Sex Differences in Language Dysfunction in Schizophrenia
Abstract
OBJECTIVE: Normal sex differences in language functions are disrupted in schizophrenia. However, identification of specific language components most vulnerable in schizophrenia and how they may differ by sex remain unexamined. The current study investigated this issue in the domains of phonology, semantics, and grammar, which have been closely linked with neuroanatomic regions for which sex differences have been identified. METHOD: Thirty-one outpatients with DSM-III-R schizophrenia and 27 healthy subjects comparable within sex on age, handedness, parental socioeconomic status, and ethnicity were systematically ascertained from a Boston catchment area. The subjects were administered an extensive language battery in the context of a comprehensive neuropsychological battery that included measures of phonology, semantics, and grammar. RESULTS: Male patients performed significantly worse than their healthy counterparts on all three domains, with phonology least affected. In contrast, language function was relatively preserved in the female patients, compared to their healthy counterparts, with phonology most affected. Across domains, the effect sizes in comparisons of male patients and healthy male subjects had a twofold difference, whereas the difference in effect sizes in comparisons of female patients and healthy female subjects was less in all areas. CONCLUSIONS: Findings were consistent with prior evidence of overall language dysfunction in schizophrenia and may have implications for understanding sex differences in neuroanatomic abnormalities in regions associated with phonological processing.
Speech disturbances have long been identified as a characteristic trait of schizophrenia, with reports dating back to the time of Kraepelin (1) and Bleuler (2). Earlier conceptualizations based on aphasia models (1) were later replaced by theories that language dysfunction in schizophrenia reflected an underlying disorder of thought process (2, 3). Over the past few decades, there has been increased interest in clarifying whether schizophrenia involves a primary language problem that is independent of formal thought disorder (i.e., deviant language that can be separated from abnormal thoughts [4]). Although it remains unclear to what extent language abnormalities and thought disorder are dissociable (5), there is increasing behavioral evidence that schizophrenia involves a primary disruption in language (e.g., references 6, 7). This conceptualization is further substantiated by findings in schizophrenia of structural and functional brain abnormalities in regions traditionally linked with language functions, such as the superior temporal gyrus (8, 9) and its subcomponents (10, 11), e.g., the planum temporale (12–14) and Heschl’s gyrus (12, 15).
Language is a complex cognitive process comprising several components that generally include 1) phonology (production and processing of individual speech sounds), 2) semantics (meaning of words), 3) orthographics (visual representation of words), 4) lexical processing (integration of phonology, semantics, and syntax), 5) pragmatics (social/global uses of language), 6) articulation (vocal expression through motor acts), 7) grammar (language structure), and 8) prosody (e.g., melody/tone) (see references 16–18). With the advent of neuroimaging techniques, researchers have begun to examine the neural correlates of various aspects of language (19, 20). There is extensive behavioral evidence of sex differences across many of these language components in healthy adults (21, 22). Generally, adult women exhibit a relative advantage over men in verbal/associational fluency (22), lexical and prelexical processing (23), efficiency of phonological processing (24), and perceptual speed (25). These behavioral differences are consistent with results from imaging studies showing normal sexual brain dimorphisms in regions subserving language, such as the superior temporal gyrus (26–28) and Broca’s area (28, 29), which are larger (relative to cerebrum size) in women than men. Functional imaging studies of phonological processing have revealed greater bilateral activation of Broca’s area in women and greater left-lateralized activation in men (30, 31).
Schizophrenia is well known to be marked by abnormalities in temporoparietal and frontal brain regions (for review, see reference 11), which are believed to disrupt language functions and higher-order cognitive problems (e.g., frontal/executive systems dysfunction) that affect language functions, respectively. In light of these neuroanatomic abnormalities, it is not surprising that schizophrenia is characterized by cognitive dysfunction across a variety of domains (20, 32, 33), making it difficult to disentangle the degree to which language or other cognitive problems may be due to thought disorder versus impairments in other affected cognitive domains (34), such as attention (35–37), frontal/executive control (38, 39), processing speed (40), information processing (41), and intellectual functioning (for review, see reference 42). This difficulty highlights the importance of considering potentially confounding cognitive factors when examining language dysfunction in schizophrenia.
It is interesting to note that language functions (e.g., semantic fluency [43]) and related brain regions (e.g., Heschl’s gyrus and the planum temporale [12, 44, 45]), in which sex differences have been reported in healthy adults, are aberrant in schizophrenia. Moreover, as with healthy adults, research with schizophrenia patients has demonstrated sex differences in some aspects of verbal functions (e.g., reference 46). Male schizophrenia patients performed more poorly on measures of verbal learning, verbal fluency (47), and verbal memory (48, 49), compared to female schizophrenia subjects, although findings were not consistent (49–51). Male patients had an advantage on spatial relative to language tasks, compared to female patients, who performed similarly across these domains (52).
