Increased Risk of Schizophrenia From Additive Interaction Between Infant Motor Developmental Delay and Obstetric Complications: Evidence From a Population-Based Longitudinal Study
Abstract
Objective:
Obstetric complications and developmental delay are well-established risk factors for schizophrenia. The authors investigated whether these risk factors interact in an additive manner to further increase risk for schizophrenia.
Method:
The study population encompassed all individuals born in Helsinki between 1962 and 1969 who had developmental records archived in the Helsinki City Archives. Through linkage between the Finnish Population Register, the Finnish Hospital Discharge Register, and the Child Health Archives, child health cards were traced for 189 individuals who had received a diagnosis of schizophrenia and 189 healthy comparison subjects, individually matched to case subjects on gender and year of birth. Child health cards from the Child Health Archives contain detailed prospective developmental data from birth as well as an indicator of fetal distress, as measured by the Apgar score. Detailed developmental data from the first year of life were extracted.
Results:
Delayed attainment of milestones in infancy significantly increased the risk of later development of schizophrenia in a dose-response manner. There was no significant main effect of obstetric complications on risk for schizophrenia and no significant association between obstetric complications and subsequent developmental delay. However, the additive effect of obstetric complications and delayed attainment of developmental milestones significantly increased the risk of schizophrenia beyond that associated with each factor independently (odds ratio=4.6, 95% confidence interval=1.3–17.2).
Conclusions:
These data provide evidence that underlying neurodevelopmental vulnerability, as indexed by delayed attainment of milestones, combined with obstetric adversity significantly increases the risk of schizophrenia in adulthood.
There has been a resurgence of interest in early-life risk factors for schizophrenia (1). Recent results from genetics studies converge with existing evidence from epidemiology and point to a neurodevelopmental etiology for schizophrenia (2). Given the multifactorial nature of the causal pathway to schizophrenia (3), studies should move toward examining how early-life risk factors may interact with each other to set up a trajectory of aberrant development.
Evidence for childhood motor developmental abnormalities preceding schizophrenia onset are well documented in both high-risk studies and general birth cohort data (4–12). Early neuromotor deficits appear to be specific to schizophrenia (7) and exhibit a dose-response relationship with risk of later development of the illness (6, 9). Likewise, there is strong evidence for the role of obstetric complications in the etiology of schizophrenia (13–19), with a wide range of prenatal and perinatal risk factors associated with the risk of developing the illness. However, although some investigators have controlled for the presence of obstetric complications when studying developmental risk factors for schizophrenia (7), no study has yet—to our knowledge—examined a possible association between obstetric complications and developmental delay in relation to schizophrenia outcomes.
One could conceptualize three possible explanations for the relationship between these two risk factors (or risk markers) for schizophrenia. First, obstetric complications and developmental delay could be manifestations of a common underlying aberrant developmental pathway or trajectory established early in fetal life (either genetic in origin or secondary to a prenatal insult, such as infection [16] or nutritional deficiency [17]). Second, obstetric complications and developmental delay may be independent risk factors for schizophrenia. Third, the relationship could be that of a “two-hit” interaction model, whereby obstetric complications would interact with an underlying developmental vulnerability.
We sought to examine these three possibilities using a large population-based data set from Finland. Our study design has several methodological advantages. First, we minimized selection bias by using register linkage between population-based registers. Second, our use of a nested case-control design is resource efficient and combines the advantages of both case-control and cohort study designs. Third, we minimized recall bias by obtaining both obstetric and developmental information from prospectively collected child health cards.
Method
Study Design
This was a matched case-control study nested within a 7-year birth cohort. All live-born individuals in Finland are assigned a unique personal identification number at birth. This personal identification number is used in all national registers, which ensures accurate linkage of information between registers. For this study, two registers were linked: the Finnish Hospital Discharge Register and the Finnish Population Register. The Finnish Hospital Discharge Register encompasses all public and private hospitals in Finland, and the discharge diagnoses for each admission are made by the attending physician. The diagnostic validity of the Finnish Hospital Discharge Register was examined against DSM-III-R criteria and has been found to have excellent (92%–100%) specificity for diagnoses of schizophrenia (20). The Finnish Population Register records information on the date and place of birth, date of death, current residence, marital status, and first-degree relatives for each Finnish citizen. These registers are maintained in computerized databases by the National Institute for Health and Welfare in Finland.
