 28 | 23 | 12.9 | 2.9 | Both | No | Decrease in regions IV, III; males more affected than females |
| De Bellis (91) | Sexual, physical, or witnessing domestic violence | PTSD versus typical controls | 44 | 61 | 12.1 | 2.3 | Both | No | Decrease in regions IV, V–VII; males more affected than females |
| De Bellis (92) | Sexual, physical, or witnessing domestic violence | PTSD versus SES-matched controls | 28 | 66 | 11.5 | 2.9 | Both | No | Decrease in regions VII, IV–VI |
| De Bellis (144) | Sexual, physical, or witnessing domestic violence | PTSD versus SES-matched or typical controls | 61 | 122 | 11.7 | 2.6 | Both | No | Decrease in regions VII, I, VI; males more affected than females; reanalysis |
| Teicher (94) | Sexual, physical, or neglect | Inpatients with versus without abuse and controls | 28 | 23 inpatients, 115 controls | 12.2 | 3.4 | Both | No | Decrease in regions IV, V–VII; males affected by neglect, females by sexual abuse; partial reanalysis |
| Zanetti (145) | Physical or sexual | BPD with versus without physical or sexual abuse and controls | 10 (4 without physical or sexual abuse) | 20 controls | 29.1 | 9.1 | Both | No | BPD versus controls NS; increase in regions V, VII in BPD with versus without abuse |
| Rusch (146) | Sexual | BPD with versus without sexual abuse and controls | 20 (10 without physical or sexual abuse) | 20 controls | 27.6 | 6.8 | Female | No | Decrease in region V in BPD versus controls; decrease in regions V, VI in BPD with versus without abuse |
| Kitayama (147) | Sexual, physical, or witnessing domestic violence | PTSD versus typical controls | 9 | 9 | 37.3 | 9.4 | Female | Yes | Decrease in region V and total area |
| Jackowski (95) | Sexual, physical, or witnessing domestic violence | PTSD versus typical controls | 17 | 15 | 10.6 | 2.3 | Both | No | Decreased FA in middle and posterior |
| Andersen (93) | Sexual | No diagnosis required; 27% with history of PTSD | 26 | 17 | 19.8 | 1.4 | Female | No | Decrease in region III; sensitive period, ages 9–10 |
| Carrion (148) | Sexual, physical, or witnessing domestic violence | PTSD symptoms versus controls | 24 | 24 | 11.0 | 2.2 | Both | Yes | NS 8.7% decrease in region VII |
| Mehta (120) | Early deprivation, 24 months | Romanian orphans versus controls | 14 | 11 | 16.1 | 0.8 | Both | No | NS 6.5% decrease in absolute volume |
| Teicher (96) | Peer verbal abuse | No psychopathology | 63 | Used ratings, not groups | 21.9 | 1.9 | Both | No | Decreased FA in region VII; males and females affected to the same degree |
| Frodl (149) | CTQ score | Unaffected relatives with major depression, controls | 6 relatives, 4 controls | 15 relatives, 20 controls | 36.3 | 12.9 | Both | No | Decreased FA in region VII controls with versus without abuse; increase in FA in region VII, relatives with versus without abuse |
| Number of Subjects | Age (Years) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| First Author (Reference) | Types of Maltreatment | Diagnostic Requirement | Exposed | Comparison | Mean | SD | Gender | Medication | Main Hippocampus Findingsb |
| Adults | |||||||||
| Bremner (97) | Physical or sexual | PTSD versus healthy controls | 17 | 17 | 41.3 | 6.6 | Female | Yes | L decreased 12% |
| Stein (99) | Sexual | PTSD or dissociative identity disorder versus SES-matched controls | 21 | 21 | 31.1 | 6.4 | Female | Yes | L decreased 5% |
| Driessen (103) | CTQ score | BPD versus healthy controls | 21 | 21 | 29.6 | 6.5 | Female | Yes | L, R decreased 16% |
| Vythilingam (16) | Physical or sexual | Major depression with versus without abuse and controls | 21 | 11 major depression, 14 controls | 31.4 | 6.9 | Female | No | L decreased 15% in major depression with physical or sexual abuse versus control; NS for major depression without physical or sexual abuse versus control |
| Schmahl (104) | Physical or sexual | BPD with abuse versus comparison without BPD | 10 | 23 | 30.3 | 8.0 | Female | Yes | L decreased 11%, R 16% |
| Bremner (63) | Sexual | Abuse with PTSD, abuse without PTSD, and controls | 10 with PTSD, 12 without PTSD | 11 | 34.9 | 7.5 | Female | No | L, R, decreased 19% for sexual abuse with PTSD versus control; NS for sexual abuse without PTSD versus control |
| Brambilla (102) | Physical or sexual | BPD versus healthy controls | 10 | 20 | 33.0 | 8.9 | Both | No | L, R decreased 6.8%, most marked in BPD with abuse |
| Pederson (150) | CTQ severe to extreme, pubertal | Abuse with PTSD, abuse without PTSD, controls | 17 with PTSD, 17 without PTSD | 17 | 25 | 6 | Female | ? | NS 2.8% decrease on L for abuse with PTSD versus control; NS 6.3% decrease on L for abuse without PTSD versus control |
| Vermetten (100) | Physical or sexual | Dissociative identity disorder with PTSD versus comparison | 15 | 23 | 37.8 | 9.0 | Female | Yes | L, R decreased 19.2% |
| Cohen (114) | ELSQ high versus low, 0–12 years | No psychopathology | 122 | 84 | 39.9 | 17.2 | Both | No | L, R decreased (p=0.07 and p=0.06) |
| Zetzsche (151) | Physical or sexual | BPD with versus without physical or sexual abuse and controls | 14 BPD with 11 BPD without physical or sexual abuse | 25 | 26.7 | 6.7 | Female | Yes | L decreased 5% (p=0.07), R decreased 6% (p=0.03) for BPD versus control; NS for BPD with versus without physical or sexual abuse |
| Andersen (93) | Sexual | No diagnosis required; 27% with history of PTSD | 26 | 17 | 19.8 | 1.4 | Female | No | Decreased 6.8% bilaterally; sensitive periods, ages 3–5, 11–13 |
| Bonne (152) | Sexual, physical, emotional | PTSD with versus without abuse, controls | 11 | 11 PTSD, 22 controls | 35.9 | 10.4 | Both | No | Decreased 9% bilaterally for PTSD versus control; NS for PTSD with versus without abuse |