The literature includes some inconsistencies that raise questions about the general proposition of sex differences in cognition in schizophrenia (53). For example, some studies have found worse cognitive performance among female patients (50) or an absence of significant sex differences on measures of verbal IQ (51, 54) and language (49, 54). However, in a number of these latter studies, adjustments were made for age at illness onset, which may have attenuated potential sex effects, given that age of onset is known to be earlier in male patients than in female patients (55). That is, accounting for sex effects in age at onset may have also accounted for sex effects in cognition. Moreover, the disproportionate representation of chronically disabled patients in some samples may have attenuated sex effects, given that these samples would include more severely ill women with cognitive impairments (46, 56).
We previously demonstrated sex differences in a global construct of verbal functioning among adult schizophrenia patients that parallel findings in studies of healthy individuals in which female subjects outperformed male subjects (46). The current study is novel in that we explore the possibility that schizophrenia involves problems in “lower-level” language processes, such as phonology, that may be differentially influenced by sex. Moreover, we are unaware of any studies that systematically examined 1) which specific components of language are most vulnerable in schizophrenia from a psycholinguistic viewpoint; 2) to what extent these components may be differentially influenced by sex; and 3) whether the pattern holds when patients are compared to healthy same-sex subjects, with adjustment for potential confounding cognitive factors. Our assumption is that identifying sex differences in specific language domains would have implications for understanding sex differences in brain abnormalities in language-associated regions. We sought to clarify these questions by examining the individual language domains of phonology, semantics, and grammar. We selected these domains because they are most closely linked with the language-associated brain regions for which normal volumetric sex differences have been identified (e.g., the planum temporale, superior temporal gyrus, Heschl’s gyrus, Broca’s area [27–29]) and in which disruptions in normal sexual brain dimorphisms in schizophrenia have been demonstrated (e.g., references 9, 15, 45).
Phonology and semantics were of particular interest in the light of mounting evidence that while semantics (e.g., semantic fluency) and phonology (e.g., phonemic fluency) are both disrupted in schizophrenia, semantics are affected more so (for a quantitative review, see reference 57) (58, 59). This finding likely reflects the dual disruption in schizophrenia of frontal and temporoparietal functions associated with verbal fluency and is noteworthy given that a reverse pattern is typical in healthy comparison subjects. One implication is that although higher-order frontal/executive functions—namely activation/retrieval and organization/planning (which affect semantic and phonemic fluency)—play a role in language more generally and disruptions in these functions may contribute to thought disorder and disorganized speech in schizophrenia, concurrent problems arise at the lower level of semantic processing (see reference 60). We began to address this problem by investigating whether there are differential deficits by sex at the more basic levels of processing, such as phonology and grammar, compared with semantic processing. This cognitive study is the first step toward better understanding how sex differences in language dysfunction may be linked with disruptions in normal sexual brain dimorphisms in schizophrenia.
Method
Subjects
The patients were systematically ascertained from an extensive outpatient treatment system in Boston, which ensured clinical stability upon participation. The patients included 31 (17 male and 14 female) individuals who met the DSM-III-R diagnostic criteria for schizophrenia. The healthy comparison subjects (N=27; 13 male and 14 female subjects) were ascertained from the same catchment area through advertisements and bulletin board postings in local hospitals.
The comparison and overall patient groups did not significantly differ on age (patients: mean=39.1 years, SD=7.0), ethnicity (85% non-Hispanic white), education level (1 year of college), socioeconomic background (middle class), or Wide-Range Achievement Test, Revised reading subtest (WRAT-R) (61) score (mean=102, SD=10). The estimated IQ (based on WAIS-R vocabulary and block design age-scaled scores [62]) was significantly lower for male patients (mean=93, SD=13) than for female patients (mean=104, SD=14) and male comparison subjects (mean=113, SD=12). Cooperation and psychosis ratings (63) for all patients (men and women) reflected clinical stability (mild to mild/moderate symptoms). The patients were receiving a mean daily neuroleptic dose of 520 mg (SD=428) in chlorpromazine equivalents; mean daily doses were comparable for men and women. Subjects were proportionately comparable between groups within sex on age, handedness, parental socioeconomic status, and ethnicity. A more detailed description of, and data for, demographic and clinical characteristics stratified by sex is provided elsewhere (46).
Written informed consent was obtained from all subjects after a full explanation of the study procedures. The study was approved by Human Studies Committees at Harvard Medical School and Massachusetts Mental Health Center.