Study Population
The base study population encompassed all individuals born in Helsinki between January 1, 1962, and December 31, 1969.
Case Definition
The primary outcome of interest was defined as having ever received a diagnosis of schizophrenia or schizophrenia spectrum disorder up to December 31, 2000.
Psychiatric outcomes were determined by linkage between the Finnish Hospital Discharge Register and the Finnish Population Register. Psychiatric diagnoses are recorded in the Finnish Hospital Discharge Register using the ICD system. Diagnoses before 1987 were determined using ICD-8, diagnoses between 1987 and 1995 were determined using ICD-9, and diagnoses since 1995 have been determined using ICD-10.
Our outcome measure was a broad classification of schizophrenia spectrum disorders that encompassed the following ICD categories: ICD-8/9 codes 295 (schizophrenia), 297 (delusional syndrome), 298 (reactive psychosis), and 299 (childhood origin psychoses) as well as ICD-10 codes F20 (schizophrenia), F21 (schizotypal disorder), F22 (delusional syndrome), F23 (psychotic episode), F24 (induced delusional syndrome), F25 (schizoaffective syndrome), F28 (other psychoses), and F29 (psychosis not otherwise specified).
Exposure Measurements
Child health care and monitoring in Finland are provided by public health nurses and primary care physicians. Infants receive an initial home visit from a public health nurse when they are 8–14 days old, after which they attend a child health center monthly for the first year of life. At every visit, nurses complete a standard form with measurements and information about milestones. This form, known as a child health card (or Terveyskortti), was introduced into general use in 1962. Child health cards are stored alphabetically by year of birth in the Child Health Archives. For this study, the records of all case subjects were manually identified, and the age (in months) at which children achieved the following motor milestones was extracted: 1) uttering first sound, 2) keeping head up, 3) grabbing at objects, 4) turning over, 5) sitting unsupported, 6) demonstrating thumb-finger grip (pincer grip), 7) standing with support, 8) walking with support, 9) standing without support, and 10) walking without support.
Obstetric Complications
The Apgar score (21) is calculated by rating newborns on a scale of 0–2 points for each of five separate indices of fetal well-being: heart rate, color, tone, breathing, and reflexes. An Apgar score between 7 and 10 is considered normal (22), and a low Apgar score in a full-term, otherwise well infant indicates obstetric distress (21). A score between 0 and 3 has been associated with poor neurological outcomes, such as cerebral palsy (23), and a score <5, used in combination with other markers of asphyxia, has been shown to identify individuals at risk of developing seizures (24). The Apgar score is reported at 1 minute and 5 minutes after birth. We used the score taken at 5 minutes because it is more predictive of long-term outcome than the score taken at 1 minute (22). In this study, we defined a score ≤8 as a low score because there were too few individuals with a score <7 to give us enough power to carry out a valid analysis.
Selection of Comparison Subjects
The next Helsinki-born child of the same gender and with a different surname listed after each case subject in the Child Health Archives and without a diagnosis of schizophrenia or schizophrenia spectrum disorder up to the year 2000 was selected as a comparison subject. The child health cards are stored in alphabetical order by year of birth. If the next card also belonged to a case subject, the previous card was selected instead. Comparison subjects were therefore matched to case subjects on gender and date of birth.
Analysis Strategy
All statistical analyses were conducted using STATA, version 10 (25). Descriptive frequencies for all exposure variables and the amount of missing data for each variable were calculated. A chi-square test was used to determine whether there was a significant difference in missing data between case and comparison subjects. Logistic regression models that took account of the matching variables of sex and year of birth were run separately for each developmental milestone variable to determine whether the variable was associated with schizophrenia. Our baseline group consisted of those individuals who achieved these milestones earliest. Models were fitted with the exposure as a categorical variable and then refitted with the exposure as a linear variable. The odds ratios and 95% confidence intervals (CIs) reported are from the categorical models, and the p values reported are based on the Wald test from the linear model. All exposure variables were tested for linearity using a likelihood ratio test; any evidence against the linear model being a good fit was noted.
