MRI and PET Study of Deficits in Hippocampal Structure and Function in Women With Childhood Sexual Abuse and Posttraumatic Stress Disorder
Abstract
OBJECTIVE: Animal studies have suggested that early stress is associated with alterations in the hippocampus, a brain area that plays a critical role in learning and memory. The purpose of this study was to measure both hippocampal structure and function in women with and without early childhood sexual abuse and the diagnosis of posttraumatic stress disorder (PTSD). METHOD: Thirty-three women participated in this study, including women with early childhood sexual abuse and PTSD (N=10), women with abuse without PTSD (N=12), and women without abuse or PTSD (N=11). Hippocampal volume was measured with magnetic resonance imaging in all subjects, and hippocampal function during the performance of hippocampal-based verbal declarative memory tasks was measured by using positron emission tomography in abused women with and without PTSD. RESULTS: A failure of hippocampal activation and 16% smaller volume of the hippocampus were seen in women with abuse and PTSD compared to women with abuse without PTSD. Women with abuse and PTSD had a 19% smaller hippocampal volume relative to women without abuse or PTSD. CONCLUSIONS: These results are consistent with deficits in hippocampal function and structure in abuse-related PTSD.
Childhood sexual abuse is a major public health problem that affects 16% of women in this country at some time before their 18th birthday (1) and is a common cause of posttraumatic stress disorder (PTSD) (2, 3). Despite the magnitude of the problem, little is known about possible changes in brain structure and function in abuse-related PTSD. In addition to symptoms such as nightmares, hyperarousal, impaired social functioning, and sleep disturbance, alterations in several facets of memory are a prominent part of the presentation of patients with PTSD. These memory alterations include amnesia, flashbacks, impaired learning and concentration, and intrusive memories. These observations have led to the hypothesis that brain regions involved in memory, including the hippocampus and prefrontal cortex, mediate symptoms of PTSD (4).
The hippocampus plays a critical role in learning and memory (5). Patients with hippocampal lesions perform poorly on tests of verbal declarative memory, including tasks involving recall of a paragraph or learning a list of words (6), with the degree of dysfunction correlating with hippocampal volume and cell loss (7–10). Although studies have suggested that the left hippocampus plays a greater role in verbal memory, verbal memory impairment has been shown to be more severe with bilateral hippocampal lesions, compared to unilateral lesions (10), indicating that both hippocampi play a role in verbal memory.
Studies in animals have shown that the hippocampus is sensitive to stress. Animals exposed to stress showed deficits in hippocampal-based memory function (11, 12), alterations in hippocampal morphology (13, 14), and inhibition of neurogenesis (or the growth of new neurons) (15) that were reversible with treatment (16). A variety of mechanisms have been proposed for these findings, including elevated levels of glucocorticoids released during stress (17, 18), stress-related inhibition of brain-derived neurotrophic factor (19, 20), and changes in serotonergic function (21), although mechanisms continue to be debated (22).
These studies led to efforts to determine if stress-induced hippocampal damage is seen in PTSD patients (23). Patients with PTSD from both combat and early childhood abuse had deficits in verbal declarative memory function, including tasks such as paragraph recall and word list learning (24–29). One study found that patients with combat-related PTSD had an 8% reduction in right hippocampal volume, as measured with magnetic resonance imaging (MRI), that was correlated with deficits in memory function (30). Other studies found smaller left hippocampal volumes in abused women with PTSD (31, 32) and smaller bilateral hippocampal volumes in patients with combat-related PTSD (33). Two studies found reduced hippocampal N-acetylaspartate, a marker of hippocampal integrity, in combat-related PTSD (34, 35), although one of those studies did not find a corresponding volume reduction (35). Studies in children (36, 37) and patients with new-onset PTSD did not find smaller hippocampal volumes (38). Some studies have also found smaller hippocampal volume in depression, another disorder that has been linked to stress (39–41). These studies suggest that deficits in the hippocampus may be associated with chronic PTSD.
Studies of the hippocampus in PTSD have left several unanswered questions. It is not known whether structural changes in the hippocampus are specific to abuse and PTSD or are a nonspecific effect of childhood abuse alone. Although deficits in hippocampal-based verbal declarative memory function measured with neuropsychological testing are suggestive of hippocampal dysfunction, it is not known whether deficits in hippocampal function (best measured with functional neuroimaging in conjunction with the performance of hippocampal-based memory tasks) are associated with PTSD. We therefore conducted a study to look at hippocampal structure (measured with MRI) in women with and without early childhood sexual abuse and with and without the diagnosis of PTSD. We also looked at hippocampal function (with positron emission tomography [PET]) during the performance of verbal declarative memory tasks in women with abuse with and without PTSD.
Method
Subject Recruitment and Assessment
Thirty-three women with and without early childhood sexual abuse and PTSD participated in the study. Abused women had a history of severe childhood (before the age of 18 years) sexual abuse, including rape, genital fondling, or attempted rape. The subjects included women with childhood sexual abuse with PTSD (N=10), women with abuse without PTSD (N=12), and women without abuse or PTSD (N=11). The women in this study represented a different group from the subjects we have previously reported on in studies of hippocampal volume in PTSD (30, 31). All women participated in the MRI component of the study. Only women with abuse, with or without PTSD, participated in the PET part of the study. All subjects were recruited through newspaper advertisements placed in local newspapers. The advertisements stated that women volunteers with a history of early life stress were requested for brain imaging studies. A second advertisement stated that healthy women volunteers were requested for brain imaging studies. The diagnosis of PTSD was established with the Structured Clinical Interview for DSM-IV (SCID) (42) conducted by a master’s-level psychologist. Interrater reliability for the SCID at our site was high (intraclass correlation coefficient [ICC]=0.95). All subjects gave written informed consent for participation. Subjects were assessed with a history and physical examination, laboratory testing, and ECG to determine if they were free of medical illnesses that could affect brain structure or function. Individuals with serious medical illnesses were excluded. The subjects were not actively abusing substances or alcohol (within the past 6 months) and were free of all medications for at least 4 weeks before the study. The subjects’ medications were not discontinued for the purposes of participating in the study. Subjects with a serious medical or neurological illness, organic mental disorders, comorbid psychotic disorders, retained metal, or a history of head trauma, loss of consciousness, cerebral infectious disease, or dyslexia were excluded. All subjects were right-handed. No difference in age was found between the women with abuse and PTSD (mean=35 years, SD=6), the women with abuse without PTSD (mean=32 years, SD=8), and the women without abuse or PTSD (mean=38 years, SD=7). Likewise, the three groups did not differ in years of education (women with abuse and PTSD: mean=15 years, SD=2; women with abuse without PTSD: mean=17 years, SD=3; and women without abuse or PTSD: mean=15 years, SD=2).