Diagnostic Assessment
Consensus diagnoses, based on DSM-III-R diagnostic criteria, were made by experienced diagnosticians (J.M.G. and L.J.S.) who were unaware of the patients’ neuropsychological data. The diagnosticians reviewed information obtained from structured research interviews with the Schedule for Affective Disorders and Schizophrenia (64) and from systematic record reviews. The healthy comparison subjects received a brief clinical interview and were administered the MMPI-168 to screen for current psychopathological disorders; they were included if the T score was less than or equal to 70 (65).
Neuropsychological Assessment
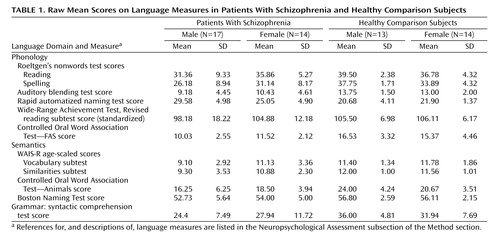
An extensive language battery, including tests of phonology, semantics, and grammar (Table 1), was administered in the context of a comprehensive neuropsychological battery (see reference 46). The phonology composite measure included 1) Roeltgen’s Nonwords Reading Test (66), total correct (read aloud a list of nonwords); 2) Roeltgen’s Nonwords Spelling Test, total correct (write down the proper spelling of a list of nonwords read to the subject one at a time); 3) auditory blending test, total correct (decipher words read to the subject in a broken form, e.g., “re…cor…der” = recorder); 4) rapid automatized naming test (67), total time (name the letters, numbers, colors, or pictures presented on cards); 5) WRAT-R (61), standardized score (single word reading); and 6) Controlled Oral Word Association Test—FAS (68), total correct (generate as many words as possible that begin with a designated letter [F, A, or S] within a 60-second time period). The semantics composite measure included 1) WAIS-R Vocabulary subtest (69), age-scaled score (provide definitions of vocabulary words); 2) WAIS-R Similarities subtest (69), age-scaled score (deduce how two words are alike/the same); 3) Boston Naming Test (70), total correct spontaneous responses (confrontation naming of 60 objects illustrated in line drawings); and 4) Controlled Oral Word Association Test—Animals (68), total correct (generate as many words as possible that are names of animals within a 60-second time period). Use of age-scaled scores for the WAIS-R subtests is standard in the literature. The grammar index consisted of the syntactic comprehension test from the 20-minute short form of Caplan and Hildebrandt’s task (71), total correct (identify the subject and object of the actions on the basis of various placement of prepositional phrases). Examiners had extensive experience in clinical assessment and neuropsychological testing with schizophrenia patients. Although the examiners were not blind to the patients’ status, they did not conduct the diagnostic interviews. The internal consistency reliability measures (Cronbach alpha for standardized variables) associated with the language measures composing the phonology (α=0.72) and semantics (α=0.81) domains were excellent. Language domains were created by standardizing all of the variables to a mean of 0 and standard deviation of 1 and averaging these standardized scores within each function. The grammar index was similarly standardized in order to use it in analyses of specificity with the other domains.
Clinical Description of the Language Assessments
The following patient description illustrates typical language dysfunctions related to our three domains of interest. A 31-year-old male patient with DSM-III-R chronic schizophrenia of 10 years’ duration was disorganized and delusional and had psychomotor symptoms at the time of assessment. He had 11 years of education and a current IQ of 92, average for a person with schizophrenia. His current IQ was higher than his ability on semantic processing tasks such as naming visual objects (Boston Naming Test) and defining words orally (WAIS-R vocabulary subtest). His performance on relatively basic tasks tapping into phonological processing and automatic knowledge, including a single-word oral reading test (WRAT-R reading subtest), was also impaired, even though performance on this test is typically preserved in schizophrenia and often better than performance on comparably normed IQ tests. This finding suggested that he had some premorbid developmental language impairment. In addition, he had gross difficulty in generating words on a verbal fluency test (Controlled Oral Word Association Test—FAS), producing approximately five words and then quitting after he perseverated on the last one. This latter performance was likely associated with a disorder of cognitive control and prefrontal/executive impairments superimposed on preexisting deficiencies. Regarding sentence comprehension, he (as well as a number of other patients) was able to correctly identify subjects and objects receiving actions in simple sentences. However, he and other patients had difficulty identifying subjects and objects of actions in complex sentences, such as “The boy that the old man pinched caught his friend.” This pattern reflects a common difficulty in abstract conceptual and sequential thinking in patients with schizophrenia. In summary, typical of many male patients in our sample, this patient’s expression of semantic knowledge, grammar, and phonological fluency were all impaired beyond what would be predicted from his IQ. These impairments became more pronounced when the patient had to search his own lexicon and initiate and sustain responses (such as on verbal fluency tests) or utilize abstract reasoning and interpret linguistic relationships. The pattern of performance suggests a combination of premorbid developmental language impairment and current dysfunction in executive control consistent with the relatively more severe linguistic impairments observed in the men with schizophrenia.