Developmental Delay
Individuals were categorized as being delayed on a particular milestone if they reached that milestone later than the age at which the majority of comparison subjects attained it. The majority was defined as between 51% and 80% of the group, depending on the distribution of the milestone in question. For example, 32% of comparison subjects achieved the sitting unsupported milestone at 6 months, 36% at 7 months, 19% at 8 months, and 13% at 9 months. For the purpose of this study, the majority was taken to be the 68% of those who achieved the milestone at 6 or 7 months. For the cumulative developmental delay analysis, only individuals with no missing values were included.
Interaction Analysis
In order to examine the additive effect of exposure to developmental delay and obstetric complications on risk of developing schizophrenia, the data were stratified on the basis of a high/low Apgar score, and we examined the effect of developmental delay within high and low score strata. Developmental delay was defined as being delayed relative to the majority of comparison subjects on at least three of the milestones. We then calculated the statistical additive interaction between developmental delay and a low Apgar score. For this we used an adaptation of an additive method that utilizes odds ratios instead of risk differences to produce an interaction contrast ratio (26). Risk differences could not be calculated in this study because the baseline risk of schizophrenia is not available in a case-control study. Ninety-five percent confidence intervals and a p value for the interaction were calculated using the LINCOM technique in STATA (25). The evidence indicates that additive interaction is the most appropriate method of assessing interactions in psychiatric epidemiology (27–29), and the majority of studies examining interactions in the etiology of psychiatric illness have used this additive approach (30–32).
Results
From the national registers, we identified 454 individuals with schizophrenia and schizophrenia spectrum disorders who were born in Helsinki between 1962 and 1969. Child health cards were located for 189 of these individuals (42%) in the Helsinki City archives. Twenty-five percent of the sample had missing data on eight or more variables. However, there was no evidence of a difference in the amount or pattern of missing data between case and comparison subjects. Incomplete record retrieval was most likely because the main maternity hospitals are located in Helsinki, and women come from regions outside of Helsinki to give birth there but attend child health clinics near their homes. In addition, during the 1960s and 1970s, there was a large expansion of the suburban areas around Helsinki, and families with young children, from all social groups, moved out of the city at that time.
Developmental Milestones
Table 1 shows the association between the age at which each of the 10 developmental milestones was attained in the first year of life and the odds of developing schizophrenia. There was good evidence for associations between schizophrenia and the ages at which five of the milestones were achieved (sitting unsupported, standing with support, walking with support, standing without support, and walking without support). Those who achieved these milestones later than individuals with the quickest rate of development had two to three times the odds of developing the illness. As seen in Table 1, none of the milestones attained before 6 months of age was associated with schizophrenia, whereas the majority of those attained after 6 months of age show a strong association with the illness.