History of childhood abuse was assessed with the Early Trauma Inventory by a master’s-level psychologist. The Early Trauma Inventory is a 56-item clinician-administered interview that assesses physical, emotional, and sexual abuse, as well as general traumatic events. The Early Trauma Inventory has been demonstrated to be reliable and valid in the assessment of childhood trauma (43); at our site the interrater reliability was high (ICC=0.99). As noted previously, all women in the abuse groups had a childhood history of severe sexual abuse, defined as rape, attempted rape, or molestation before the 18th birthday. However, women with PTSD had still greater sexual abuse severity, as measured by the Early Trauma Inventory Sexual Abuse Severity Index (abused women with PTSD: mean=948, SD=711; abused women without PTSD: mean=71, SD=136). Subjects with a history of abuse also underwent assessment of PTSD symptoms with the Civilian Mississippi Scale, a reliable and valid measure of PTSD symptom severity that is administered as a self-report measure (44). The mean Civilian Mississippi Scale score was 126 (SD=26) for the abused women with PTSD and 75 (SD=6) for the abused women without PTSD. Current dissociative state in the abused women was assessed by a master’s-level psychologist with the Clinician-Administered Dissociative States Scale, a reliable and valid measure of the severity of dissociative states (45); interrater reliability for the Clinician-Administered Dissociative States Scale at our site was high (ICC=0.92). The mean Clinician-Administered Dissociative States Scale scores were 11 (SD=10) for the abused women with PTSD and 2 (SD=3) for the abused women without PTSD.
On the basis of the SCID interview, eight (80%) of the 10 patients with PTSD fulfilled the criteria for a lifetime history of major depression and two (20%) fulfilled with criteria for current major depression. One patient (10%) met the criteria for a past history of dysthymia. Two (20%) fulfilled the criteria for a lifetime history of panic disorder with agoraphobia, two (20%) for current panic disorder with agoraphobia, and one (10%) for a lifetime history of panic disorder without agoraphobia. Four patients (40%) met the criteria for a past history of alcohol and/or substance abuse or dependence disorder; no patients had current disorders. The substance-related disorders included past alcohol dependence (30%), polysubstance dependence (10%), marijuana dependence (10%), and cocaine dependence (10%). Among the comparison group of sexually abused women without PTSD, three (25%) of 12 fulfilled the criteria for a lifetime history of major depression and none for current major depression on the basis of the SCID interview. One (8%) of 12 patients fulfilled the criteria for current panic disorder with agoraphobia. One (8%) of 12 fulfilled the criteria for lifetime obsessive-compulsive disorder, one (8%) for lifetime generalized anxiety disorder, and one (8%) for lifetime anorexia. One patient (8%) met the criteria for lifetime marijuana dependence, one (8%) for lifetime marijuana abuse, and one (8%) for lifetime cocaine dependence. None of the women without abuse or PTSD had a history of psychiatric disorder.
MRI Methods
All women underwent MRI imaging of the brain for measurement of hippocampal volume by using methods previously described (30). Coronal images were obtained throughout the brain by using a General Electric Signa 1.5-T MR imaging device (Milwaukee) with spoiled gradient recall acquisition T1-weighted images (TR=25, TE=5, NEX=2, 3-mm slice thickness without gaps). The images were transferred to a Sun Workstation (Sun Microsystems, Santa Clara, Calif.) for processing by using the ANALYZE program (Mayo Clinic, Rochester, N.Y.). Measurements of the mid-hippocampal body and whole brain volume were performed by using methods previously described (30, 31). Briefly, the hippocampus was outlined in five slices (15 mm) between the superior colliculus and the bifurcation of the basilar artery. Measurements were performed by researchers who were blind to the subject’s name and diagnosis. Interrater reliability for this method, as measured with ICCs, was as follows: left hippocampus (ICC=0.93, p<0.05); right hippocampus (ICC=0.94, p<0.05).
PET Methods
In the PET study, the abused women with PTSD were compared with those without PTSD by using PET methods previously described (46). Each subject underwent four PET scans on a single day. The subject was placed in the scanner with her head in a holder to minimize motion and was positioned with the canthomeatal line parallel to an external laser light. An intravenous line was inserted for administration of [15O]H2O. After the subject’s head was positioned within the camera gantry, a transmission scan of the head was obtained by using an external 67Ga/68Ge rod source, to correct emission data for attenuation due to overlying bone and soft tissue.
Subjects then underwent scanning during verbal declarative memory and control tasks. All tasks involved listening to a paragraph that was read aloud in a normal tone of voice over a 1-minute period. Encoding of paragraphs was selected as the task for assessment of neural correlates of verbal declarative memory function because prior studies have shown that PTSD patients have deficits on this task (24, 25). Subjects first underwent two scans while listening to two different paragraphs during which time they were instructed to count the number of times they heard the letter “d.” Then subjects underwent two scans during which they listened to one of two different paragraphs preceded by instructions to form an image in their mind and try and remember as much of the paragraph as they could. Five minutes later, subjects were asked to engage in free recall of the paragraph. Scores consisted of the number of elements of the paragraph correctly recalled. A fixed order (the control condition followed by the memory-encoding condition) was followed to minimize memory encoding during the control condition. According to the logic of the study design, differences in brain blood flow between the memory-encoding and the control conditions would be secondary to the specific effects of verbal declarative memory encoding, while keeping other factors equal, including attention, auditory perception, and comprehension of a coherent verbal narrative.