Data Analyses
A multivariate general linear model for correlated data (72) was used to test for significant sex differences across language functions, with the independent predictors of sex, group, and sex-by-group interaction. Investigators have implicated a role of executive functions specifically in language dysfunction in schizophrenia (38). We therefore covaried for attention (a core cognitive function that affects language processing independent of group effects) to control for the degree to which variability in this aspect of cognition contributes to language deficits and/or sex effects. The objective was to clarify whether sex differences in language problems in schizophrenia persist above and beyond underlying disruptions in complex attention/executive functions. We selected from our previous work the attention measure that captures a wide range of complex attention/executive functions. Attention was operationalized as a composite measure (46) of the auditory continuous performance test (total correct), visual continuous performance test (discriminability and reaction time scores), dichotic listening test (total correct digits), WAIS-R digit symbol subtest (total), and visual cancellations test (total time and errors). The interaction term was used to test whether there was a “different difference” between women and men with schizophrenia, compared with their healthy counterparts. In addition, our stringent sampling procedures controlled for much extraneous variation, which is important when identifying significant sex effects in small samples.
Table 1 presents raw mean scores for the individual measures that composed the language domains. The variables were standardized by using the standard deviations from the total sample, reflecting differences from general population averages. The mean standardized scores depicted in Figure 1 reflect effect sizes within each group.
Results
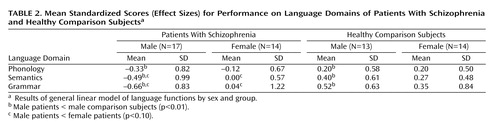
The results of the multivariate general linear model for correlated data showed a significant group effect on subjects’ performance in the three language domains with adjustment for attention (F=2.92, df=3, 81, p<0.04). The univariate general linear models showed that the interaction of sex by group was significant for semantics (t=1.94, df=98, p=0.05) and grammar (t=2.09, df=89, p<0.04) and for phonology with adjustment for attention (t=2.31, df=97, p=0.02) (Table 2). The univariate analyses showed that male patients performed significantly worse than their healthy male counterparts on all three domains, whereas female patients, compared to their healthy counterparts, had substantially smaller and nonsignificant functional reductions across all domains (Figure 1). Further, between the sexes, male patients performed worse than female patients on grammar (t=–1.77, df=25, p<0.09) and semantics (t=–1.72, df=26.2, p<0.10). Although these differences did not reach statistical significance, male patients’ performance on these domains was approximately one-half of a standard deviation below that for female patients. Male and female patients performed more similarly on phonological processing.
Women with schizophrenia performed relatively worse on phonological processing than on semantics or grammar (effect size=–0.12 for phonology versus 0.00 and 0.04 for semantics and grammar, respectively). In contrast, men with schizophrenia were the least affected on phonology (effect size=–0.33 for phonology versus –0.49 and –0.66 for semantics and grammar, respectively). Across domains, the effect sizes for the male patients, compared with the healthy male comparison subjects, had a twofold difference, whereas the differences in effect sizes for the women were less.
Discussion
This study demonstrated significant sex differences in language domains in comparisons between healthy men and women and men and women with schizophrenia. Women with schizophrenia, compared to their healthy counterparts, had relative preservation of function across domains. Phonological processing performance in women with schizophrenia was below that in healthy female subjects and was not significantly different from that in men with schizophrenia. However, grammar and semantics were sexually differentiated. Men with schizophrenia, compared to healthy men, showed impairment across the three language domains. The finding of relatively worse performance across language domains in the male patients, relative to the healthy comparison subjects, was consistent with previous findings of impaired verbal functions among patients with schizophrenia, including findings from our earlier work (46).
In the comparison of male patients with female patients, the relatively worse performance across all language domains among male patients was consistent with previous research demonstrating more impaired verbal learning and verbal fluency (47, 73) among male patients, compared to female patients. The results from the current study extended our previous findings (46) by demonstrating that although there was relative preservation of functioning among the women, there were some differences among individual language components (Figure 1). Specifically, male patients (relative to their healthy counterparts) were more substantially affected in semantics and grammar, with a relatively smaller decline observed in phonology. A reverse pattern was observed among female patients (relative to their healthy counterparts). The direction of the findings (albeit not significant) suggested a relative preservation of grammar, which was the least affected domain (nonsignificantly less than semantics), whereas phonology was the most substantially affected domain (falling below the female population average).