| Case Subjects (N=189) | Comparison Subjects (N=189) | Analysis | |||||
|---|---|---|---|---|---|---|---|
| Milestone and Month Achieved | N | % | N | % | Odds Ratio | 95% CI | p |
| Uttering first sound | 0.8 | ||||||
| First | 91 | 66.9 | 93 | 67.4 | 1.0 | ||
| Second | 45 | 33.1 | 45 | 32.6 | 1.1 | 0.6–1.8 | |
| Keeping head up | 0.9 | ||||||
| First | 28 | 21.1 | 21 | 15.7 | 1.0 | ||
| Second | 64 | 48.1 | 78 | 58.2 | 0.6 | 0.3–1.3 | |
| Third | 41 | 30.8 | 35 | 26.1 | 0.9 | 0.4–1.9 | |
| Grabbing at objects | 0.8 | ||||||
| Second | 17 | 13.1 | 7 | 5.7 | 1.0 | ||
| Third | 69 | 53.1 | 79 | 64.8 | 0.3 | 0.1–0.9 | |
| Fourth | 32 | 24.6 | 26 | 21.3 | 0.5 | 0.2–1.4 | |
| Fifth | 12 | 9.2 | 10 | 8.2 | 0.5 | 0.1–1.7 | |
| Turning over | 0.1 | ||||||
| Third | 22 | 16.8 | 20 | 14.9 | 1.0 | ||
| Fourth | 43 | 32.8 | 60 | 44.8 | 0.7 | 0.3–1.4 | |
| Fifth | 36 | 27.5 | 34 | 25.4 | 0.9 | 0.4–2.2 | |
| Sixth | 30 | 22.9 | 20 | 14.9 | 1.5 | 0.6–3.6 | |
| Sitting unsupported | 0.002 | ||||||
| Sixth | 18 | 15.1 | 40 | 32.3 | 1.0 | ||
| Seventh | 43 | 36.1 | 44 | 35.5 | 2.1 | 1.0–4.3 | |
| Eighth | 36 | 30.3 | 24 | 19.4 | 3.7 | 1.7–8.3 | |
| Ninth | 22 | 18.5 | 16 | 12.9 | 3.4 | 1.4–8.2 | |
| Demonstrating pincer grip | 0.4 | ||||||
| Sixth | 15 | 17.2 | 8 | 9.4 | 1.0 | ||
| Seventh | 26 | 29.9 | 36 | 42.4 | 0.4 | 0.1–1.1 | |
| Eighth | 22 | 25.3 | 26 | 30.6 | 0.4 | 0.1–1.4 | |
| Ninth | 24 | 27.6 | 15 | 17.7 | 1.1 | 0.3–3.6 | |
| Standing with support | 0.01 | ||||||
| Seventh | 18 | 16.7 | 34 | 30.1 | 1.0 | ||
| Eighth | 28 | 25.9 | 32 | 28.3 | 1.7 | 0.8–3.9 | |
| Ninth | 30 | 27.8 | 24 | 21.2 | 2.7 | 1.2–6.0 | |
| Tenth | 23 | 21.3 | 15 | 13.3 | 3.1 | 1.2–7.8 | |
| Eleventh | 9 | 8.3 | 8 | 7.1 | 2.3 | 0.7–7.1 | |
| Walking with support | 0.005 | ||||||
| Seventh | 7 | 6.9 | 12 | 11.3 | 1.0 | ||
| Eighth | 15 | 14.8 | 28 | 26.4 | 0.9 | 0.3–2.9 | |
| Ninth | 26 | 25.7 | 30 | 28.3 | 1.5 | 0.5–4.8 | |
| Tenth | 25 | 24.8 | 17 | 16.0 | 3.1 | 0.9–10.4 | |
| Eleventh | 19 | 18.8 | 14 | 13.2 | 2.3 | 0.7–7.9 | |
| Twelfth | 9 | 8.9 | 5 | 4.7 | 3.6 | 0.8–16.1 | |
| Standing without support | 0.004 | ||||||
| Ninth | 8 | 9.5 | 21 | 22.6 | 1.0 | ||
| Tenth | 19 | 22.6 | 25 | 26.9 | 2.2 | 0.7–6.4 | |
| Eleventh | 30 | 35.7 | 27 | 29.0 | 3.4 | 1.2–9.3 | |
| Twelfth | 18 | 21.4 | 16 | 17.2 | 3.2 | 1.0–9.9 | |
| Thirteenth | 9 | 10.7 | 4 | 4.3 | 9.0 | 1.8–44.2 | |
| Walking without support | 0.09 | ||||||
| Ninth | 6 | 5.1 | 6 | 5.3 | 1.0 | ||
| Tenth | 9 | 7.8 | 13 | 11.1 | 0.7 | 0.2–2.9 | |
| Eleventh | 22 | 18.9 | 26 | 22.2 | 0.9 | 0.3–3.7 | |
| Twelfth | 38 | 32.8 | 46 | 39.3 | 0.9 | 0.2–3.1 | |
| Thirteenth | 41 | 35.3 | 26 | 22.2 | 1.8 | 0.5–6.4 | |
TABLE 1. Association Between Age of Achieving Developmental Milestones in Infancy and Risk of Later Developing Schizophrenia in a Finnish Cohorta
Cumulative Developmental Delay
There was evidence of a cumulative effect of developmental delay, such that the odds of developing schizophrenia increased with each additional delayed milestone in children with delays relative to those without delays (Table 2). The odds ratio for this cumulative developmental variable fitted as a linear variable was 1.2 (95% CI=1.1–1.3, p<0.05). In other words, with every additional milestone on which the child was delayed, the odds of developing schizophrenia increased by 20%.