| Weniger (153) | Physical or sexual | PTSD, dissociative disorders, and controls | 10 PTSD 13 dissociative disorders | 25 | 32. | 7.1 | Female | Yes | Decreased 18% bilaterally for PTSD versus control; NS for dissociative disorders versus control |
| Lenze (154) | CECA score | Remitted major depression with versus without abuse, controls | 19 | 12 Remitted major depression, 24 controls | 48.5 | 14.9 | Female | Yes | Decreased L for remitted major depression versus control; abuse NS contribution |
| Soloff (155) | Physical or sexual | BPD with versus without physical or sexual abuse, controls | 20 with 14 with-out physical or sexual abuse | 30 | 26.6 | 7.9 | Both | No | Decreased R, L for BPD versus control; NS for BPD with versus without physical or sexual abuse |
| Weniger (101) | Physical or sexual | BPD, controls | 24 | 25 | 32.5 | 6.5 | Female | Yes | Decreased 12% bilaterally (with or without comorbid PTSD) |
| Gatt (156) | ELSQ score | No psychopathology | 89 | Used ratings, not groups | 36.2 | 12.7 | Both | No | Decreased gray matter volume R, L with ELSQ ratings and MET polymorphism of BDNF |
| Frodl (98) | CTQ score | Major depression, healthy controls | 43 | 42 | 44.1 | 12.4 | Both | Yes | NS gray matter volume; emotional neglect: decreased white matter volume on L in females, L and R in males |
| Thomaes (157) | Physical or sexual | Complex PTSD, controls | 33 | 30 | 35.5 | 11.0 | Female | Yes | R decreased (p<0.04); R inverse correlation with abuse severity (p<0.02) |
| Landré (158) | Sexual | PTSD, unexposed controls | 17 | 17 | 24.8 | 4.7 | Female | No | NS |
| Sala (159) | Physical or sexual | BPD, matched controls | 15 BPD (6 physical or sexual abuse) | 15 | 33.5 | 7.9 | Both | Yes | R decreased 12.7% for BPD versus control; R, L decreased for BPD with versus without physical or sexual abuse |
| Everaerd (160) | List of Threatening Life Events | No psychopathology; 5HTTLPR genotyping | 357 | Used ratings, not groups | 23.7 | 5.6 | Both | No | Gene-by-abuse-by-gender; decreased R, L for males with S′-allele and severe adversity (p<0.002) |
| Teicher (42) | CTQ and ACE scores | No diagnosis required; 46% exposed history major depression | 104 | 89 | 21.9 | 2.1 | Both | No | Decreased 6% in L subfields dentate gyrus and CA3; not related to major depression or PTSD |
| Dannlowski (43) | CTQ score | No psychopathology | 148 | Used ratings, not groups | 33.8 | 10.4 | Both | No | R decreased (p<0.05) |
| Carballedo (161) | CTQ score | No psychopathology, with versus without family of history major depression | 20 positive, 20 negative family history | Used median split ratings | 36.5 | 13.1 | Both | No | Decreased L, R hippocampal heads in subjects with emotional abuse and positive family history |
| Children and adolescents | |||||||||
| De Bellis (91) | Sexual, physical, or witnessing domestic violence | PTSD versus typical controls | 44 | 61 | 12.1 | 2.3 | Both | No | NS 2.2% increase |
| Carrion (148) | Sexual, physical, or witnessing domestic violence | PTSD symptoms versus controls | 24 | 24 | 11.0 | 2.2 | Both | Yes | NS 7.6% decrease |
| De Bellis (162) | Sexual, physical, or witnessing domestic violence | PTSD versus typical controls | 9 | 9 | 10.6 | 1.6 | Both | Yes | NS at baseline or while followed longitudinally for >2 years |
| Chugani (163) | Early deprivation, mean 38 months | Romanian orphans versus epilepsy control | 10 | 7 | 10.3 | 3.9 | Both | No | Decreased PET glucose metabolism in L temporal region, including hippocampus |
| De Bellis (92) | Sexual, physical, or witnessing domestic violence | PTSD versus SES-matched controls | 28 | 66 | 11.5 | 2.9 | Both | No | NS 1.8% decrease |
| Tupler (107) | Sexual, physical, or witnessing domestic violence | PTSD versus SES-matched or typical controls | 61 | 122 | 11.7 | 2.6 | Both | No | NS gray matter volume; increased white matter volume; reanalysis |
| Carrion (106) | Sexual, physical, or witnessing domestic violence | PTSD symptoms | 15 | 0 | 10.4 | 8–14 | Both | Yes | Inverse correlation (r=–0.48) between volume and cortisol level over 12–18 months |
| Mehta (120) | Early deprivation, 24 months | Romanian orphans versus controls | 14 | 11 | 16.1 | 0.8 | Both | No | L, R decreased 16% absolute, NS after adjusted for brain volume |
| Rao (44) | Early life adversity | Major depression, high risk, and controls | 30 major depression 22 high risk, 35 controls | Ratings of exposure within each group | 14.9 | 1.8 | Both | No | Decreased R, L with early life adversity in high risk and controls; hippocampal volume partially mediated risk for major depression with early life adversity |
| Carrion (164) | Sexual, physical, or witnessing domestic violence | PTSD symptoms versus controls | 16 | 11 | 13.9 | 2.0 | Both | Yes | Abnormal (decreased) R BOLD response on verbal memory task |
| Maheu (165) | Caregiver deprivation—emotional neglect | Orphans or foster care versus controls | 11 | 19 | 13.5 | 2.6 | Both | No | Abnormal (increased) L BOLD response to fearful and angry versus neutral faces |
| Tottenham (121) | Early deprivation, 63 months | Orphans versus healthy controls | 34 | 28 | 8.9 | 2.1 | Both | ? | NS 2.5% decrease on L in late adoptees (after 15 months) |
| Edmiston (166) | CTQ score | No psychopathology | 42 | Used ratings, not groups | 15.33 | 1.37 | Both | No | Decreased with total scores on R, L in females; decreased with emotional neglect on R, L in males and females |
| Lupien (122) | Mothers with chronic major depression | Exposed versus controls | 17 | 21 | 10 | Both | No | NS | |
There are also associations between exposure to early maltreatment and the attenuated structural or functional development of the neocortex (93, 108–113), including the anterior cingulate (109, 114–116), the orbitofrontal (89, 116, 117) and dorsolateral prefrontal cortex (88, 115), and the visual and auditory cortex (Table 3).