One of the limitations of this study was the relatively small sample size. Previous literature showed a relative advantage for normal women compared with men regarding phonological processing, although in our sample normal men and women were comparable. This was most likely attributable to limited power due to small sample size, particularly given that there is greater variability within than between the sexes in the normal population. The present study, however, focused on how male and female patients differed from their healthy counterparts on language domains. In fact, sample sizes within sex were similar, and there were significant differential sex effects. This may in part be due to the fact that the samples were tightly comparable within sex on important sociodemographic and other potential confounders that may affect language function. There were no significant sex differences in chlorpromazine equivalents or other clinical factors (46). Moreover, because the study was designed to measure particular domains of language, the psychometric properties of these domains were strong.
Overall, the current findings suggested a divergent pattern of sex differences, namely a more pronounced “decline” in anterior (e.g., phonology) and posterior (e.g., semantics) language functions in male than in female patients (in comparisons of patients with their healthy counterparts). This finding was strikingly consistent with research implicating sex differences in structural brain volumes in comparisons of normal sexual dimorphisms to patients’ sexual dimorphisms in regions associated with language functions, such as Heschl’s gyrus, planum temporale, Broca’s area, and superior temporal gyrus (9, 12, 15, 27, 45, 74, 75). For example, in an earlier study, sex differences in planum temporale asymmetry were found, with male patients demonstrating exaggerated leftward planum temporale asymmetry, compared with healthy male subjects, and female patients demonstrating more right asymmetry, compared with healthy female subjects (45). The current findings are also consistent with recent functional neuroimaging studies demonstrating relatively greater right hemisphere (30, 31) or more symmetrical (76) involvement in phonological processing in healthy women than in men, although not consistently (e.g., references 77, 78), and reduced lateralization of phonological processing in male patients relative to healthy comparison subjects (79, 80) and female patients (81), again although not consistently (82).
It is possible that phonology—an aspect of language that ordinarily tends to be more left-lateralized functionally and more reliant on anterior functions (e.g., frontal/executive control) than aspects of language that are more evenly distributed across hemispheres (e.g., semantics) and believed to be reliant on both anterior (frontaland posterior (temporal) language regions—is less affected in male patients, given the association with increased left asymmetry in planum temporale in male patients (45). In contrast, findings in female patients, who as females are expected to show greater frontal and temporal symmetry and have shown greater asymmetry with increases in the right planum temporale (45), suggest the potential for enhanced ability to recruit right hemisphere posterior regions for semantic task performance.
 |
 |
Received Nov. 16, 2004; revision received May 5, 2005; accepted May 13, 2005. From the Harvard Medical School Department of Psychiatry at Beth Israel Deaconess Medical Center, Division of Public Psychiatry, Massachusetts Mental Health Center, Boston; Harvard Medical School Department of Psychiatry, Division of Psychiatric Neuroscience at Massachusetts General Hospital, Boston; Harvard Medical School Department of Psychiatry, Brigham & Women’s Hospital; Harvard and MIT Athinoula Martinos Center for Biomedical Imaging at Massachusetts General Hospital, Boston; Harvard Institute of Psychiatric Epidemiology and Genetics, Department of Epidemiology, Harvard School of Public Health, Boston; Institute of Behavioral Genomics, Department of Psychiatry, University of California San Diego, La Jolla, Calif.; and Harvard Medical School Department of Medicine, Division of Women’s Health, Connors Center for Women’s Health and Gender Biology, Brigham & Women’s Hospital. Address correspondence and reprint requests to Dr. Goldstein, Division of Women’s Health, Brigham & Women’s Hospital, One Brigham Circle, 3rd Floor; 1620 Tremont St., Boston, MA 02120; jill_[email protected] (e-mail).Supported by NIMH grants SDA K21 MH-00096 (J.M.G.), NIMH grant RO1 MH-56956, which was supported in part by the NIH Office of Research on Women’s Health (J.M.G.), and an NIMH postdoctoral fellowship in the Clinical Research Training Program in Biological Psychiatry, Department of Psychiatry, Harvard Medical School (T32 MH-16259) (D.J.W.).
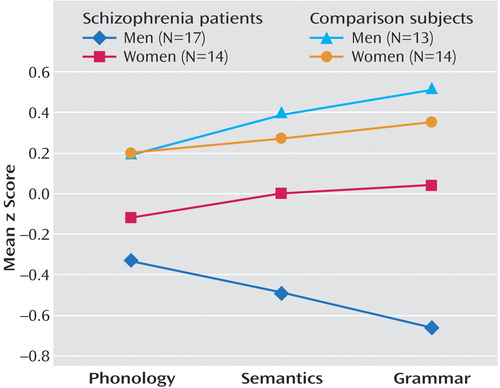
Figure 1Mean Standardized Scores on Language Domains for Male and Female Subjects With Schizophrenia and Same-Sex Healthy Comparison Subjects a
aResults of general linear model of language functions by sex and group revealed that male patients had significantly lower standardized scores than male comparison subjects on measures of phonology (t=2.31, df=97, p=0.02), semantics (t=1.94, df=98, p=0.05), and grammar (t=2.09, df=89, p<0.04).
