Diffusion Tensor Imaging Findings in First-Episode and Chronic Schizophrenia Patients
Abstract
Objective: Comparisons of diffusion tensor imaging (DTI) data between first-episode and chronic schizophrenia patients assessed in different studies have led to the suggestion that the decreased fractional anisotropy observed in chronic schizophrenia patients is less pronounced in first-episode patients. However, such comparisons of imaging data generated across studies are susceptible to numerous confounders, which may limit the interpretation of any differences. In order to address these issues and determine whether the DTI abnormalities of chronic schizophrenia are present at illness onset, the authors conducted a DTI investigation of the largest cohort of first-episode schizophrenia patients yet reported in conjunction with a group of chronic schizophrenia patients and healthy subjects for comparison. Method: Fractional anisotropy data generated by diffusion tensor imaging with a 3-T Siemens Allegra head-dedicated MRI system were compared between 40 first-episode schizophrenia patients and 39 age- and gender-matched healthy comparison subjects and between 40 chronic schizophrenia patients and 40 age- and gender-matched healthy comparison subjects. The following regions of interest were assessed: forceps minor (bilaterally), forceps major (bilaterally), inferior longitudinal fasciculus (bilaterally), and the genu and splenium of the corpus callosum. Results: In most regions, chronic schizophrenia patients showed significant or trend-level lower fractional anisotropy than healthy comparison subjects, whereas the first-episode schizophrenia patients showed only trend-level lower fractional anisotropy in the inferior longitudinal fasciculus. Conclusions: The cross-sectional data reported here suggest less widespread changes in white matter at illness onset in schizophrenia which progress in more chronic states. More definitive conclusions will require follow-up imaging of first-episode schizophrenia patients.
Disruptions in white matter tract structure are becoming an accepted part of the pathophysiology of schizophrenia on the basis of mounting evidence from genetic (1 – 4) and histopathological (5 , 6) studies. The technique of diffusion tensor imaging (DTI) has provided indirect in vivo evidence for disruptions in the coherence and organization of white matter structures in schizophrenia. Although inconsistencies exist, the vast majority of DTI studies report significant and widespread alterations of fractional anisotropy (FA) in the white matter of chronic schizophrenia patients (see reference 7 for a review). However, the study of patients with chronic illness cannot address questions about the timing of the observed white matter changes and their possible interactions with continued brain white matter development in adulthood (8 , 9) . Indeed, patients with chronic schizophrenia have been exposed for long periods to various confounders, such as antipsychotic medication, substance use, medical comorbidity (particularly medical conditions related to second-generation antipsychotics), and potentially exaggerated aging effects. Therefore, examination of DTI abnormalities closer to illness onset is important in the effort to disentangle the primary white matter pathology of schizophrenia from secondary changes that may occur over time. This is especially true in light of the evidence for a progressive reduction in white matter volume following illness onset and its relationship to outcome (10) .
Cumulative data from the nine DTI studies of first-episode and early-onset schizophrenia published to date are contradictory as to whether the DTI abnormalities reported in patients with chronic schizophrenia are present early in the illness. When drawing any conclusions from these data about the relative expression of DTI findings early in the course of illness compared with findings in more chronic illness, there are three main issues to consider. First, there are inconsistencies as to where and how widespread these DTI abnormalities are early in the illness. Some studies report well-circumscribed DTI findings (11 – 15) , whereas others report more widespread findings (16 , 17) or no abnormalities at all (18 , 19) . Second, several studies demonstrate directly conflicting results in the same structures. For example, Hao et al. (16) reported reduced FA in the hippocampus bilaterally in first-episode schizophrenia patients, whereas Begre et al. (19) found no such changes. Kumra et al. (13) found FA reduction in the frontal and occipital lobes, but in a subsequent study (14) identified FA reductions only in the anterior cingulate region of early-onset schizophrenia patients. Third, comparisons of DTI data generated from some of these studies have been made with DTI data from chronic schizophrenia patients generated in different studies (11 , 18) . Such comparisons have led to the suggestion that the decreased FA observed in chronic schizophrenia patients is less pronounced in first-episode patients, leading to further conjecture about the occurrence of progressive and exaggerated age-dependent changes in schizophrenia. Although this supposition is supported by direct evidence of progressive white matter volume reductions following illness onset (10) , it is contradicted by longitudinal magnetization transfer imaging studies showing white matter changes present at baseline in first-episode schizophrenia patients but no significant differences in the magnitude of these differences from healthy comparison subjects over time (20) . Nonetheless, the comparison of DTI data between first-episode and chronic schizophrenia patients assessed in different studies is inherently flawed. Moreover, limited sample sizes may contribute to some of the contradictory findings in first-episode patients with schizophrenia.