| Number of Subjects | Age (Years) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| First Author (Reference) | Types of Maltreatment | Diagnostic Requirement | Exposed | Comparison | Mean | SD | Gender | Medication | Main Cortical Findingsb |
| De Bellis (91) | Sexual, physical, or witnessing domestic violence | PTSD versus typical controls | 44 | 61 | 12.1 | 2.3 | Both | No | Increased prefrontal cerebrospinal fluid (volume loss) |
| De Bellis (109) | Sexual, physical, or witnessing domestic violence | PTSD versus typical controls | 11 | 11 | 10.2 | 29 | Both | No | Decreased N-acetyl aspartate/creatine ratio in anterior cingulate |
| Carrion (108) | Sexual, physical, or witnessing domestic violence | PTSD symptoms versus controls | 24 | 24 | 11.0 | 2.2 | Both | Yes | Decreased frontal asymmetry |
| Chugani (163) | Early deprivation, mean=38 months | Romanian orphans versus epilepsy control | 10 | 7 | 10.3 | 3.9 | Both | No | Decreased PET glucose metabolism in R, L orbital frontal gyrus, infralimbic prefrontal cortex |
| De Bellis (92) | Sexual, physical, or witnessing domestic violence | PTSD versus SES-matched controls | 28 | 66 | 11.5 | 2.9 | Both | No | Increased prefrontal cerebrospinal fluid (volume loss) |
| De Bellis (167) | Sexual, physical, or witnessing domestic violence | PTSD versus typical controls | 43 | 61 | 12.1 | 2.3 | Both | No | Increased R, L superior temporal gyrus gray matter volume; reanalysis |
| De Bellis (144) | Sexual, physical, or witnessing domestic violence | PTSD versus SES-matched or typical controls | 61 | 122 | 11.7 | 2.6 | Both | No | Increased prefrontal cerebrospinal fluid (volume loss); reanalysis |
| Brambilla (102) | Physical or sexual | BPD versus healthy controls | 10 | 20 | 33.0 | 8.9 | Both | No | NS in temporal lobes and dorsolateral prefrontal cortex |
| Richert (168) | Sexual, physical, or witnessing domestic violence | PTSD symptoms versus controls | 23 | 24 | 11.0 | 2.2 | Both | Yes | Increased middle inferior ventral prefrontal gray matter volume; reanalysis (108) |
| Cohen (114) | ELSQ high versus low, 0–12 years | No psychopathology | 122 | 84 | 39.9 | 17.2 | Both | No | Decreased anterior cingulate total volume |
| Kitayama (169) | Physical, sexual | PTSD versus healthy controls | 8 | 13 | 39.3 | 8.2 | Both | ? | Decreased R anterior cingulate volume |
| Andersen (93) | Sexual versus healthy controls | No diagnosis required; 27% with history of PTSD | 26 | 17 | 19.8 | 1.4 | Female | No | Decreased total frontal gray matter volume; sensitive period, ages 14–16 |
| Tomoda (111) | Sexual versus healthy controls | No diagnosis required; most without axis I, II disorders | 23 | 14 | 19.7 | 1.4 | Female | No | Decreased occipital gray matter volume in BA 17–18; sensitive period, before age 12; partial reanalysis (93) |
| Tomoda (115) | Harsh corporal punishment versus healthy controls | No diagnosis required; most without axis I, II disorders | 23 | 22 | 21.7 | 2.0 | Both | No | Decreased gray matter volume in dorsolateral, anterior cingulate, and medial prefrontal |
| Carrion (148) | Sexual, physical, or witnessing domestic violence | PTSD symptoms versus controls | 24 | 24 | 11.0 | 2.2 | Both | Yes | Increased R, L inferior and superior prefrontal gray matter volume; reanalysis (108) |
| Carrion (170) | Sexual, physical, or witnessing domestic violence | PTSD symptoms versus controls | 30 | 15 | 13.2 | 2.1 | Both | No | Decreased L ventral and inferior prefrontal gray matter volume; inverse correlation between prebedtime cortisol level and L ventral gray matter volume |
| van Harmelen (171) | Emotional abuse or neglect | Major depression or anxiety disorders versus controls | 84 | 97 | 37.5 | 10.4 | Both | Yes | Decreased L dorsomedial prefrontal gray matter volume, independent of psychopathology |
| Sheu (88) | Harsh corporal punishment versus controls | No diagnosis required; 63% no lifetime history | 19 | 23 | 21.9 | 2.1 | Both | No | Increased T2 relaxation time (decreased regional cerebral blood volume) in R, L dorsolateral prefrontal |
| Hanson (89) | Physical versus health controls | No diagnosis required | 31 | 41 | 11.8 | 1.1 | Both | Yes | Decreased R orbital frontal, dorsolateral, temporal, and left and right parietal lobes |
| Frodl (98) | CTQ score | Major depression and healthy controls | 43 | 42 | 44.1 | 12.4 | Both | Yes | Physical neglect: decreased prefrontal gray matter volume |
| Thomaes (157) | Physical or sexual | Complex PTSD and controls | 33 | 30 | 35.5 | 11.0 | Female | Yes | Decreased R dorsal anterior cingulate, R orbitofrontal |
| Landré (158) | Sexual | PTSD and unexposed controls | 17 | 17 | 24.8 | 4.7 | Female | No | NS regional measures of cortical thickness |
| Tomoda (112) | Parental verbal abuse versus healthy controls | No diagnosis required, 48% with history of mood disorder | 21 | 19 | 21.2 | 2.2 | Both | No | Increased L superior temporal gyrus gray matter volume |
| Edmiston (166) | CTQ score | No psychopathology | 42 | Used ratings not groups | 15.33 | 1.37 | Both | No | Decreased dorsolateral, orbitofrontal, subgenual prefrontal gray matter volume |
| Gerritsen (172) | List of Threatening Life Events | No psychopathology; BDNF polymorphism | 568 | Used ratings not groups | 23.4 | 5.4 | Both | No | Decreased anterior cingulate and medial orbitofrontal at 1.5-T but not 3-T; G×E BDNF versus events for subgenual anterior cingulate |