1. Kraepelin E: Dementia Praecox and Paraphrenia. Translated by Barclay RM; edited by Robertson GM. Edinburgh, E & S Livingstone, 1919Google Scholar
2. Bleuler E: Dementia Praecox or the Group of Schizophrenias (1908). Translated by Zinkin J. New York, International Universities Press, 1950Google Scholar
3. Critchley M: Case studies of aphasia and schizophrenic patients. Br J Psychiatry 1964; 110:353–364Crossref, Medline, Google Scholar
4. Chaika E: Thought disorder or speech disorder in schizophrenia? Schizophr Bull 1982; 8:587–594Crossref, Medline, Google Scholar
5. Cutting J, Murphy D: Schizophrenic thought disorder: a psychological and organic interpretation. Br J Psychiatry 1988; 152:310–319Crossref, Medline, Google Scholar
6. Cutting J: The Psychology of Schizophrenia. Edinburgh, Churchill Livingstone, 1985Google Scholar
7. Thomas P, Fraser W: Linguistics, human communication and psychiatry. Br J Psychiatry 1994; 165:585–592Crossref, Medline, Google Scholar
8. Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Lee C-U, Ciszewski AA, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW: Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry 2003; 160:156–164Link, Google Scholar
9. Reite M, Sheeder J, Teale P, Adams M, Richardson D, Simon J, Jones RH, Rojas DC: Magnetic source imaging evidence of sex differences in cerebral lateralization in schizophrenia. Arch Gen Psychiatry 1997; 54:433–440Crossref, Medline, Google Scholar
10. McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME: MRI anatomy of schizophrenia. Biol Psychiatry 1999; 45:1099–1119Crossref, Medline, Google Scholar
11. Shenton ME, Dickey CC, Frumin M, McCarley RW: A review of MRI findings in schizophrenia. Schizophr Res 2001; 49:1–52Crossref, Medline, Google Scholar
12. Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, Snyderman D, Yurgelun-Todd D, Kikinis R, Jolesz FA, Shenton ME: Planum temporale and Heschl gyrus volume reduction in schizophrenia. Arch Gen Psychiatry 2000; 57:692–699Crossref, Medline, Google Scholar
13. Kwon JS, McCarley RW, Hirayasu Y, Anderson JE, Fischer IA, Kikinis R, Jolesz FA, Shenton ME: Left planum temporale volume reduction in schizophrenia. Arch Gen Psychiatry 1999; 56:142–148Crossref, Medline, Google Scholar
14. DeLisi LE, Hoff AL, Neale C, Kushner M: Asymmetries in the superior temporal lobe in male and female first-episode schizophrenic patients: measures of the planum temporale and superior temporal gyrus by MRI. Schizophr Res 1994; 12:19–28Crossref, Medline, Google Scholar
15. Rojas DC, Teale P, Sheeder J, Simon J, Reite M: Sex-specific expression of Heschl’s gyrus functional and structural abnormalities in paranoid schizophrenia. Am J Psychiatry 1997; 154:1655–1662Abstract, Google Scholar
16. Chomsky N: Language and Mind. New York, Harcourt Brace Jovanovich, 1972Google Scholar
17. Akmajian A, Demers R, Farmer A, Harnsih R: Linguistics: An Introduction to Language and Communication. Cambridge, Mass, MIT Press, 1995Google Scholar
18. Langacker R: Language and Its Structure: Some Fundamental Linguistic Concepts. New York, Harcourt Brace Jovanovich & World, 1968Google Scholar
19. Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD: Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage 1999; 10:15–35Crossref, Medline, Google Scholar
20. Kuperberg GR, McGuire PK, Bullmore ET, Brammer MJ, Rabe-Hesketh S, Wright IC, Lythgoe DJ, Williams SC, David AS: Common and distinct neural substrates for pragmatic, semantic, and syntactic processing of spoken sentences: an fMRI study. J Cogn Neurosci 2000; 12:321–341Crossref, Medline, Google Scholar
21. Halpern DF: Sex Differences in Cognitive Abilities. Hillsdale, NJ, Lawrence Erlbaum Associates, 1992Google Scholar
22. Collaer ML, Hines M: Human behavioral sex differences: a role for gonadal hormones during early development? Psychol Bull 1995; 118:55–107Crossref, Medline, Google Scholar
23. Majeres RL: Sex differences in phonological processes: speeded matching and word reading. Mem Cognit 1999; 27:246–253Crossref, Medline, Google Scholar