Ten seconds before administration of [15O]H2O, subjects received instructions related to the paragraph to be read. The reader then began the reading of the paragraph, which lasted a total of 60 seconds. At the same time the reader began reading, the subject received a bolus injection of 30 mCi of [15O]H2O, followed 10 seconds later by a PET scan acquisition that was 80 seconds in length. The onset of the PET scan acquisition was timed to correspond to the point of maximum increase in uptake of tracer into the brain. With the bolus injection method of [15O]H2O (which has a half-life of 110 seconds), the tracer peaks at 10 seconds, with 90% of counts obtained in the first 60 seconds after the peak, which was the time during which the paragraph was read. PET imaging was performed on a Posicam PET camera (Positron Corp., Houston) (in-plane resolution after filtering=6 mm full width at half maximum).
Images were reconstructed and analyzed on a SunSparc Workstation (Sun Microsystems, Santa Clara, Calif.) by using statistical parametric mapping (SPM 96) (Wellcome Department of Cognitive Neuroscience, Institute of Neurology, University College London). Images for each patient set were realigned to the first scan of the study session. The mean concentration of radioactivity in each scan was obtained as an area-weighted sum of the concentration for each slice and adjusted to a nominal value of 50 ml/min per 100 g. The data underwent transformation into a common anatomical space and were smoothed with a three-dimensional Gaussian filter to 16 mm full width at half maximum. Regional blood flow with global blood flow as a covariate was compared between the control and active conditions in sexually abused women with and without PTSD. The interaction between group (PTSD versus non-PTSD) and condition (control versus memory encoding) was also examined. Statistical analyses yielded image data sets in which the values assigned to individual voxels correspond to the t statistic (47). Statistical images were displayed with values of z score units. A threshold z score of 3.09 (p<0.001) was used to examine areas of activation within the hypothesized region (hippocampus). The threshold of the z score corresponds to a p value of <0.001 for a one-tailed t test. Since the current study was a replication of a prior study in combat veterans with PTSD, the direction of change in blood flow was hypothesized a priori, which justified the use of a one-tailed test. SPM 96 employs a fixed-effects analysis that may limit the ability to generalize from the findings for the study group. Location of areas of activation were identified as the distance from the anterior commissure in millimeters, with x, y, and z coordinates, using a standard stereotaxic atlas (48).
Data Analysis
Repeated-measures analysis of variance with side (left and right hippocampus) as the repeated measure was used to compare hippocampal volume between groups. Linear regression was used to compare the relationship of left and right hippocampal volume with left hippocampal blood flow during the memory task and variables hypothesized to be related to these parameters, including PTSD symptom level as measured with the Civilian Mississippi Scale and dissociative symptom level as measured with the Clinician-Administered Dissociative States Scale. Analysis of covariance was used to compare hippocampal volumes between groups, with adjustment for whole brain size.
Results
Hippocampal Structure Measured With MRI
Repeated-measures analysis of variance with side (left versus right hippocampal volume) as the repeated measure showed a significant difference in left and right hippocampal volumes between the abused women with PTSD, the abused women without PTSD, and the women without abuse or PTSD (F=4.96, df=2, 29, p=0.01). (Duncan’s multiple range test showed that the abused women with PTSD had a smaller mean volume of both the left and right hippocampus than both the abused women without PTSD and the women without abuse or PTSD [p<0.05].) The mean hippocampal volume of the abused women with PTSD was 16% smaller than that of the abused women without PTSD and 19% smaller than that of the women without abuse or PTSD. The mean left hippocampal volume of the abused women with PTSD (mean=973 mm3, SD=162) was 15% smaller than that of the abused women without PTSD (mean=1150 mm3, SD=189) and 17% smaller than that of the women without abuse or PTSD (mean=1160 mm3, SD=205). The mean right hippocampal volume of the abused women with PTSD (mean=915 mm3, SD=179) was 16% smaller than that of the abused women without PTSD (mean=1101 mm3, SD=174) and 22% smaller than that of the women without abuse or PTSD (mean=1180 mm3, SD=213) (Figure 1). There was no difference in whole brain volume between the abused women with PTSD (mean=1,157,160 mm3, SD=80,549), the abused women without PTSD (mean=1,200,905 mm3, SD=56,836), and the women without abuse or PTSD (mean=1,192,597 mm3, SD=117,879). The finding of smaller hippocampal volumes in abused women with PTSD, compared to the other groups, was significant after adjustment for differences in whole brain volumes by using analysis of covariance (p<0.05).
Hippocampal Function Measured With PET
For the memory tasks performed during the PET study there were no significant differences between the abused women with and without PTSD in performance for recall of the paragraph after the memory-encoding condition, as measured by the number of correctly recalled elements (mean=46, SD=16, versus mean=50, SD=19).