In order to address these issues and determine whether the DTI abnormalities of chronic schizophrenia are present at illness onset, we conducted a DTI investigation of the largest cohort of first-episode schizophrenia patients yet reported in conjunction with a group of chronic schizophrenia patients and healthy subjects for comparison. For subjects in this study, scanning and image analysis procedures were the same, allowing direct comparisons of first-episode and chronic schizophrenia patients in white matter regions of interest that have previously been reported to show FA reductions in chronic schizophrenia patients.
Method
Subjects
Chronic and first-episode schizophrenia patients were recruited from inpatient, outpatient, day treatment, and vocational rehabilitation services at Mount Sinai Hospital (New York City), Pilgrim Psychiatric Center (West Brentwood, N.Y.), Bronx VA Medical Center (New York City), Hudson Valley Veterans Affairs Medical Center (Montrose, N.Y.), and Queens Hospital Center (New York City). The study was approved by each center’s institutional review board. All participants provided informed consent after the study procedures were fully explained to them. All potential participants with schizophrenia underwent an assessment of capacity to provide informed consent by a psychiatrist independent of the study. For inclusion in the study, schizophrenia patients had to be at least 18 years of age and have a DSM-IV diagnosis of schizophrenia or schizoaffective disorder based on the Comprehensive Assessment of Symptoms and History (21) . First-episode schizophrenia patients were identified as those whose illness onset was within 3 years of enrollment in the study, as determined by the Symptom Onset in Schizophrenia Inventory (22) . Healthy comparison subjects with no DSM-IV axis I disorders (as determined by the Comprehensive Assessment of Symptoms and History) were recruited from the New York City area.
Patients with schizophrenia and healthy subjects were excluded if they had a positive urine screen for any drugs of abuse, a medical diagnosis that may produce white matter changes (e.g., HIV infection, multiple sclerosis), a history of any brain disorder that may produce cognitive impairment or behavioral symptoms (e.g., head injury, cerebrovascular disease), or an unstable medical condition (e.g., poorly controlled diabetes or hypertension, symptomatic coronary artery disease). Each subject was carefully screened with a structured assessment of symptoms, review of medical history, physical examination, laboratory studies (including CBC, routine chemistry studies, liver enzyme assessment, and thyroid function tests), urine toxicology screen, and ECG to ensure that they met the inclusion criteria.
Image Acquisition
All scans were acquired with a 3-T Siemens Allegra head-dedicated MRI system. This system is equipped with the highest-performance head gradient system on the market, with a maximum gradient strength of 40 mT/m and slew rate 400 mT/m per second. This allows reduced susceptibility-based distortions in single-shot echo-planar imaging due to the available ultrashort echo-spacing, which benefits the DTI imaging sequence that we used. It also allows very high b-values (up to 10,000 seconds/mm 2 ) in diffusion-weighted imaging due to a powerful gradient system. The protocol for the structural scans consisted of a three-plane sagittal localizer from which all other structural scans were prescribed. The following structural scans were conducted: axial three-dimensional, magnetization-prepared, rapid-acquisition gradient-echo scans (repetition time=2500 msec, echo time=4.4 msec, field of view=21 cm, matrix=256×256, 208 slices, thickness=0.82 mm); turbo-spin echo T 2 -weighted axial scans (repetition time=5380 msec, echo time=99 msec, field of view=18.3 cm×21 cm, matrix=512×448, turbo factor=11, 28 slices, thickness=3 mm, skip=1 mm); and DTI scans using a pulsed-gradient spin-echo sequence with echo-planar imaging acquisition (repetition time=4100 msec, echo time=80 msec, field of view=21 cm, matrix =128×128, 28 slices, thickness=3 mm, skip=1 mm, b-factor=1250 seconds/mm 2 , 12 gradient directions, five averages). Total imaging time for the structural scans averaged about 50 minutes. We inspected the images immediately after each scan, and if motion was detected, the scans were repeated.