24. Majeres RL: Sex differences in phonetic processing: speed of identification of alphabetical sequences. Percept Mot Skills 1997; 85(3, part 2):1243-1251Google Scholar
25. Harshman RA, Hampson E, Berenbaum SA: Individual differences in cognitive abilities and brain organization, part I: sex and handedness differences in ability. Can J Psychol 1983; 37:144–192Crossref, Medline, Google Scholar
26. Filipek PA, Richelme C, Kennedy DN, Caviness VS Jr: The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex 1994; 4:344–360Crossref, Medline, Google Scholar
27. Schlaepfer TE, Harris GJ, Tien AY, Peng L, Lee S, Pearlson GD: Structural differences in the cerebral cortex of healthy female and male subjects: a magnetic resonance imaging study. Psychiatry Res Neuroimaging 1995; 61:129–135Crossref, Medline, Google Scholar
28. Harasty J, Double KL, Halliday GM, Kril JJ, McRitchie DA: Language-associated cortical regions are proportionally larger in the female brain. Arch Neurol 1997; 54:171–176Crossref, Medline, Google Scholar
29. Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Faraone SV, Tsuang MT: Normal sexual dimorphism of the adult human brain assessed by in-vivo magnetic resonance imaging. Cereb Cortex 2001; 11:490–497Crossref, Medline, Google Scholar
30. Shaywitz B, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Fletcher JM, Shankweiler DP, Katz L: Sex differences in the functional organization of the brain for language. Nature 1995; 373:607–609Crossref, Medline, Google Scholar
31. Pugh KR, Shaywitz BA, Shaywitz SE, Shankweiler DP, Katz L, Fletcher JM, Skudlarski P, Fulbright RK, Constable RT, Bronen RA, Lacadie C, Gore JC: Predicting reading performance from neuroimaging profiles: the cerebral basis of phonological effects in printed word identification. J Exp Psychol Hum Percept Perform 1997; 23:299–318Crossref, Medline, Google Scholar
32. Andreasen NC, Paradiso S, O’Leary DS: “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull 1998; 24:203–218Crossref, Medline, Google Scholar
33. Heinrichs RW, Zakzanis KK: Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 1998; 12:426–445Crossref, Medline, Google Scholar
34. Rodriguez-Ferrera S, McCarthy RA, McKenna PJ: Language in schizophrenia and its relationship to formal thought disorder. Psychol Med 2001; 31:197–205Crossref, Medline, Google Scholar
35. Seidman LJ: Schizophrenia and brain dysfunction: an integration of recent neurodiagnostic findings. Psychol Bull 1983; 94:195–238Crossref, Medline, Google Scholar
36. Nuechterlein KH, Dawson ME: Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull 1984; 10:160–203Crossref, Medline, Google Scholar
37. Cornblatt BA, Keilp JG: Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull 1994; 20:31–46Crossref, Medline, Google Scholar
38. Barr WB, Bilder RM, Goldberg E, Kaplan E, Mukherjee S: The neuropsychology of schizophrenic speech. J Commun Disord 1989; 22:327–349Crossref, Medline, Google Scholar
39. Grove WM, Andreasen NC: Language and thinking in psychosis: is there an input abnormality? Arch Gen Psychiatry 1985; 42:26–32Crossref, Medline, Google Scholar
40. Brebion G, Amador X, Smith MJ, Gorman JM: Memory impairment and schizophrenia: the role of processing speed. Schizophr Res 1998; 30:31–39Crossref, Medline, Google Scholar
41. Schwartz S: Is there a schizophrenic language? Behav Brain Sci 1982; 5:579–626Crossref, Google Scholar
42. Aylward E, Walker E, Bettes B: Intelligence in schizophrenia: meta-analysis of the research. Schizophr Bull 1984; 10:430–459Crossref, Medline, Google Scholar
43. Gourovitch ML, Goldberg TE, Weinberger DR: Verbal fluency deficits in patients with schizophrenia: semantic fluency is differentially impaired as compared with phonologic fluency. Neuropsychology 1996; 10:573–577Crossref, Google Scholar
44. Shapleske J, Rossell S, Woodruff PWR, David AS: The planum temporale: a systematic, quantitative review of its structural functional and clinical significance. Brain Res Brain Res Rev 1999; 29:26–49Crossref, Medline, Google Scholar
45. Goldstein JM, Seidman LJ, O’Brien LM, Horton NJ, Kennedy DN, Makris N, Caviness VS Jr, Faraone SV, Tsuang MT: Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch Gen Psychiatry 2002; 59:154–164Crossref, Medline, Google Scholar