| Carballedo (161) | CTQ score | No psychopathology, with versus without family history of major depression | 20 positive 20 negative family history | Used median split ratings | 36.5 | 13.1 | Both | No | Decreased L dorsolateral and medial prefrontal, R anterior cingulate with emotional abuse and positive family history |
| Tomoda (128) | Witnessed domestic violence versus healthy controls | No diagnosis required, 59% past psychiatric history | 22 | 30 | 21.7 | 2.2 | Both | No | Decreased gray matter volume and thickness in R lingual gyrus (BA 18), decreased thickness R, L V2 and L occipital pole; sensitive period, ages 11–13 |
While maltreatment may be associated with alterations in the striatum/basal ganglia (87, 88, 114) and cerebellum (118, 119), most studies have not reported structural differences in the amygdala (91–93, 97, 102, 114). However, increased amygdala volume has been reported in children with institutional deprivation or rearing by chronically depressed mothers (120–122), while smaller amygdala volumes have been observed in adults with childhood trauma and borderline personality disorder or dissociative identity disorder (100, 101, 103, 104). Nevertheless, there is good evidence of enhanced amygdala reactivity in maltreated individuals (17, 43, 46, 72).
Second, there appear to be sensitive periods when these regions are maximally susceptible to the effects of stress. Following this path of inquiry, our group examined the relationship between age at exposure to sexual abuse and observed alterations in brain morphology in a preliminary sample of young adult women (93). We found the hippocampus to be maximally susceptible to maltreatment in women exposed between the ages of 3 and 5 years. However, when maltreatment occurred at ages 9–10, the midportion of the corpus callosum was maximally susceptible, and at ages 14–16, the prefrontal cortex was affected. Thus, there appear to be specific windows of vulnerability in development that determine the negative effects of exposure. These observations are supported by translational research showing that synaptic density in the hippocampus but not the prefrontal cortex of rats is sensitive to the effects of early (preweaning) stress, while the opposite is true with regard to peripubertal stress (123, 124). Rao et al. (125) provided additional support for an early hippocampal sensitive period in humans, reporting that degree of parental nurturance at age 4, but not at age 8, predicted hippocampal volume at age 14.
Third, the effects of maltreatment on brain functioning may not appear immediately after exposure (124). Several studies have reported reductions in the gray matter volume of the hippocampus in adults with a history of maltreatment but not in maltreated children (Table 2). This pattern of results is consistent with translational studies showing that effects of early stress on the hippocampus first emerge during the transition between puberty and adulthood (124). The delay between exposure and neurobiological change may be particularly relevant, as a comparable time lag often occurs between exposure and emergence of depression or posttraumatic stress disorder (126).
Fourth, maltreatment also appears to affect the development of sensory systems and pathways that process and convey the adverse experience. For example, parental verbal abuse is associated with decreased fractional anisotropy in the arcuate fasciculus, which interconnects Wernicke’s and Broca’s areas (127), and with alterations in gray matter volume in the auditory cortex (112). Conversely, witnessing domestic violence is associated with a reduction in gray matter volume in the primary and secondary visual cortex (128) and with decreased fractional anisotropy in the inferior longitudinal fasciculus, which interconnects the visual cortex and the limbic system to shape our emotional and memory response to things that we see (129).
Figure 3 places these findings in context by showing that many of the identified neuroanatomical abnormalities are interconnected and are components of a circuit regulating response to potentially threatening stimuli. Briefly, the thalamus and sensory cortex process threatening sights and sounds and convey this information to the amygdala (130). Prefrontal regions, particularly the ventromedial and orbitofrontal cortex, modulate amygdala response, perhaps turning it down with the realization that something is not actually a threat or, in other cases, irrationally amplifying it (130). The hippocampus also processes this information and plays a key role in retrieving relevant explicit memories (130). The amygdala integrates this information and signals the paraventricular nucleus of the hypothalamus, which in turn regulates autonomic (e.g., heart rate) and pituitary-adrenal hormonal responses and signals the locus ceruleus, which regulates the intracerebral noradrenergic response. The hippocampus, through the subiculum and bed nucleus of the stria terminalis, also modulates paraventricular response, particularly to psychological stressors (131).
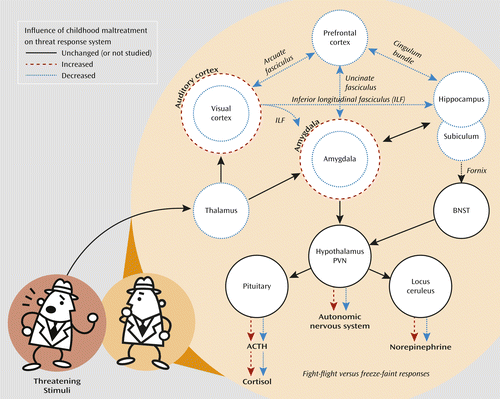
a Childhood maltreatment alters development of regions and pathways within this circuit, which serves to reprogram response to subsequent stressors, resulting in either exaggerated or blunted responses. Based primarily on LeDoux (130). ACTH=adrenocorticotropic hormone; BNST=bed nucleus of stria terminalis; PVN=paraventricular nucleus of hypothalamus.