Image Processing
Raw DTI data were transferred to an offline workstation for postprocessing. In-house software written in MATLAB, version 6.5 (MathWorks, Inc., Natick, Mass.), was used to compute the FA and vector maps. The FA images were then converted to analyze format. The software program MEDx, version 3.4.3 (Medical Numerics, Inc., Sterling, Va.), was used to inspect and define the regions of interest (ROIs) on the FA images from which the FA data were obtained. Given that ROIs were based on the FA maps and not on the anatomical maps, minor distortion differences would not be expected to affect the localization of the ROIs (as compared with techniques that transfer ROIs identified on anatomical scans to the DTI-FA images). Image analysts blind to diagnosis placed ROIs in eight separate white matter tracts using identifiable landmarks and with reference to the Mori MRI atlas of human white matter (23) . Six of these ROIs were identified from an axial slice oriented through the striatum and parallel to the anterior commissure-posterior commissure (AC-PC) line ( Figure 1 ) (see Mori atlas [23] , p. 83): the forceps minor (bilaterally) was sampled by placing one ROI where the anterior limb of the internal capsule meets the genu of the corpus callosum followed by a second contiguously placed ROI anteriorly (each ROI measured 202 mm 3 ); the genu and splenium of the corpus callosum were each sampled with a single ROI placed in the middle of these structures (each ROI measured 81 mm 3 ); the forceps major (bilaterally) was sampled with three contiguously placed ROIs (each ROI measured 73 mm 3 ) following the tract as precisely as possible, with the first ROI placed where the splenium of the corpus callosum meets the sagittal stratum. The inferior longitudinal fasciculus ( Figure 1 ) was identified from a separate axial slice oriented slightly oblique so as to follow as closely as possible the main axis of the inferior longitudinal fasciculus in the medial temporal lobe as identified from the Mori atlas ( 23 , p. 199). The first ROI in the inferior longitudinal fasciculus was placed at the anterior portion of the cerebral peduncles, followed by four contiguous ROIs placed posteriorly, tracking the inferior longitudinal fasciculus (each ROI measured 73 mm 3 ). For each white matter tract sampled, the reported FA value represented the average of all ROIs placed in that tract. Quality control measures included the inspection of each image with traced ROIs by two senior investigators (C.T. and D.C.) for the quality of the image and the correct placement of ROIs. Moreover, interrater reliability measures were calculated for the two image analysts who traced these ROIs in each white matter tract in 100 randomly selected subjects. All intraclass correlation coefficients exceeded 0.86 (forceps minor: right=0.97, left=0.97; forceps major: right=0.92, left=0.96; genu of the corpus callosum=0.96; splenium of the corpus callosum=0.92; inferior longitudinal fasciculus, right=0.87, left=0.88).
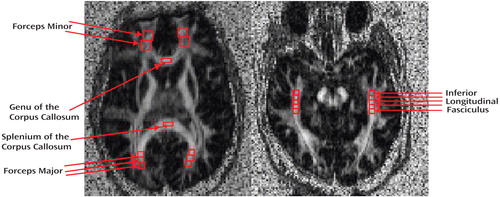
Analyses
In order to construct the best-fitting comparison groups for both schizophrenia groups, each first-episode and chronic schizophrenia subject was individually matched for age (within 2 years), gender, handedness, and ethnicity (when possible) to a healthy comparison subject.
Independent t tests were performed on FA data from each of the eight ROIs to assess differences between the first-episode and chronic schizophrenia patients and their respective healthy comparators. The first-episode schizophrenia patients and their matched healthy comparators were compared in one set of analyses and the chronic schizophrenia patients and their matched healthy comparators in another. Holm’s procedure (24) for multiple comparisons was used for increased power compared with the Bonferroni procedure, with the same strong familywise control of type I error.
The association between age and FA was investigated by correlational analysis for the chronic schizophrenia group and the group of their healthy matched comparators.