| Case Subjects (N=141) | Comparison Subjects (N=135) | Analysisa | ||||
|---|---|---|---|---|---|---|
| Number of Delayed Milestones | N | % | N | % | Odds Ratio | 95% CI |
| 0 | 17 | 12.1 | 20 | 14.8 | 1.0 | |
| 1 | 18 | 12.8 | 29 | 21.0 | 0.8 | 0.3–1.9 |
| 2 | 19 | 13.5 | 28 | 20.7 | 0.8 | 0.3–2.1 |
| 3 | 24 | 17.0 | 18 | 13.3 | 1.7 | 0.7–4.1 |
| 4 | 23 | 16.3 | 11 | 8.1 | 2.6 | 0.9–7.1 |
| 5 | 16 | 11.4 | 8 | 5.9 | 2.5 | 0.8–7.7 |
| ≥6 | 24 | 17.0 | 21 | 16.0 | 1.4 | 0.6–3.6 |
TABLE 2. Cumulative Developmental Delay in Achieving Milestones in Infancy and Risk of Later Developing Schizophrenia in a Finnish Cohort
Obstetric Complications
Data on Apgar scores were available for 156 case subjects and 145 comparison subjects (Table 3). The median score was 10 for comparison subjects and 9 for case subjects. Thirty (19.2%) case subjects and 21 (14.5%) comparison subjects had a low score (≤8). Although the effect was in the expected direction, there was no significant main association between Apgar score and risk of schizophrenia. A low score was not associated with developmental delay on any of the motor milestones examined or with cumulative developmental delay.
| Case Subjects (N=156) | Comparison Subjects (N=145) | |||
|---|---|---|---|---|
| Apgar Score | N | % | N | % |
| 1 | 3 | 1.9 | 1 | 0.69 |
| 5 | 1 | 0.6 | 0 | 0.0 |
| 6 | 3 | 1.9 | 2 | 1.4 |
| 7 | 8 | 5.1 | 5 | 3.5 |
| 8 | 15 | 9.6 | 13 | 8.9 |
| 9 | 55 | 35.3 | 38 | 26.2 |
| 10 | 71 | 45.5 | 86 | 59.3 |
TABLE 3. Apgar Scores at 5 Minutes After Birth Across Schizophrenia Case Subjects and Comparison Subjects in a Finnish Cohorta
Additive Interaction Between Obstetric Complications and Infant Developmental Delay
Information was available on both developmental milestone attainment and Apgar score for 142 case subjects and 133 comparison subjects. The odds of developing schizophrenia for individuals in each of three risk categories, relative to those with no developmental delay plus a normal Apgar score, were as follows: no developmental delay plus low Apgar score, odds ratio=1.2, 95% CI=0.5–2.5; developmental delay plus normal Apgar score, odds ratio=1.5, 95% CI=0.9–2.6; and developmental delay plus low Apgar score, odds ratio=4.6, 95% CI=1.3–17.2 (Figure 1). Thus, exposure to both obstetric complications and developmental delay produced a significant 4.6-fold increase in the odds of later developing schizophrenia compared with a nonsignificant 1.5-fold increase in the odds of schizophrenia with a developmental delay only and a nonsignificiant 1.2-fold increase in the odds of schizophrenia with exposure to obstetric complications only. The statistical additive interaction between obstetric complications and developmental delay was positive, indicating that exposure to obstetric complications and developmental delay interact on the additive scale (formula: odds ratio [AB]–odds ratio [A]–odds ratio [B]+1; case subjects: 4.6–1.5–1.2+1=2.9). This interaction was significant (beta=1.9, 95% CI=0.4–3.5, p=0.02).