Abused women without PTSD had increased blood flow in the left hippocampus during verbal memory encoding relative to the control task, and abused women with PTSD had failure of left hippocampal activation (Figure 2). No significant difference in blood flow during the control task was found between groups; however, significantly greater increases in blood flow during verbal memory encoding, relative to the control task, were found in the left hippocampus in the women without PTSD than in the women with PTSD (F=14.93, df=1, 20, p<0.001; Talairach coordinates x=–12, y=–36, z=4) (Figure 3). These differences were statistically significant after adjustment for left hippocampal volume, suggesting that failure of activation in the abused women with PTSD was not secondary to smaller hippocampal volume in those patients. Other areas of activation during verbal memory encoding in the women without PTSD (at the p<0.001 threshold of significance) included the right superior temporal gyrus (Brodmann’s area 22), right inferior frontal gyrus (Brodmann’s area 45), bilateral somatosensory cortex (Brodmann’s areas 40, 43, 4), and cerebellum; areas of decreased blood flow in the women without PTSD included the left superior and middle frontal gyrus (Brodmann’s areas 8, 9), anterior cingulate (Brodmann’s area 32), and left fusiform and left inferior temporal gyrus (Brodmann’s area 20). Increased blood flow in the PTSD group was found in the bilateral middle (Brodmann’s areas 10, 44, 45) and inferior (Brodmann’s areas 45, 46) frontal gyrus, anterior cingulate (Brodmann’s area 32), left inferior parietal lobule (Brodmann’s area 40), left superior temporal gyrus (Brodmann’s areas 39, 21, 22), and right visual association cortex (Brodmann’s area 19); areas of decreased blood flow in the women with PTSD included the orbitofrontal cortex (Brodmann’s area 11), right superior frontal gyrus (Brodmann’s area 10), and cerebellum. Direct between-group contrasts showed that, in addition to greater hippocampal activation during memory encoding, women without PTSD had greater blood flow increases in the cerebellum and greater decreases in the bilateral inferior frontal gyrus (Brodmann’s areas 44, 45) during memory encoding relative to the control condition (Table 1).
Relationship Between Brain and Behavior
We also examined the relationship between left hippocampal volume and behavioral measures of dissociation (Clinician-Administered Dissociative States Scale), PTSD symptom severity (Civilian Mississippi Scale), and abuse severity (Early Trauma Inventory). Linear regression demonstrated a significant relationship between higher dissociative symptom level as measured with the Clinician-Administered Dissociative States Scale and smaller left hippocampal volume (R2=0.30; t=–2.16, df=1, p<0.05) (Figure 4). Linear regression also showed a relationship between greater PTSD symptom severity, as measured with the Civilian Mississippi Scale, and smaller right hippocampal volume (R2=0.27; t=–2.36, df=1, p<0.05). There was no correlation between left hippocampal volume and left hippocampal blood flow during activation tasks in either the women with PTSD or those without PTSD. There was no relationship between left hippocampal blood flow and dissociative or PTSD symptom levels.
Discussion
The findings of the current study are consistent with deficits in both hippocampal structure and function in PTSD related to early childhood sexual abuse in women. In this study, abused women with PTSD had a 16% lower mean hippocampal volume than abused women without PTSD and a 19% lower mean hippocampal volume than women without abuse or PTSD. These differences were significant after adjustment for differences in whole brain volume. The women with PTSD also showed a failure of left hippocampal activation during a verbal memory task (paragraph encoding) that was significant after adjustment for hippocampal atrophy. Measures of symptoms of dissociation were correlated with smaller left hippocampal volume, and measures of PTSD symptoms were correlated with smaller right hippocampal volume.
These findings are similar to those of prior studies of patients with PTSD. The current study represents the third independent replication of smaller hippocampal volume in PTSD from our own group. These findings also provide evidence that smaller hippocampal volume in patients is not seen in all individuals with a history of childhood abuse but is seen only in individuals who have both a history of abuse and the diagnosis of PTSD. This finding corroborates a previous study in combat veterans (33) and suggests a correlation between changes in the brain and PTSD symptoms. To our knowledge, this is the first report showing failure of hippocampal activation during a memory task (during the encoding of a paragraph) in PTSD, which adds corroborative evidence for deficits in the hippocampus in PTSD. The findings provide a structural and functional neuroanatomical correlate for the deficits in neuropsychological testing of declarative memory found in patients with PTSD and provide support for the hypothesis that stress is associated with hippocampal damage or dysfunction.
The deficits in hippocampal structure and function appear to be specific to the diagnosis of PTSD and not a nonspecific outcome of exposure to childhood abuse. A number of studies have shown that PTSD is associated with long-term changes in brain structures and systems that mediate memory and the stress response (23, 49). However, only 15% of individuals exposed to a traumatic stressor (defined as a threat to the life of self or significant other, accompanied by intense fear, horror, or distress) will develop chronic symptoms of PTSD. A critical question in the field is why, among individuals exposed to a given level of stressor, some develop chronic PTSD symptoms and others do not. It is possible that abused women who develop stress-induced neurological deficits, including hippocampal deficits, develop PTSD, while abused women who do not develop hippocampal deficits do not develop PTSD. Another possibility is that, in abused women who develop PTSD, the stress of the disorder, including repeated traumatic reminders with activation of stress responses, leads to hippocampal damage and/or inhibition of neurogenesis (15, 16). A third possibility is that individuals with baseline hippocampal deficits have a genetic vulnerability to develop PTSD after being abused (49). The findings of the current study are consistent with the idea that abuse per se in the absence of PTSD does not lead to deficits in hippocampal structure or function. Additional research is needed to improve our understanding of why some individuals develop PTSD in response to a stressor and others do not.
Several aspects of this study deserve comment. We did not find a significant difference in memory performance on the paragraph recall component of the study between women with and without PTSD, which contradicts prior studies of verbal declarative memory function in PTSD (24–29). However, prior studies that focused on neuropsychological testing alone had larger numbers of subjects. PTSD patients in the current study did show a pattern of poorer performance (8% difference) that might be statistically significant in a larger group of subjects.
We performed several correlations of dissociation (Clinician-Administered Dissociative States Scale), PTSD symptoms (Mississippi scale), and abuse severity (Early Trauma Inventory) with left and right hippocampal volume. These analyses showed a correlation between smaller left hippocampal volume and dissociation (Clinician-Administered Dissociative States Scale) and smaller right hippocampal volume and PTSD (Mississippi scale). Because of the multiple comparisons involved in these correlations, the findings should be considered preliminary. However, the finding of a correlation between smaller left hippocampal volume and dissociation replicates a finding of a prior study in abuse-related PTSD (32).