Results
A total of 232 participants underwent DTI scanning: 115 healthy volunteers, 40 first-episode schizophrenia patients, and 77 chronic schizophrenia patients. Data from 63 of the chronic schizophrenia patients and 55 of the healthy comparison subjects have been previously published (25) and demonstrated widespread areas of reduced anisotropy in the schizophrenia patients, including in the frontal white matter, the corpus callosum, and the frontal longitudinal fasciculus, using significance probability mapping.
The necessity of age-matching resulted in a reduction of the number of subjects available for the final and most appropriate analysis. Table 1 summarizes demographic and clinical data for all participants included in the analysis. At the time of assessment, the first-episode schizophrenia patients were within 1 year, on average, of the onset of their illness. The chronic schizophrenia patients had been ill for considerably longer. Both patient groups completed fewer years of education than their matched healthy comparators. The chronic schizophrenia patients were somewhat better matched for ethnicity than the first-episode patients.
Figure 2 presents the group means for FA values × 1000 in each of the eight ROIs for the first-episode schizophrenia subjects and their matched healthy comparators and for the chronic schizophrenia patients and their matched healthy comparators. As shown in Table 2 , t tests revealed that chronic schizophrenia patients had significantly lower FA than healthy comparison subjects in the right forceps minor and the left inferior longitudinal fasciculus and trend-level lower FA in the left forceps minor, the right forceps major, and the splenium of the corpus callosum after Holm corrections. First-episode schizophrenia patients showed no significant FA reductions relative to their matched healthy comparators and trend-level FA reductions only in the left and right inferior longitudinal fasciculus after Holm corrections. Comparing effect sizes for the differences between first-episode schizophrenia patients and healthy comparators and chronic schizophrenia patients and healthy comparators demonstrated similar FA decreases in the inferior longitudinal fasciculus and the forceps major only ( Table 2 ). Unlike chronic schizophrenia patients, first-episode patients demonstrated no appreciable FA decreases relative to healthy comparison subjects in the genu of the corpus callosum or the forceps minor.

a Vertical bars represent 95% confidence intervals.
Scatterplots and linear fitting of the data correlating age and FA are shown for chronic schizophrenia patients and healthy comparison subjects concurrently in Figure 3 . Significant inverse correlations between age and FA were widespread in the healthy comparison group. The chronic schizophrenia patients demonstrated similar relationships between FA and aging in the forceps minor, a greater FA decline with age in the forceps major and the inferior longitudinal fasciculus, and a weaker relationship between FA and age in the corpus callosum.
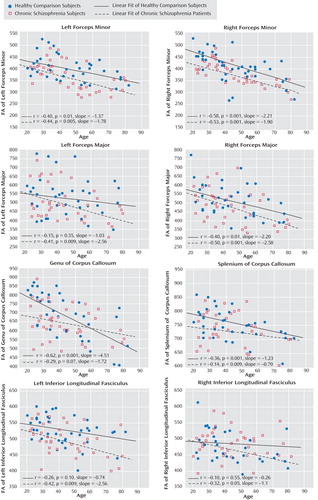
Discussion
The results of this study demonstrate modest widespread reductions in FA in the white matter of chronic schizophrenia patients. Given the modest nature of these changes, they reached statistical significance only in the right forceps minor and the left inferior longitudinal fasciculus and were trend level in the remaining six tracts sampled. In contrast, no significant FA reductions were observed in first-episode schizophrenia patients, but trend-level FA decreases were observed in the left and right inferior longitudinal fasciculus. Comparison of effect sizes for these FA changes demonstrated marked differences between first-episode and chronic schizophrenia patients in the forceps minor and the genu of the corpus callosum. Whereas chronic schizophrenia patients show small to medium-sized FA decreases in these tracts, first-episode schizophrenia patients show none at all. However, in the forceps major, the splenium of the corpus callosum, and the inferior longitudinal fasciculus, the small to medium-sized FA reductions are similar between first-episode and chronic schizophrenia patients. Taken together, these data suggest that in some brain regions modest white matter changes are present early in the course of schizophrenia and are comparable to those observed in more chronic stages of the illness. In other brain regions, however, white matter changes are not observed at illness onset; in these regions, it may require time for ongoing pathological processes to produce observable changes.