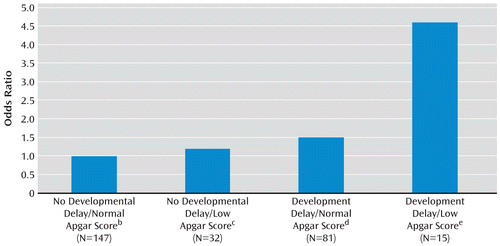
FIGURE 1. Additive Interaction Between Obstetric Complications (as Indicated by Low Apgar Score) and Developmental Delaya
a Apgar scores ≤8 were considered low.
b Reference group.
c 95% confidence interval (CI)=0.5–2.5.
d 95% CI=0.9–2.6.
e 95% CI=1.3–17.2.
Discussion
Our findings support previous evidence that delayed motor development in the first year of life is a relatively robust risk factor for later development of schizophrenia. In our sample, children who went on to develop schizophrenia were delayed in their attainment of motor milestones compared with children who did not develop schizophrenia. This association was present for milestones attained after age 6 months. There was no evidence that very early milestones were associated with later development of schizophrenia. The milestones that showed the strongest association with schizophrenia were those related to sitting, standing, and walking, as evidenced by strong linear trends for these milestones. Individuals who sat, stood, or walked later than the earliest developers had, on average, a two- to threefold increase in the odds of developing schizophrenia in adulthood. There was also evidence of a dose-response effect across all the milestones examined, such that the greater the number of milestones on which an individual was delayed, the higher the odds of developing schizophrenia.
Only two previous studies of schizophrenia included detailed prospective information on specific motor milestones in the first year of life: the Northern Finland 1966 Birth Cohort (6, 10) and the Copenhagen Perinatal Cohort (12). Our findings of delayed development across a range of early motor milestones are consistent with those from both these prior studies. The data from the Northern Finland 1966 Birth Cohort showed a dose-response effect between standing/walking and schizophrenia; the later the children stood or walked, the higher their odds of developing schizophrenia (6, 10). Evidence from the Copenhagen Perinatal Cohort (12) showed that infants who developed schizophrenia attained early milestones, such as sitting without support, later than those who did not develop the illness.
The present study, similar to previous work (5, 6, 10, 12), also shows that those who develop schizophrenia do not form a readily identifiable developmental subgroup; examination of the distribution of any of the milestones presented in this study shows that individuals with schizophrenia, as a group, have a slight leftward shift in the distribution of attainment of milestones (5, 9). This indicates the difficulty of using motor delay in childhood to identify those who will go on to develop schizophrenia in the general population. Childhood motor developmental delay, as a proxy of presumed underlying neural abnormalities, is neither a necessary nor a sufficient cause of schizophrenia, and these deficits have weak positive predictive power (6, 8, 10, 12), as the majority of children with motor developmental delay do not go on to develop schizophrenia. However, deviations seen in the expected developmental trajectory will inform us about additional vulnerability factors that operate at critical windows in development. Our data show that the underlying vulnerability indicated by developmental delay can be amplified by the possible neurotoxic effects of obstetric complications increasing the risk of adverse outcomes.
Our study has a number of strengths. First, our nested case-control design ensured that an adequate number of cases were identified and that comparison subjects were representative of the population from which the case subjects were drawn. Second, the developmental milestone information was collected prospectively and independently of outcome status. Third, the developmental information we used was considerably more detailed than the information used in the majority of previous studies, and it was collected by trained public health nurses, using standardized measures and stored on standardized health cards, all of which gives our exposure measures a high degree of validity. A limitation of our work is the failure to find child health cards on all Helsinki-born case subjects identified from the registers. However, we have no reason to suspect that those who attended child health clinics outside the city of Helsinki differ in their risk of schizophrenia compared with those who attended clinics in Helsinki. A second limitation is the amount of missing data on individual variables on the child health cards. This is likely a result of mothers missing appointments for their infants. These assessments were frequent in the first year of life (approximately monthly), and it is quite possible that mothers were unable to make every appointment. However, there was no difference in the amount or pattern of this missing information from the child health cards for case and comparison subjects, and therefore it seems unlikely to have biased the results. A third limitation is the nonspecificity of the Apgar score in relation to the cause of obstetric complications. Knowledge of the exact cause of the obstetric complications would be informative about the specific mechanisms that may be involved in the effects we report. However, Apgar score is a recognized global measure of obstetric distress, and as such is likely to be more sensitive to the fetal condition than measurement of any one individual complication.