The PET study involving measurement of hippocampal activation with memory tasks did not include a comparison group of women without abuse or psychiatric disorder. Therefore, we do not have information about the performance of this task in normal women. However, we subsequently studied brain activation using this same task in normal subjects (men and women) and demonstrated hippocampal activation (data not shown); this result suggests the validity of this task for measuring hippocampal activation in normal individuals (unpublished 2002 data of J.D. Bremner, et al.).
The women with abuse-related PTSD in the current study showed the typical dilemmas of multiple comorbidities of past history of alcohol or substance abuse/dependence and depression. Although we are confident that current or even recent alcohol or substance abuse/dependence did not confound our results, it is possible that past histories could be a confounding factor. Other studies based on national samples have shown that women with PTSD had a 28% rate of a past history of alcohol abuse/dependence, compared with 14% in women without PTSD (3). Women with PTSD also had a 46% lifetime rate of comorbid depression, compared with 10% for women without PTSD. In addition, women with PTSD had higher rates of all anxiety disorders, including panic disorder. We did not exclude women with comorbid affective and anxiety disorders or past alcohol abuse/dependence, as we felt that these criteria would result in selection of subjects who were not representative of women with abuse-related PTSD at large. We did exclude women with a history of schizophrenia, eating disorders, or bipolar disorder and women with current alcohol/substance use disorders. Bremner (23) has argued that these commonly comorbid disorders should be viewed as representing a group of “trauma-spectrum” disorders that have their basis in a stress-mediated effect on the brain. Friedman and Yehuda (50) have argued against exclusion of patients with comorbid depression from PTSD studies, based on the absence of biological findings to support differences in PTSD patients with and without histories of comorbid depression and doubts about the truly distinct nature of depression as a separate entity in PTSD patients when this disorder follows the development of PTSD.
The current study used a declarative memory task (paragraph recall) to study hippocampal activation. However, despite the evidence from lesion studies that the hippocampus plays an important role in new learning and memory (6), prior PET studies have not in fact consistently found activation in the hippocampus during memory tasks (51–57). Some researchers have argued that the hippocampus is activated only during successful retrieval, during learning of novel material, or during encoding (but not retrieval) of memories (58). To our knowledge, the current study is the first study to use paragraph encoding as a probe of hippocampal function. Paragraph encoding involves a complex synthesis of speech comprehension, assessment of lingual structure and syntax, comparison with prior experiences, and visual imagery. The complex role of paragraph encoding is consistent with the integrative role in memory formation hypothesized for the hippocampus (5). Memory encoding (as opposed to retrieval) was selected, as most prior PET studies have found more robust hippocampal activations with encoding tasks. For these reasons, and because we found robust deficits in paragraph recall in patients with PTSD, we used encoding of a paragraph as the probe of hippocampal function.
This study adds to a growing literature indicating hippocampal dysfunction in PTSD. In our own research we have used a multifaceted approach to study brain areas, including structural imaging, specific cognitive probes of function in individual brain regions (such as memory encoding as a probe of hippocampal function), and stimulus of specific PTSD symptoms (e.g., stimulation of traumatic recall with scripts of childhood abuse). These studies have provided convergent evidence that common brain areas, including the hippocampus, mediate both the cognitive and the symptomatic disturbances in PTSD. These studies and others have implicated in addition to the hippocampus an interconnected network of brain areas such as the medial prefrontal cortex, including the anterior cingulate proper (Brodmann’s area 32) and other areas of the anterior cingulate (subgenual area [Brodmann’s area 24] and the subcallosal area [Brodmann’s area 25]) (46, 59, 60); the inferior frontal gyrus; the inferior and middle temporal gyri; the posterior cingulate; and the visual association cortex (61–68). Given the known role of the hippocampus in mediating emotional processing of complex visual stimuli (69, 70), it is not surprising that the same region is involved in both cognitive and symptomatic processes in PTSD.
 |
Received Feb. 22, 2002; revisions received July 10 and Nov. 5, 2002; accepted Nov. 8, 2002. From the Departments of Psychiatry and Behavioral Sciences, Radiology, and Medicine (Cardiology), and the Emory Center for Positron Emission Tomography, Emory University School of Medicine, Atlanta; the Atlanta Veterans Affairs Medical Center, Decatur, Ga.; the Mood and Anxiety Disorders Program, NIMH, Bethesda, Md.; and the Departments of Psychiatry, Medicine (Cardiology), and Radiology, Yale University School of Medicine, New Haven, Conn. Address correspondence to Dr. Bremner, Emory Clinical Neuroscience Research Unit, 1256 Briarcliff Rd., Atlanta GA 30306; [email protected] (e-mail). This study was supported NIMH grants 1R01MH-56120-01A1 and HL-059619-02, VA Career Development Award to Dr. Bremner, a General Clinical Research Center Clinical Associate Physician Award to Dr. Bremner, and a grant from the VA National Center for Posttraumatic Stress Disorder. The authors thank Helen Sayward, M.S., for assistance in image processing and analysis; Susan Insall, R.N., M.S.W., Sandi Capelli, R.N., and Jacque Piscitelli, M.S.W., for assistance in patient recruitment, assessment, and scanning; Chris Cooper, C.N.M.T., for assistance in PET image acquisition; and Ryan Wagner for assistance in preparation of radiopharmaceuticals.
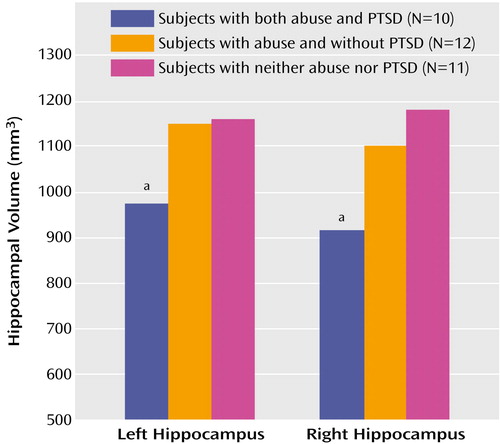
Figure 1. Left and Right Hippocampal Volumes as Measured With MRI in Women With and Without Childhood Sexual Abuse and PTSD
aWomen with abuse and PTSD differed significantly from women with abuse and without PTSD and women without abuse or PTSD.