Hence, childhood maltreatment, by affecting the development of key components of this system, reprograms response to subsequent stressors. The influence of maltreatment on autonomic and hypothalamic-pituitary-adrenal response to psychological stressors has been evaluated in a series of studies using the Trier Social Stress Test. Heim et al. (132) first reported that women with a history of physical or sexual abuse had heightened cortisol, ACTH, and heart rate response to stress challenge. Subsequent studies have generally painted a different picture, with evidence emerging for a blunting of cortisol response in adults with a history of maltreatment (133–136). Nevertheless, some individuals show an augmented response, consistent with an enhanced fight-or-flight reaction, and others show a blunted response, consistent with freezing. This divergent pattern of response may be influenced by the type (137) and timing (138) of maltreatment.
Psychosocial Correlates of Exposure
Simultaneous to disruptions in brain development that occur with exposure to mistreatment are alterations in the development of psychological structures. Alterations have been observed in the form of poor self-concept, feelings of worthlessness, and negative views of the world. Furthermore, victims of maltreatment show deficits in what is called deontic reasoning (reasoning about duties and obligations we owe one another), which puts victims at increased risk for future victimization (139). Victims of maltreatment are also more likely to show insecure attachment, associated with diminished expectations of support as well as poor emotion regulation capacities (140).
Treatment Implications
The first question is whether interventions exist that can reduce a child’s risk of abuse and neglect. The Nurse-Family Partnership has been shown in randomized controlled trials to reduce the incidence of abuse (particularly physical abuse) and neglect of first-born children of high-risk mothers (141). There is also emerging evidence for the efficacy of other interventions against the emergence or reoccurrence of physical abuse. However, no interventions have been shown to be effective in reducing risk for sexual abuse, emotional abuse, witnessing domestic violence, or recurrence of neglect (141).
The second question is whether preemptive interventions exist that can reduce long-term risk for psychiatric illness in maltreated children prior to the emergence of psychopathology. This is an important but largely unexplored area. Third, are there good acute treatments with long-term benefits for maltreated children with psychopathology? Trauma-focused cognitive-behavioral therapy for sexually abused children with symptoms of posttraumatic stress has the most evidence of efficacy (141), but long-term outcome studies are sparse. Assessing and treating parents may also be critical, as maltreatment is often associated with parental psychopathology and parenting problems (26). Recent efforts to develop neurobiologically informed treatments provide preliminary evidence that lower posttreatment cortisol levels may be associated with reduced effects on hippocampal development (106).
Finally, what can be recommended for adults with ecophenotypic variants of major depression, anxiety disorders, substance abuse, or posttraumatic stress? Results of a recent meta-analysis show that depressed individuals with a history of maltreatment respond more poorly to treatment (13), suggesting that standard first-line recommendations for depression may be inadequate for these individuals. The finding that the cognitive-behavioral analysis system of psychotherapy was more effective than nefazodone in maltreated individuals with chronic depression (40) is intriguing, but research is needed to ascertain whether these findings apply to other medications, to other systems of therapy, and to maltreated individuals with less chronic conditions. Integrative trauma-focused treatments have been developed for maltreated individuals with substance abuse that are more helpful than standard treatments, although the results have been far from ideal (83). Childhood maltreatment is often associated with development of insecure attachment patterns (24), and mentalization-based therapy appears to have beneficial effects in patients with insecure attachment patterns across a range of disorders, including major depression, substance abuse, and borderline personality disorder (142). Efforts to reduce allostatic load and inflammation (19) may also be of benefit for maltreated individuals.
Recent recommendations for adults with maltreatment-related posttraumatic stress are to adopt a sequential approach that begins with safety, education, stabilization, skill building, and development of the therapeutic alliance before endeavoring to revisit or rework the trauma, as this may be destabilizing (143). Overall, we suspect that unknowingly mixing maltreated and nonmaltreated subtypes in treatment trials may have left us with an incomplete understanding of risks and benefits. Stratifying study subjects by maltreatment history may provide more definitive insights and delineate a clearer course of action for each subtype.
Conclusions
Childhood maltreatment is a complex etiological agent that appears to vary in impact according to the timing, type, and severity of exposure, coupled with a number of susceptibility and resilience cofactors. We propose using the term ecophenotype to delineate these psychiatric conditions. We specifically recommend, as a first step, adding the specifier “with maltreatment history” or “with early life stress” to the disorders discussed here so that these populations can be studied separately or stratified within samples. This will lead to a richer understanding of differences in clinical presentation, genetic underpinnings, biological correlates, treatment response, and outcomes. Doing so may also help resolve inconsistencies in the literature resulting from unassessed differences in the percentage of maltreated subjects within a given study.