In our introduction, we proposed three explanations of the association between infant motor developmental delay and schizophrenia. The first hypothesis was that obstetric complications and developmental delay are manifestations of a common underlying aberrant cause (either genetic in origin or secondary to a prenatal insult, such as infection [16] or nutritional deficiency [17]). The second hypothesis was that obstetric complications and developmental delay are independent risk factors, and the third hypothesis was that there is an interaction between the two risk factors. The first hypothesis (common cause) is not supported by our data, since our findings show that obstetric complications do not predict developmental delay and indicate that these two risk factors for schizophrenia are not outcomes of the same underlying common cause. The second hypothesis is not supported because we found an additive effect of exposure to obstetric complications, such that when coupled with delayed motor development, the risk of schizophrenia is significantly increased relative to the risk from developmental delay without exposure to obstetric complications. Therefore, our data support the third hypothesis: an underlying vulnerability compounded by additional environmental adversity.
This is, to our knowledge, the first study to provide evidence that obstetric complications interact with developmental delay to further increase vulnerability to schizophrenia. There are a number of possible mechanisms that may explain our result. First, our findings may point to a gene-environment interaction, since motor developmental delay could be an expression of underlying genetic vulnerability (4, 8). Second, our data could point to a genetic interaction in which the genes predisposing to a difficult birth interact with the genes for motor development or to a gene-environment correlation whereby the effects of obstetric complications and motor development seen here are under the same genetic control. However, evidence that the effect of obstetric complications on risk for schizophrenia is not a proxy for genetic risk has been shown in a number of studies (33–38). A third possibility is that developmental delay is not actually a genetically mediated risk factor but instead reflects some aspect of prenatal development, and our findings may represent a synergistic interaction between two or more environmental risk factors. A fourth possibility is that a single environmental process is at work, whereby an environmental factor, such as exposure to a prenatal event or stressor, could increase the likelihood of both obstetric complications and developmental delay. However, our two risk factors were not significantly associated with each other, indicating that they are probably not part of a single underlying neurodevelopmental state and that two vulnerability processes may be at work in tandem to synergistically increase risk of schizophrenia. We recommend moving away from considering risk factors in isolation and focusing on investigating aberrant trajectories of development from prenatal life through childhood and adolescence.
1. : Risk for schizophrenia: broadening the concepts, pushing back the boundaries. Schizophr Res 2005; 79:5–13Crossref, Medline, Google Scholar
2. : Rethinking schizophrenia. Nature 2010; 468:187–193Crossref, Medline, Google Scholar
3. : Schizophrenia. Lancet 2009; 374:635–645Crossref, Medline, Google Scholar
4. : Infants at risk for schizophrenia: sequelae of a genetic neurointegrative defect: a review and replication analysis of pandysmaturation in the Jerusalem Infant Development Study. Arch Gen Psychiatry 1992; 49:221–235Crossref, Medline, Google Scholar
5. : Child development risk factors for adult schizophrenia in the British 1946 Birth Cohort. Lancet 1994; 344:398–402Crossref, Medline, Google Scholar
6. : Early developmental milestones in adult schizophrenia and other psychoses: a 31-year follow-up of the Northern Finland 1966 Birth Cohort. Schizophr Res 2001; 52:1–19Crossref, Medline, Google Scholar
7. : Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry 2002; 59:449–456Crossref, Medline, Google Scholar
8. : Childhood developmental abnormalities in schizophrenia: evidence from high-risk studies. Schizophr Res 2003; 60:239–258Crossref, Medline, Google Scholar