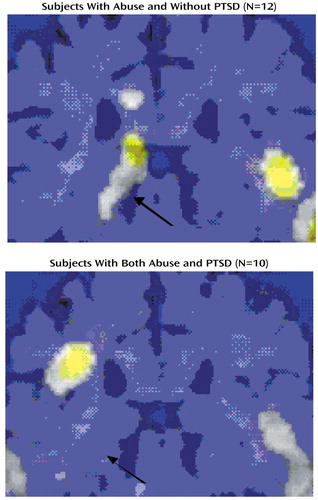
Figure 2. Hippocampal Areas of Significant Increases in Cerebral Blood Flow During a Verbal Memory Encoding Task, Relative to a Control Condition, in Women With Childhood Sexual Abuse Without and With PTSDa
aCerebral blood flow was measured with [ 15O]H2O positron emission tomography while subjects listened to a paragraph read aloud and counted the number of times they heard the letter “d” (control condition) and while subjects attempted to recall as much as they could of a different paragraph that had just been read aloud (verbal memory encoding task). Each panel shows a statistical parametric map overlaid on an MRI template on which areas of significant activation (z score>3.00, p<0.001) are highlighted in yellow. Areas of significant increases in blood flow were found in the left hippocampus in sexually abused women without PTSD but not in sexually abused women with PTSD (z>3.09, p<0.001) (arrow).
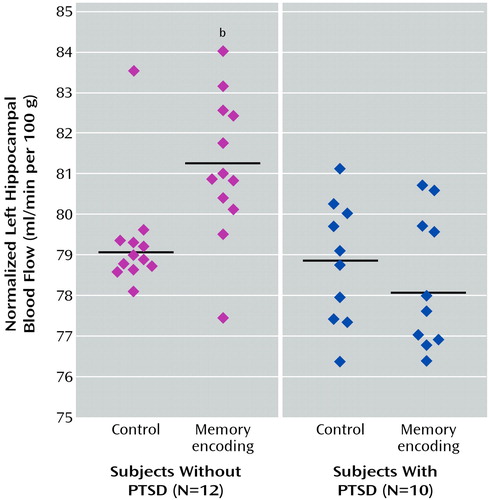
Figure 3. Left Hippocampal Blood Flow Normalized to Global Blood Flow During a Verbal Memory Encoding Task, Relative to a Control Condition, in Women With a History of Childhood Sexual Abuse Without and With PTSDa
aCerebral blood flow was measured with [ 15O]H2O positron emission tomography while subjects listened to a paragraph read aloud and counted the number of times they heard the letter “d” (control condition) and while subjects attempted to recall as much as they could of a different paragraph that had just been read aloud (verbal memory encoding task).
bSignificantly greater increase in blood flow during the verbal memory encoding task in subjects without PTSD, compared to subjects with PTSD (F=14.93, df=1, 20, p<0.001). The difference was statistically significant after adjustment for left hippocampal volume, suggesting that failure of activation was not secondary to smaller hippocampal volume in patients with PTSD.
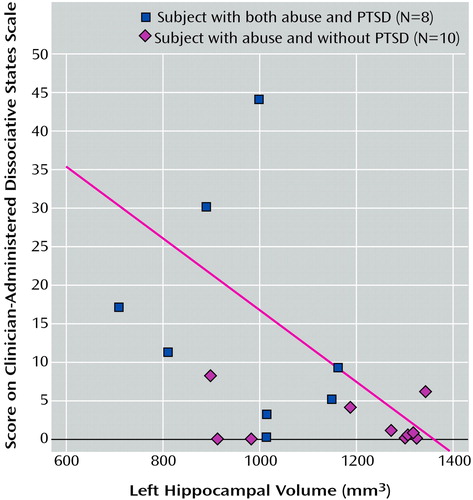
Figure 4. Relationship Between Left Hippocampal Volume and Dissociative Symptom Level in Women With a History of Childhood Sexual Abuse With and Without PTSDa
aLogistic regression showed a significant relationship between higher dissociative symptom level, as measured with the Clinician-Administered Dissociative States Scale, and smaller left hippocampal volume, as measured with MRI (R2=0.30; t=–2.16, df=1, p<0.05).
1. McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, Ryden J, Derogatis LR, Bass EG: Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA 1997; 277:1362-1368Crossref, Medline, Google Scholar
2. Saigh PA, Bremner JD (eds): Posttraumatic Stress Disorder: A Comprehensive Text. New York, Allyn & Bacon, 1999Google Scholar
3. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB: Posttraumatic Stress Disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995; 52:1048-1060Crossref, Medline, Google Scholar
4. Bremner JD, Southwick SM, Charney DS: The neurobiology of posttraumatic stress disorder: an integration of animal and human research, in Posttraumatic Stress Disorder: A Comprehensive Text. Edited by Saigh PA, Bremner JD. New York, Allyn & Bacon, 1999, pp 103-143Google Scholar
5. Zola-Morgan SM, Squire LR: The primate hippocampal formation: evidence for a time-limited role in memory storage. Science 1990; 250:288-290Crossref, Medline, Google Scholar
6. Scoville WB, Milner B: Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 1957; 20:11-21Crossref, Medline, Google Scholar
7. Sass KJ, Sass A, Westerveld M, Lencz T, Novelly RA, Kim JH, Spencer DD: Specificity in the correlation of verbal memory and hippocampal neuron loss: dissociation of memory, language, and verbal intellectual ability. J Clin Exp Neuropsychol 1992; 14:662-672Crossref, Medline, Google Scholar
8. Sass KJ, Westerveld M, Buchanan CP, Spencer SS, Kim JH, Spencer DD: Degree of hippocampal neuron loss determines severity of verbal memory decrease after left anteromesiotemporal lobectomy. Epilepsia 1994; 35:1179-1186Crossref, Medline, Google Scholar
9. Lencz T, McCarthy G, Bronen RA, Scott TM, Inserni JA, Sass KJ, Novelly RA, Kim JH, Spencer DD: Quantitative magnetic resonance imaging studies in temporal lobe epilepsy: relationship to neuropathology and neuropsychological function. Ann Neurol 1992; 31:629-637Crossref, Medline, Google Scholar
10. Sass KJ, Spencer DD, Kim JH, Westerveld M, Novelly RA, Lencz T: Verbal memory impairment correlates with hippocampal pyramidal cell density. Neurology 1990; 40:1694-1697Crossref, Medline, Google Scholar
11. Diamond DM, Fleshner M, Ingersoll N, Rose GM: Psychological stress impairs spatial working memory: relevance to electrophysiological studies of hippocampal function. Behav Neurosci 1996; 110:661-672Crossref, Medline, Google Scholar
12. Luine V, Villages M, Martinex C, McEwen BS: Repeated stress causes reversible impairments of spatial memory performance. Brain Res 1994; 639:167-170Crossref, Medline, Google Scholar
13. Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM: Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci 1989; 9:1705-1711Crossref, Medline, Google Scholar
14. Sapolsky RM, Uno H, Rebert CS, Finch CE: Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci 1990; 10:2897-2902Crossref, Medline, Google Scholar
15. Gould E, McEwen BS, Tanapat P, Galea LAM, Fuchs E: Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 1997; 17:2492-2498Crossref, Medline, Google Scholar
16. Malberg JE, Eisch AJ, Nestler EJ, Duman RS: Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 2000; 20:9104-9110Crossref, Medline, Google Scholar
17. Sapolsky RM: Why stress is bad for your brain. Science 1996; 273:749-750Crossref, Medline, Google Scholar
18. Lawrence MS, Sapolsky RM: Glucocorticoids accelerate ATP loss following metabolic insults in cultured hippocampal neurons. Brain Res 1994; 646:303-306Crossref, Medline, Google Scholar
19. Nibuya M, Morinobu S, Duman RS: Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 1995; 15:7539-7547Crossref, Medline, Google Scholar
20. Smith MA, Makino S, Kvetnansky R, Post RM: Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNA in the hippocampus. J Neurosci 1995; 15:1768-1777Crossref, Medline, Google Scholar
21. McEwen BS, Angulo J, Cameron H, Chao HM, Daniels D, Gannon MN, Gould E, Mendelson S, Sakai R, Spencer R, Woolley C: Paradoxical effects of adrenal steroids on the brain: protection versus degeneration. Biol Psychiatry 1992; 31:177-199Crossref, Medline, Google Scholar
22. Leverenz JB, Wilkinson CW, Wamble M, Corbin S, Grabber JE, Raskind MA, Peskind ER: Effect of chronic high-dose exogenous cortisol on hippocampal neuronal number in aged nonhuman primate. J Neurosci 1999; 19:2356-2361Crossref, Medline, Google Scholar
23. Bremner JD: Does Stress Damage the Brain? Understanding Trauma-Related Disorders From a Mind-Body Perspective. New York, WW Norton, 2002Google Scholar
24. Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, Innis RB, McCarthy G, Charney DS: Deficits in short-term memory in posttraumatic stress disorder. Am J Psychiatry 1993; 150:1015-1019Link, Google Scholar
25. Bremner JD, Randall PR, Capelli S, Scott T, McCarthy G, Charney DS: Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Res 1995; 59:97-107Crossref, Medline, Google Scholar
26. Uddo M, Vasterling JT, Brailey K, Sutker PB: Memory and attention in posttraumatic stress disorder. J Psychopathology Behav Assess 1993; 15:43-52Crossref, Google Scholar
27. Yehuda R, Keefe RSE, Harvey PD, Levengood RA, Gerber DK, Geni J, Siever LJ: Learning and memory in combat veterans with posttraumatic stress disorder. Am J Psychiatry 1995; 152:137-139Link, Google Scholar
28. Vasterling JJ, Brailey K, Constans JI, Sutker PB: Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology 1998; 12:125-133Crossref, Medline, Google Scholar
29. Gilbertson MW, Gurvits TV, Lasko NB, Orr SP, Pitman RK: Multivariate assessment of explicit memory function in combat veterans with posttraumatic stress disorder. J Trauma Stress 2001; 14:413-420Crossref, Medline, Google Scholar
30. Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB: MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995; 152:973-981Link, Google Scholar
31. Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure CM, Capelli S, McCarthy G, Innis RB, Charney DS: MRI-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: a preliminary report. Biol Psychiatry 1997; 41:23-32Crossref, Medline, Google Scholar
32. Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B: Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 1997; 27:951-959Crossref, Medline, Google Scholar
33. Gurvits TG, Shenton MR, Hokama H, Ohta H, Lasko NB, Gilberson MW, Orr SP, Kikinis R, Lolesz FA, McCarley RW, Pitman RK: Magnetic resonance imaging study of hippocampal volume in chronic combat-related posttraumatic stress disorder. Biol Psychiatry 1996; 40:192-199Crossref, Google Scholar
34. Freeman TW, Cardwell D, Karson CN, Komoroski RA: In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. Magn Reson Med 1998; 40:66-71Crossref, Medline, Google Scholar