1 : Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. Psychiatr Serv 2002; 53:1001–1009Link, Google Scholar
2 : Prospectively ascertained child maltreatment and its association with DSM-IV mental disorders in young adults. Arch Gen Psychiatry 2010; 67:712–719Crossref, Medline, Google Scholar
3 : Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry 2010; 67:113–123Crossref, Medline, Google Scholar
4 : Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med 2009; 163:1135–1143Crossref, Medline, Google Scholar
5 : A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry 2007; 64:49–56Crossref, Medline, Google Scholar
6 : Adverse childhood experiences and prescribed psychotropic medications in adults. Am J Prev Med 2007; 32:389–394Crossref, Medline, Google Scholar
7 : Examining the unique relationships between anxiety disorders and childhood physical and sexual abuse in the National Comorbidity Survey-Replication. Psychiatry Res 2010; 177:150–155Crossref, Medline, Google Scholar
8 : Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry 2000; 57:953–959Crossref, Medline, Google Scholar
9 : Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics 2003; 111:564–572Crossref, Medline, Google Scholar
10 : Childhood trauma in borderline personality disorder. Am J Psychiatry 1989; 146:490–495Link, Google Scholar
11 : Reported pathological childhood experiences associated with the development of borderline personality disorder. Am J Psychiatry 1997; 154:1101–1106Link, Google Scholar
12 : Schizophrenia and other psychotic disorders in a cohort of sexually abused children. Arch Gen Psychiatry 2010; 67:1114–1119Crossref, Medline, Google Scholar
13 : Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am J Psychiatry 2012; 169:141–151Link, Google Scholar
14 : Prevalence and clinical impact of childhood trauma in patients with severe mental disorders. J Nerv Ment Dis 2011; 199:156–161Crossref, Medline, Google Scholar
15 : Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry 2002; 51:288–297Crossref, Medline, Google Scholar
16 : Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 2002; 159:2072–2080Link, Google Scholar
17 : Childhood trauma history differentiates amygdala response to sad faces within MDD. J Psychiatr Res 2011; 45:886–895Crossref, Medline, Google Scholar
18 : Adverse childhood experiences and the risk of premature mortality. Am J Prev Med 2009; 37:389–396Crossref, Medline, Google Scholar
19 : Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav 2012; 106:29–39Crossref, Medline, Google Scholar
20 : Developmental traumatology: the psychobiological development of maltreated children and its implications for research, treatment, and policy. Dev Psychopathol 2001; 13:539–564Crossref, Medline, Google Scholar
21 : The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 2008; 33:693–710Crossref, Medline, Google Scholar
22 : Clinical characteristics and treatment outcome of depression in patients with and without a history of emotional and physical abuse. J Psychiatr Res 2010; 44:302–309Crossref, Medline, Google Scholar
23 : Towards a scientific taxonomy of depression. Dialogues Clin Neurosci 2008; 10:301–308Medline, Google Scholar
24 : Best practices in psychotherapy for children and adolescents, in Treating Complex Traumatic Stress Disorders. Edited by Courtois CAFord JD. New York, Guilford, 2009, pp 59–81Google Scholar
25 : Childhood trauma and children’s emerging psychotic symptoms: a genetically sensitive longitudinal cohort study. Am J Psychiatry 2011; 168:65–72Link, Google Scholar
26 : Psychopathology in the relatives of depressed-abused children. Child Abuse Negl 1998; 22:171–181Crossref, Medline, Google Scholar
27 : Effects of early stress on brain structure and function: implications for understanding the relationship between child maltreatment and depression. Dev Psychopathol 2001; 13:451–471Crossref, Medline, Google Scholar
28 : The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse Negl 2004; 28:771–784Crossref, Medline, Google Scholar
29 : An update on multiple personality disorder. Hosp Community Psychiatry 1987; 38:363–373Abstract, Google Scholar
30 : Abuse histories in 102 cases of multiple personality disorder. Can J Psychiatry 1991; 36:97–101Crossref, Medline, Google Scholar
31 : Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) Study. Am J Prev Med 1998; 14:245–258Crossref, Medline, Google Scholar
32 : Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. JAMA 2001; 286:3089–3096Crossref, Medline, Google Scholar
33 : A registry-based twin study of depression in men. Arch Gen Psychiatry 1998; 55:468–472Crossref, Medline, Google Scholar
34 : Childhood abuse and lifetime psychopathology in a community sample. Am J Psychiatry 2001; 158:1878–1883Link, Google Scholar
35 : The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Med 2012; 9:e1001349Crossref, Medline, Google Scholar
36 : Does sexual violence contribute to elevated rates of anxiety and depression in females? Psychol Med 2002; 32:991–996Crossref, Medline, Google Scholar
37 : Impact of childhood life events and trauma on the course of depressive and anxiety disorders. Acta Psychiatr Scand 2012; 126:198–207Crossref, Medline, Google Scholar
38 : Childhood adversity and anxiety versus dysthymia co-morbidity in major depression. Psychol Med 2002; 32:1239–1249Crossref, Medline, Google Scholar
39 : Depression with atypical features in the National Comorbidity Survey: classification, description, and consequences. Arch Gen Psychiatry 2003; 60:817–826Crossref, Medline, Google Scholar
40 : Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci USA 2003; 100:14293–14296Crossref, Medline, Google Scholar
41 : Childhood maltreatment and differential treatment response and recurrence in adult major depressive disorder. J Consult Clin Psychol 2012; 80:342–353Crossref, Medline, Google Scholar
42 : Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA 2012; 109:E563–E572Crossref, Medline, Google Scholar
43 : Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 2012; 71:286–293Crossref, Medline, Google Scholar
44 : Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry 2010; 67:357–364Crossref, Medline, Google Scholar
45 : Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. Am J Psychiatry 2012; 169:693–703Link, Google Scholar
46 : Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Soc Cogn Affect Neurosci 2013; 8:362–369Crossref, Medline, Google Scholar
47 : The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry 2011; 68:444–454Crossref, Medline, Google Scholar
48 : Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 2009; 12:342–348Crossref, Medline, Google Scholar
49 : Duplication of the SLIT3 locus on 5q35.1 predisposes to major depressive disorder. PLoS ONE 2010; 5:e15463Crossref, Medline, Google Scholar
50 : Developmental trauma disorder: toward a rational diagnosis for children with complex trauma histories. Psychiatr Ann 2005; 35:401–408Crossref, Google Scholar