9. : Childhood similarities and differences between schizophrenia and bipolar disorder, in Schizophrenia: Challenging the Orthodox. Edited by MacDonald CSchulze KMurray RMWright P. London, Taylor and Francis, 2004Google Scholar
10. : The persistence of developmental markers in childhood and adolescence and risk for schizophrenic psychoses in adult life: a 34-year follow-up of the Northern Finland 1966 Birth Cohort. Schizophr Res 2004; 71:213–225Crossref, Medline, Google Scholar
11. : The antecedents of schizophrenia: a review of birth cohort studies. Schizophr Bull 2009; 35:603–623Crossref, Medline, Google Scholar
12. : Early developmental milestones and risk of schizophrenia: a 45-year follow-up of the Copenhagen Perinatal Cohort. Schizophr Res 2010; 118:41–47Crossref, Medline, Google Scholar
13. : Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry 2002; 159:1080–1092Link, Google Scholar
14. : The role of obstetric events in schizophrenia. Schizophr Bull 2006; 32:3–8Crossref, Medline, Google Scholar
15. : Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr Bull 2008; 34:1083–1094Crossref, Medline, Google Scholar
16. : Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 2010; 167:261–280Link, Google Scholar
17. : Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study. Arch Gen Psychiatry 2010; 67:889–894Crossref, Medline, Google Scholar
18. : The environment and susceptibility to schizophrenia. Prog Neurobiol 2011; 93:23–58Crossref, Medline, Google Scholar
19. : Obstetric complications and schizophrenia, in the Origins of Schizophrenia. Edited by Brown ASPatterson PH. New York, Columbia University Press (in press)Google Scholar
20. : Accuracy of register-based schizophrenia diagnoses in a genetic study. Eur Psychiatry 1998; 13:57–62Crossref, Medline, Google Scholar
21. : A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg 1953; 32:260–267Crossref, Medline, Google Scholar
22.
23. : Apgar scores as predictors of chronic neurologic disability. Pediatrics 1981; 68:36–44Medline, Google Scholar
24. : Can asphyxiated infants at risk for neonatal seizures be rapidly identified by current high-risk markers? Pediatrics 1996; 97:456–462Medline, Google Scholar
25.
26. : Causal explanation with a risk factor framework, in Psychiatric Epidemiology: Searching for the Causes of Mental Disorders. Edited by Susser ESwartz SMorabia ABromet EJ. New York, Oxford University Press, 2006, pp422–440Google Scholar
27. : Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull 2008; 34:1066–1082Crossref, Medline, Google Scholar
28. : Gene-environment interactions and depression. JAMA 2009; 302:1860–1861Crossref, Medline, Google Scholar
29. : Interpretation of interactions: guide for the perplexed. Br J Psychiatry 2010; 197:170–171Crossref, Medline, Google Scholar
30. : Do urbanicity and familial liability coparticipate in causing psychosis? Am J Psychiatry 2003; 160:477–482Link, Google Scholar
31. : Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry 2009; 166:1025–1030Link, Google Scholar
32. : Cannabis use and childhood trauma interact additively to increase the risk of psychotic symptoms in adolescence. Psychol Med 2010; 40:1627–1634Crossref, Medline, Google Scholar
33. : Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry 2002; 159:1514–1520Link, Google Scholar
34. : Severity of obstetric complications and risk of adult schizophrenia in male patients: a case-control study. J Matern Fetal 2003; 14:35–38Medline, Google Scholar
35. : Pregnancy, delivery, and neonatal complications in a population cohort of women with schizophrenia and major affective disorders. Am J Psychiatry 2005; 162:79–91Link, Google Scholar
36. : Fetal growth restriction and schizophrenia: a Swedish twin study. Twin Res 2005; 8:402–408Crossref, Google Scholar
37. : Negative association between a history of obstetric complications and the number of neurological soft signs in first-episode schizophrenic disorder. Psychiatry Res 2007; 149:273–277Crossref, Medline, Google Scholar
38. : The effects of genetic liability for schizophrenia and maternal smoking during pregnancy on obstetric complications. Schizophr Res 2007; 93:229–236Crossref, Medline, Google Scholar