35. Schuff N, Neylan TC, Lenoci MA, Du AT, Weiss DS, Marmar CR, Weiner MW: Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry 2001; 50:952-959Crossref, Medline, Google Scholar
36. De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND: AE Bennett Research Award: Developmental traumatology, part II: brain development. Biol Psychiatry 1999; 45:1271-1284Crossref, Medline, Google Scholar
37. De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G: A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry 2001; 50:305-309Crossref, Medline, Google Scholar
38. Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, Shalev AY: Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry 2001; 158:1248-1251Link, Google Scholar
39. Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW: Hippocampal atrophy in major depression. Proc Natl Acad Sci USA 1996; 93:3908-3913Crossref, Medline, Google Scholar
40. Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS: Hippocampal volume reduction in major depression. Am J Psychiatry 2000; 157:115-117Link, Google Scholar
41. Bremner JD: Structural changes in the brain in depression and relationship to symptom recurrence. CNS Spectrums 2002; 7:129-139Crossref, Medline, Google Scholar
42. Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1987Google Scholar
43. Bremner JD, Vermetten E, Mazure CM: Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Dep Anxiety 2000; 12:1-12Crossref, Medline, Google Scholar
44. Kulka RA, Schlenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR, Weiss DS: Trauma and the Vietnam War Generation: Report of Findings From the National Vietnam Veterans Readjustment Study. New York, Brunner/Mazel, 1990Google Scholar
45. Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM: Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 1998; 11:125-136Crossref, Medline, Google Scholar
46. Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS: Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry 1999; 156:1787-1795Abstract, Google Scholar
47. Friston K, Frith C, Liddle P, Frackowiak R: Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab 1991; 11:690-699Crossref, Medline, Google Scholar
48. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
49. Pitman RK, Shin LM, Rauch SL: Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. J Clin Psychiatry 2001; 62:47-54Medline, Google Scholar
50. Friedman MJ, Yehuda R: Posttraumatic stress disorder and comorbidity: psychobiological approaches to differential diagnosis, in Neurobiological and Clinical Consequences of Stress: From Normal Adaptation to PTSD. Edited by Friedman MJ, Charney DS, Deutch AY. New York, Raven Press, 1995, pp 429-446Google Scholar
51. Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS: Conscious recollection and the human hippocampal formation: evidence from positron emission tomography. Proc Natl Acad Sci USA 1996; 93:321-325Crossref, Medline, Google Scholar
52. Grasby PM, Frith CD, Friston KJ, Bench C, Frackowiak RSJ, Dolan RJ: Functional mapping of brain areas implicated in auditory-verbal memory function. Brain 1993; 116:1-20Crossref, Medline, Google Scholar
53. Tulving E, Kapur S, Markowitsch HJ, Craik FIM, Habib R, Houle S: Neuroanatomical correlates of retrieval in episodic memory: auditory sentence recognition. Proc Natl Acad Sci USA 1994; 91:2012-2015Crossref, Medline, Google Scholar
54. Nyberg L, McIntosh AR, Cabeza R, Habib R, Houle S, Tulving E: General and specific brain regions involved in encoding and retrieval of events: what, where, and when. Proc Natl Acad Sci USA 1996; 93:11280-11285Crossref, Medline, Google Scholar
55. Buckner RL, Petersen SE, Ojemann JG, Miezin FM, Squire LR, Raichle ME: Functional anatomical studies of explicit and implicit memory retrieval tasks. J Neurosci 1995; 15:12-29Crossref, Medline, Google Scholar
56. Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RSJ, Dolan RJ: Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature 1994; 368:633-635Crossref, Medline, Google Scholar
57. Andreasen NC, O’Leary DS, Arndt S, Cizadlo T, Hurtig R, Rezai K, Watkins GL, Boles Ponto LL, Hichwa RD: Short-term and long-term verbal memory: a positron emission tomography study. Proc Natl Acad Sci USA 1995; 92:5111-5115Crossref, Medline, Google Scholar
58. Schacter DL, Reiman E, Uecker A, Polster MR, Yun LS, Cooper LA: Brain regions associated with retrieval of structurally coherent visual information. Nature 1995; 376:587-590Crossref, Medline, Google Scholar
59. Bremner JD, Innis RB, Ng CK, Staib L, Duncan J, Bronen R, Zubal G, Rich D, Krystal JH, Dey H, Soufer R, Charney DS: PET measurement of cerebral metabolic correlates of yohimbine administration in posttraumatic stress disorder. Arch Gen Psychiatry 1997; 54:246-256Crossref, Medline, Google Scholar
60. Bremner JD, Staib L, Kaloupek D, Southwick SM, Soufer R, Charney DS: Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder (PTSD): a positron emission tomography study. Biol Psychiatry 1999; 45:806-816Crossref, Medline, Google Scholar
61. Rauch SL, van der Kolk BA, Fisler RE, Alpert NA, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK: A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry 1996; 53:380-387Crossref, Medline, Google Scholar
62. Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, Macklin ML, Pitman RK: Visual imagery and perception in posttraumatic stress disorder: a positron emission tomographic investigation. Arch Gen Psychiatry 1997; 54:233-237Crossref, Medline, Google Scholar
63. Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK: Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am J Psychiatry 1999; 156:575-584Abstract, Google Scholar
64. Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM: Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry 1999; 45:817-826Crossref, Medline, Google Scholar
65. Zubieta J-K, Chinitz JA, Lombardi U, Fig LM, Cameron OG, Liberzon I: Medial frontal cortex involvement in PTSD symptoms: a SPECT study. J Psychiatr Res 1999; 33:259-264Crossref, Medline, Google Scholar
66. Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS: Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry 2001; 158:1920-1922Link, Google Scholar
67. Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK: Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 2000; 47:769-776Crossref, Medline, Google Scholar
68. Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL: An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry 2001; 50:932-942Crossref, Medline, Google Scholar
69. Phillips RG, LeDoux JE: Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 1992; 106:274-285Crossref, Medline, Google Scholar
70. Kim JJ, Fanselow MS: Modality-specific retrograde amnesia of fear. Science 1992; 256:675-677Crossref, Medline, Google Scholar