These DTI findings may reflect a variety of neuropathological phenomena. For example, FA is associated with axonal myelination in some disease states (26 , 27) and with axonal density or axonal coherence in other disease states (28 , 29) . Given these associations, data from our study may reflect a progressive loss of axonal myelination, density, or coherence in some white matter regions subsequent to illness onset.
Alternatively, pathological changes may be present in those white matter regions that appear normal at illness onset but may be too subtle to be detected with DTI. In either case, the more profound FA reductions in the forceps minor and the genu of the corpus callosum observed in the chronic schizophrenia patients may be the result of chronic progressive processes occurring after illness onset. This possibility would be consistent with MRI evidence for a progressive reduction in frontal white matter volume in patients with schizophrenia over a relatively short period following illness onset (10) .
If indeed there is a progression in white matter abnormalities over time in schizophrenia, one must also consider secondary influences confounding any primary schizophrenia-related changes. For example, age-related changes in FA may contribute to these progressive white matter changes in schizophrenia patients. Indeed, age-related declines of FA in frontal white matter (30 , 31) and other regions (32) have been reported in healthy populations. Moreover, widespread greater-than-normal age-related FA reductions have been observed in white matter in schizophrenia patients (32) . In contrast, our study found no exaggerated aging effects on FA in chronic schizophrenia patients relative to healthy comparison subjects in the forceps minor or the genu of the corpus callosum, tracts that showed striking differences between first-episode and chronic schizophrenia patients. In addition, other investigators have failed to observe significant negative relationships between FA and length of illness in schizophrenia patients (33 – 36) .
While these data seem to contradict the notion of an accelerated aging effect on FA in schizophrenia patients after illness onset, there are alternative explanations. First, these time-related changes in white matter integrity in schizophrenia may not be linear in the way we fit the data. It may be that more complex curvilinear functions better characterize the relationships between age and FA in schizophrenia. In addition, chronic treatment with antipsychotic medication may also influence white matter imaging findings in schizophrenia patients. For example, Bartzokis et al. (37) showed that schizophrenia patients treated with risperidone had significantly more white matter volume than patients treated with fluphenazine, and both groups differed significantly from healthy comparison subjects in opposite directions. However, other research groups found no association between antipsychotic treatment and FA (33 , 35 , 36) .
Furthermore, it is possible that these white matter changes are not homogeneous across patient subtypes and that more aggressive changes occur only in those with more severe disease. Indeed, the chronic schizophrenia patients imaged in this study represent a diverse population, from eight different treatment locations, ranging from a long-term institutional setting to other supervised residential settings to more ambulatory care centers and vocational rehabilitation programs. In contrast, most of the first-episode patients had recently been discharged from their first psychiatric admission at one of two hospitals and referred to either monthly outpatient care or a partial hospitalization program at one of two centers. Thus, it is possible that a much larger sample of first-episode patients from a greater diversity of treatment settings would be required to ensure representation of poor-outcome patients with more widespread white matter changes at illness onset. Data from DTI investigations showing anisotropy reductions to be more extensive in schizophrenia patients with poor outcomes (38) support this possibility.
Other investigators have identified DTI changes in first-episode schizophrenia patients in some brain regions that our study did not (13 , 17) . These discrepancies may be explained by differences in anisotropy analysis and brain regions sampled. For example, Federspiel et al. (17) , using intervoxel coherence instead of FA, found lower intervoxel coherence in frontal white matter regions bilaterally. Kumra et al. (13) did use FA measures and found significant reductions in the frontal white matter of adolescent patients with early-onset schizophrenia. However, these FA frontal lobe white matter decreases were not homogeneous and were noted to be very small above the AC-PC line, becoming much larger at the level of the AC-PC line. In the present study, we measured FA in a frontal lobe white matter ROI (forceps minor) located 6 mm above the AC-PC line, which may explain the discrepancy with the Kumra et al. findings.
This study has some limitations that require mention. First, the ROI method we used for anisotropy analysis may have contributed to the discrepant findings between the first-episode and chronic schizophrenia patients. The placement of ROIs is subjective, and errors may have occurred while defining ROIs in the white matter structures we sampled. However, it is unlikely that these findings are due to such error, because raters used ROIs of the same size and applied strict criteria for placement, and interrater reliability was high.