51 : Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry 2009; 66:1201–1209Crossref, Medline, Google Scholar
52 : Toward an empirical definition of pediatric PTSD: the phenomenology of PTSD symptoms in youth. J Am Acad Child Adolesc Psychiatry 2002; 41:166–173Crossref, Medline, Google Scholar
53 : The clinical correlates of reported childhood sexual abuse: an association between age at trauma onset and severity of depression and PTSD in adults. J Child Sex Abuse 2010; 19:156–170Crossref, Medline, Google Scholar
54 : A developmental approach to complex PTSD: childhood and adult cumulative trauma as predictors of symptom complexity. J Trauma Stress 2009; 22:399–408Crossref, Medline, Google Scholar
55 : The concept of post-traumatic mood disorder and its implications for adolescent suicidal behavior. Minerva Pediatr 2008; 60:1393–1399Medline, Google Scholar
56 : Dissociation, PTSD, and substance abuse: an empirical study. J Trauma Dissociation 2012; 13:115–126Crossref, Medline, Google Scholar
57 : Relationships among childhood trauma, posttraumatic stress disorder, and dissociation in men living with HIV/AIDS. J Trauma Dissociation 2012; 13:102–114Crossref, Medline, Google Scholar
58 : Alexithymia in PTSD: psychometric and fMRI studies. Ann N Y Acad Sci 2006; 1071:397–400Crossref, Medline, Google Scholar
59 : Disorders of extreme stress: the empirical foundation of a complex adaptation to trauma. J Trauma Stress 2005; 18:389–399Crossref, Medline, Google Scholar
60 : Complex PTSD in victims exposed to sexual and physical abuse: results from the DSM-IV field trial for posttraumatic stress disorder. J Trauma Stress 1997; 10:539–555Crossref, Medline, Google Scholar
61 : Structural and functional brain changes in posttraumatic stress disorder. J Clin Psychiatry 2004; 65(suppl 1):11–17Medline, Google Scholar
62 : Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002; 5:1242–1247Crossref, Medline, Google Scholar
63 : MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry 2003; 160:924–932Link, Google Scholar
64 : Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 2008; 299:1291–1305Crossref, Medline, Google Scholar
65 : Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology 2010; 35:1684–1692Crossref, Medline, Google Scholar
66 : Relationship of childhood sexual and physical abuse to anxiety disorders. J Nerv Ment Dis 1995; 183:309–314Crossref, Medline, Google Scholar
67 : Childhood maltreatment linked to greater symptom severity and poorer quality of life and function in social anxiety disorder. Depress Anxiety 2009; 26:1027–1032Crossref, Medline, Google Scholar
68 : Childhood maltreatment and social anxiety disorder: implications for symptom severity and response to pharmacotherapy. Depress Anxiety 2012; 29:131–138Crossref, Medline, Google Scholar
69 : The human dimension: how the prefrontal cortex modulates the subcortical fear response. Rev Neurosci 2007; 18:191–207Crossref, Medline, Google Scholar
70 : [Neuroimaging and neurobiology of social anxiety]. Riv Psichiatr 2010; 45:349–360 (Italian)Medline, Google Scholar
71 : The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010; 35:169–191Crossref, Medline, Google Scholar
72 : Heightened neural reactivity to threat in child victims of family violence. Curr Biol 2011; 21:R947–R948Crossref, Medline, Google Scholar
73 : The long-term effects of childhood maltreatment experiences on subsequent illicit drug use and drug-related problems in young adulthood. Addict Behav 2011; 36:95–102Crossref, Medline, Google Scholar
74 : The impact of childhood maltreatment on young adults’ substance abuse. Am J Drug Alcohol Abuse 2007; 33:139–146Crossref, Medline, Google Scholar
75 : Childhood maltreatment histories, alcohol and other drug use symptoms, and sexual risk behavior in a treatment sample of adolescents. Am J Public Health 2012; 102(suppl 2):S250–S257Crossref, Medline, Google Scholar
76 : High prevalence of childhood emotional, physical, and sexual trauma among a Canadian cohort of HIV-seropositive illicit drug users. AIDS Care 2011; 23:714–721Crossref, Medline, Google Scholar
77 : Psychological distress in childhood trauma survivors who abuse drugs. Am J Drug Alcohol Abuse 2002; 28:1–13Crossref, Medline, Google Scholar
78 : Predicting personality pathology among adult patients with substance use disorders: effects of childhood maltreatment. Addict Behav 1998; 23:855–868Crossref, Medline, Google Scholar
79 : Childhood trauma and injection drug use among high-risk youth. J Adolesc Health 2009; 45:300–302Crossref, Medline, Google Scholar
80 : Clinical outcomes of traumatized youth in adolescent substance abuse treatment: a longitudinal multisite study. J Psychoactive Drugs 2008; 40:77–84Crossref, Medline, Google Scholar
81 : The impact of early trauma and abuse on residential substance abuse treatment outcomes for women. J Subst Abuse Treat 2008; 34:90–100Crossref, Medline, Google Scholar
82 : Impact of victimization on substance abuse treatment outcomes for adolescents in outpatient and residential substance abuse treatment. Am J Addict 2006; 15(suppl 1):34–42Crossref, Medline, Google Scholar
83 : Integrated treatment for the survivor of childhood trauma who is chemically dependent. J Psychoactive Drugs 1994; 26:369–378Crossref, Medline, Google Scholar
84 ;
85 : Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI Study. Addict Biol 2010; 15:504–516Crossref, Medline, Google Scholar
86 : Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci USA 2011; 108:15037–15042Crossref, Medline, Google Scholar
87 : Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol Psychiatry 2009; 66:206–213Crossref, Medline, Google Scholar
88 : Harsh corporal punishment is associated with increased T2 relaxation time in dopamine-rich regions. Neuroimage 2010; 53:412–419Crossref, Medline, Google Scholar
89 : Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci 2010; 30:7466–7472Crossref, Medline, Google Scholar
90 : Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Ann N Y Acad Sci 1997; 821:160–175Crossref, Medline, Google Scholar
91 : Developmental traumatology, part II: brain development. Biol Psychiatry 1999; 45:1271–1284Crossref, Medline, Google Scholar
92 : Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry 2002; 52:1066–1078Crossref, Medline, Google Scholar
93 : Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci 2008; 20:292–301Link, Google Scholar
94 : Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry 2004; 56:80–85Crossref, Medline, Google Scholar
95 : Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry Res 2008; 162:256–261Crossref, Medline, Google Scholar
96 : Hurtful words: association of exposure to peer verbal abuse with elevated psychiatric symptom scores and corpus callosum abnormalities. Am J Psychiatry 2010; 167:1464–1471Link, Google Scholar