While the cross-sectional data we report in this study suggest that changes in white matter are less widespread at illness onset in schizophrenia and progress in more chronic states, more definitive conclusions will require follow-up imaging of first-episode patients. We are currently conducting follow-up studies of our first-episode cohort to determine whether the DTI findings reported here are progressive and related to illness outcome. Such investigations may demonstrate critical points of intervention early in the illness that may affect long-term outcome.
1. Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA: Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA 2001; 98:4746–4751Google Scholar
2. Sugai T, Kawamura M, Iritani S, Araki K, Makifuchi T, Imai C, Nakamura R, Kakita A, Takahashi H, Nawa H: Prefrontal abnormality of schizophrenia revealed by DNA microarray: impact on glial and neurotrophic gene expression. Ann NY Acad Sci 2004; 1025:84–91Google Scholar
3. Aston C, Jiang L, Sokolov BP: Microarray analysis of postmortem temporal cortex from patients with schizophrenia. J Neurosci Res 2004; 77:858–866Google Scholar
4. Davis KL, Haroutunian V: Global expression-profiling studies and oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet 2003; 362:758Google Scholar
5. Hof PR, Haroutunian V, Friedrich VL Jr, Byne W, Buitron C, Perl DP, Davis KL: Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry 2003; 53:1075–1085Google Scholar
6. Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI: Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res 2004; 67:269–275Google Scholar
7. Kanaan RA, Kim JS, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK: Diffusion tensor imaging in schizophrenia. Biol Psychiatry 2005; 58:921–929Google Scholar
8. Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J: Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry 2001; 58:461–465Google Scholar
9. Bartzokis G, Nuechterlein KH, Lu PH, Gitlin M, Rogers S, Mintz J: Dysregulated brain development in adult men with schizophrenia: a magnetic resonance imaging study. Biol Psychiatry 2003; 53:412–421Google Scholar
10. Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M: Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry 2003; 60:585–594Google Scholar
11. Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S, Gunduz-Bruce H, Malhotra AK, Kane JM, Bilder RM, Lim KO: White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry 2005; 162:602–605Google Scholar
12. Szeszko PR, Robinson DG, Ashtari M, Vogel J, Betensky J, Sevy S, Ardekani BA, Lencz T, Malhotra AK, McCormack J, Miller R, Lim KO, Gunduz-Bruce H, Kane JM, Bilder RM: Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology 2008; 33:976–984Google Scholar
13. Kumra S, Ashtari M, McMeniman M, Vogel J, Augustin R, Becker DE, Nakayama E, Gyato K, Kane JM, Lim K, Szeszko P: Reduced frontal white matter integrity in early-onset schizophrenia: a preliminary study. Biol Psychiatry 2004; 55:1138–1145Google Scholar
14. Kumra S, Ashtari M, Cervellione KL, Henderson I, Kester H, Roofeh D, Wu J, Clarke T, Thaden E, Kane JM, Rhinewine J, Lencz T, Diamond A, Ardekani BA, Szeszko PR: White matter abnormalities in early-onset schizophrenia: a voxel-based diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry 2005; 44:934–941Google Scholar
15. Kyriakopoulos M, Vyas NS, Barker GJ, Chitnis XA, Frangou S: A diffusion tensor imaging study of white matter in early-onset schizophrenia. Biol Psychiatry 2008; 63:519–523Google Scholar
16. Hao Y, Liu Z, Jiang T, Gong G, Liu H, Tan L, Kuang F, Xu L, Yi Y, Zhang Z: White matter integrity of the whole brain is disrupted in first-episode schizophrenia. Neuroreport 2006; 17:23–26Google Scholar
17. Federspiel A, Begre S, Kiefer C, Schroth G, Strik WK, Dierks T: Alterations of white matter connectivity in first episode schizophrenia. Neurobiol Dis 2006; 22:702–709Google Scholar
18. Price G, Bagary MS, Cercignani M, Altmann DR, Ron MA: The corpus callosum in first episode schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry 2005; 76:585–587Google Scholar
19. Begre S, Federspiel A, Kiefer C, Schroth G, Dierks T, Strik WK: Reduced hippocampal anisotropy related to anteriorization of alpha EEG in schizophrenia. Neuroreport 2003; 14:739–742Google Scholar
20. Price G, Cercignani M, Bagary MS, Barnes TR, Barker GJ, Joyce EM, Ron MA: A volumetric MRI and magnetization transfer imaging follow-up study of patients with first-episode schizophrenia. Schizophr Res 2006; 87:100–108Google Scholar
21. Andreasen NC, Flaum M, Arndt S: The Comprehensive Assessment of Symptoms and History (CASH): an instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry 1992; 49:615–623Google Scholar
22. Perkins DO, Leserman J, Jarskog LF, Graham K, Kazmer J, Lieberman JA: Characterizing and dating the onset of symptoms in psychotic illness: the Symptom Onset in Schizophrenia (SOS) Inventory. Schizophr Res 2000; 44:1–10Google Scholar
23. Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PC: MRI Atlas of Human White Matter. Amsterdam, Elsevier, 2005Google Scholar
24. Holm S: A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6:65–70Google Scholar
25. Buchsbaum MS, Friedman J, Buchsbaum BR, Chu KW, Hazlett EA, Newmark R, Schneiderman JS, Torosjan Y, Tang C, Hof PR, Stewart D, Davis KL, Gorman J: Diffusion tensor imaging in schizophrenia. Biol Psychiatry 2006; 60:1181–1187Google Scholar
26. Hüppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, Volpe JJ: Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res 1998; 44:584–590Google Scholar
27. Filippi M, Cercignani M, Inglese M, Horsfield MA, Comi G: Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurol 2001; 56:304–311Google Scholar
28. Hong YH, Lee KW, Sung JJ, Chang KH, Song IC: Diffusion tensor MRI as a diagnostic tool of upper motor neuron involvement in amyotrophic lateral sclerosis. J Neurol Sci 2004; 227:73–78Google Scholar
29. Toosy AT, Werring DJ, Orrell RW, Howard RS, King MD, Barker GJ, Miller DH, Thompson AJ: Diffusion tensor imaging detects corticospinal tract involvement at multiple levels in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2003; 74:1250–1257Google Scholar
30. Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM: Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging 2005; 26:1215–1227Google Scholar
31. Pfefferbaum A, Adalsteinsson E, Sullivan EV: Frontal circuitry degradation marks healthy adult aging: evidence from diffusion tensor imaging. Neuroimage 2005; 26:891–899Google Scholar
32. Mori T, Ohnishi T, Hashimoto R, Nemoto K, Moriguchi Y, Noguchi H, Nakabayashi T, Hori H, Harada S, Saitoh O, Matsuda H, Kunugi H: Progressive changes of white matter integrity in schizophrenia revealed by diffusion tensor imaging. Psychiatry Res 2007; 154:133–145Google Scholar
33. Foong J, Maier M, Clark CA, Barker GJ, Miller DH, Ron MA: Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry 2000; 68:242–244Google Scholar
34. Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, Kikinis R, Jolesz FA, McCarley RW, Shenton ME: Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry 2003; 54:1171–1180Google Scholar
35. Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, Niznikiewicz M, Connor EE, Levitt JJ, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME: DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage 2005; 26:1109–1118Google Scholar
36. Minami T, Nobuhara K, Okugawa G, Takase K, Yoshida T, Sawada S, Ha-Kawa S, Ikeda K, Kinoshita T: Diffusion tensor magnetic resonance imaging of disruption of regional white matter in schizophrenia. Neuropsychobiology 2003; 47:141–145Google Scholar
37. Bartzokis G, Lu PH, Nuechterlein KH, Gitlin M, Doi C, Edwards N, Lieu C, Altshuler LL, Mintz J: Differential effects of typical and atypical antipsychotics on brain myelination in schizophrenia. Schizophr Res 2007; 93:13–22Google Scholar
38. Mitelman SA, Torosjan Y, Newmark RE, Schneiderman JS, Chu KW, Brickman AM, Haznedar MM, Hazlett EA, Tang CY, Shihabuddin L, Buchsbaum MS: Internal capsule, corpus callosum, and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophr Res 2007; 92:211–224Google Scholar