97 : Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: a preliminary report. Biol Psychiatry 1997; 41:23–32Crossref, Medline, Google Scholar
98 : Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res 2010; 44:799–807Crossref, Medline, Google Scholar
99 : Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 1997; 27:951–959Crossref, Medline, Google Scholar
100 : Hippocampal and amygdalar volumes in dissociative identity disorder. Am J Psychiatry 2006; 163:630–636Link, Google Scholar
101 : Reduced amygdala and hippocampus size in trauma-exposed women with borderline personality disorder and without posttraumatic stress disorder. J Psychiatry Neurosci 2009; 34:383–388Medline, Google Scholar
102 : Anatomical MRI study of borderline personality disorder patients. Psychiatry Res 2004; 131:125–133Crossref, Medline, Google Scholar
103 : Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry 2000; 57:1115–1122Crossref, Medline, Google Scholar
104 : Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res 2003; 122:193–198Crossref, Medline, Google Scholar
105 : Childhood maltreatment is associated with reduced volume in hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA 2012; 109:E563–E572Crossref, Medline, Google Scholar
106 : Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics 2007; 119:509–516Crossref, Medline, Google Scholar
107 : Segmented hippocampal volume in children and adolescents with posttraumatic stress disorder. Biol Psychiatry 2006; 59:523–529Crossref, Medline, Google Scholar
108 : Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry 2001; 50:943–951Crossref, Medline, Google Scholar
109 : N-Acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. Am J Psychiatry 2000; 157:1175–1177Link, Google Scholar
110 : Preliminary evidence for aberrant cortical development in abused children: a quantitative EEG study. J Neuropsychiatry Clin Neurosci 1998; 10:298–307Crossref, Medline, Google Scholar
111 : Childhood sexual abuse is associated with reduced gray matter volume in visual cortex of young women. Biol Psychiatry 2009; 66:642–648Crossref, Medline, Google Scholar
112 : Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. Neuroimage 2011; 54(suppl 1):S280–S286Crossref, Medline, Google Scholar
113 : Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry 2002; 52:1089–1101Crossref, Medline, Google Scholar
114 : Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry 2006; 59:975–982Crossref, Medline, Google Scholar
115 : Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. Neuroimage 2009; 47(suppl 2):T66–T71Crossref, Medline, Google Scholar
116 : Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biol Psychiatry 2003; 53:879–889Crossref, Medline, Google Scholar
117 : Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am J Psychiatry 1999; 156:575–584Abstract, Google Scholar
118 : Abnormal T2 relaxation time in the cerebellar vermis of adults sexually abused in childhood: potential role of the vermis in stress-enhanced risk for drug abuse. Psychoneuroendocrinology 2002; 27:231–244Crossref, Medline, Google Scholar
119 : Cerebellar volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry 2006; 60:697–703Crossref, Medline, Google Scholar
120 : Amygdala, hippocampal, and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees Study pilot. J Child Psychol Psychiatry 2009; 50:943–951Crossref, Medline, Google Scholar
121 : Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci 2010; 13:46–61Crossref, Medline, Google Scholar
122 : Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci USA 2011; 108:14324–14329Crossref, Medline, Google Scholar
123 : Stress, sensitive periods, and maturational events in adolescent depression. Trends Neurosci 2008; 31:183–191Crossref, Medline, Google Scholar
124 : Delayed effects of early stress on hippocampal development. Neuropsychopharmacology 2004; 29:1988–1993Crossref, Medline, Google Scholar
125 : Early parental care is important for hippocampal maturation: evidence from brain morphology in humans. Neuroimage 2010; 49:1144–1150Crossref, Medline, Google Scholar
126 : Length of time between onset of childhood sexual abuse and emergence of depression in a young adult sample: a retrospective clinical report. J Clin Psychiatry 2009; 70:684–691Crossref, Medline, Google Scholar
127 : Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol Psychiatry 2009; 65:227–234Crossref, Medline, Google Scholar
128 : Reduced visual cortex gray matter volume and thickness in young adults who witnessed domestic violence during childhood. PLoS ONE 2012; 7:e52528Crossref, Medline, Google Scholar
129 : Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. Neuroimage 2012; 59:1071–1079Crossref, Medline, Google Scholar
130 : Synaptic Self: How Our Brains Become Who We Are. Harmondsworth, England, Viking Penguin, 2002Google Scholar
131 : Role of the ventral subiculum in stress integration. Behav Brain Res 2006; 174:215–224Crossref, Medline, Google Scholar
132 : Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 2000; 284:592–597Crossref, Medline, Google Scholar
133 : Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry 2007; 62:1080–1087Crossref, Medline, Google Scholar
134 : Effect of childhood physical abuse on cortisol stress response. Psychopharmacology (Berl) 2011; 214:367–375Crossref, Medline, Google Scholar
135 : Cortisol response to stress in female youths exposed to childhood maltreatment: results of the Youth Mood Project. Biol Psychiatry 2009; 66:62–68Crossref, Medline, Google Scholar
136 : The salivary alpha amylase over cortisol ratio as a marker to assess dysregulations of the stress systems. Physiol Behav 2012; 106:65–72Crossref, Medline, Google Scholar
137 : Diverse patterns of neuroendocrine activity in maltreated children. Dev Psychopathol 2001; 13:677–693Crossref, Medline, Google Scholar
138 : Timing matters: long term effects of adversities from prenatal period up to adolescence on adolescents’ cortisol stress response: the TRAILS study. Psychoneuroendocrinology 2012; 37:1439–1447Crossref, Medline, Google Scholar
139 : Trauma-related predictors of deontic reasoning: a pilot study in a community sample of children. Child Abuse Negl 2008; 32:732–737Crossref, Medline, Google Scholar
140 : Attachment organization, emotion regulation, and expectations of support in a clinical sample of women with childhood abuse histories. J Trauma Stress 2008; 21:282–289Crossref, Medline, Google Scholar
141 : Interventions to prevent child maltreatment and associated impairment. Lancet 2009; 373:250–266Crossref, Medline, Google Scholar
142 : Affect Regulation, Mentalization, and the Development of the Self. New York, Other Press LLC, 2002Google Scholar
143 : Treating complex traumatic stress disorders: an evidence-based guide, in Best Practices in Psychotherapy for Adults. Edited by Courtois CAFord JD. New York, Guilford, 2009, pp 82–103Google Scholar