46. Goldstein JM, Seidman LJ, Goodman JM, Koren D, Lee H, Weintraub S, Tsuang MT: Are there sex differences in neuropsychological functions among patients with schizophrenia? Am J Psychiatry 1998; 155:1358–1364Link, Google Scholar
47. Albus M, Hubmann W, Mohr F, Scherer J, Sobizack N, Franz U, Hecht S, Borrmann M, Wahlheim C: Are there gender differences in neuropsychological performance in patients with first-episode schizophrenia? Schizophr Res 1997; 28:39–50Crossref, Medline, Google Scholar
48. Hoff AL, Riordan H, O’Donnell DW, Morris L, DeLisi LE: Neuropsychological functioning of first-episode schizophreniform patients. Am J Psychiatry 1992; 149:898–903Link, Google Scholar
49. Hoff AL, Wieneke M, Faustman WO, Horon R, Sakuma M, Blankfeld H, Espinoza S, DeLisi LE: Sex differences in neuropsychological functioning of first-episode and chronically ill schizophrenic patients. Am J Psychiatry 1998; 155:1437–1439Link, Google Scholar
50. Nasrallah H, Schwarzkopf S, Olson S, Coffman J: Gender differences in schizophrenia on MRI brain scans. Schizophr Bull 1990; 16:205–210Crossref, Medline, Google Scholar
51. Andia A, Zisook S, Heaton R, Hesselink J, Jernigan T, Kuck J, Moranville J, Braff D: Gender differences in schizophrenia. J Nerv Ment Dis 1995; 183:522–528Crossref, Medline, Google Scholar
52. Ragland JD, Gur RE, Klimas BC, McGrady N, Gur RC: Neuropsychological laterality indices of schizophrenia: interactions with gender. Schizophr Bull 1999; 25:79–89Crossref, Medline, Google Scholar
53. Hoff A, Kremen WS: Sex Differences in Neurocognitive Function in Schizophrenia. Washington, DC, American Psychiatric Publishing, 2002Google Scholar
54. Goldberg TE, Gold JM, Torrey EF, Weinberger DR: Lack of sex differences in the neuropsychological performance of patients with schizophrenia. Am J Psychiatry 1995; 152:883–888Link, Google Scholar
55. Goldstein JM, Lewine RRJ: Overview of sex differences in schizophrenia: where have we been and where do we go from here? in Women and Schizophrenia. Edited by Castle DJ, McGrath JJ, Kulkarni J. Cambridge, UK, Cambridge University Press, 2000, pp 111-153Google Scholar
56. Goldstein JM: Sampling biases in studies on gender and schizophrenia: a reply. Schizophr Bull 1993; 19:9–14Crossref, Google Scholar
57. Bokat CE, Goldberg TE: Letter and category fluency in schizophrenic patients: a meta-analysis. Schizophr Res 2003; 64:73–78Crossref, Medline, Google Scholar
58. Kremen WS, Seidman LJ, Faraone SV, Tsuang MT: Is there disproportionate impairment in semantic or phonemic fluency in schizophrenia? J Int Neuropsychol Soc 2003; 9:79–88Crossref, Medline, Google Scholar
59. Phillips TJ, James AC, Crow TJ, Collinson SL: Semantic fluency is impaired but phonemic and design fluency are preserved in early-onset schizophrenia. Schizophr Res 2004; 70:215–222Crossref, Medline, Google Scholar
60. Goldberg TE, Aloia MS, Gourovitch M, Missar D, Pickar D, Weinberger DR: Cognitive substrates of thought disorder, I: the semantic system. Am J Psychiatry 1998; 155:1671–1676Link, Google Scholar
61. Jastak S, Wilkinson GS: The Wide Range Achievement Test, Revised. Wilmington, Del, Jastak Associates, 1984Google Scholar
62. Lezak M: Neuropsychological Assessment, 3rd ed. New York, Oxford University Press, 1995Google Scholar
63. Faraone SV, Seidman LJ, Kremen WS, Pepple JR, Lyons MJ, Tsuang MT: Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: a diagnostic efficiency analysis. J Abnorm Psychol 1995; 104:286–304Crossref, Medline, Google Scholar
64. Spitzer RL, Endicott J: Schedule for Affective Disorders and Schizophrenia (SADS), 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1978Google Scholar
65. Vincent KR, Castillo IM, Hauser RI, Zapata JA, Stuart HJ, Cohn CK, O’Shanick GJ: MMPI-168 Codebook. Norwood, NJ, Ablex Publishing Corp, 1984Google Scholar
66. Roeltgen DP: Phonological error analysis, development and empirical evaluation. Brain Lang 1992; 43:190–229Crossref, Medline, Google Scholar
67. Katz WF, Curtiss S, Tallal P: Rapid automatized naming and gesture by normal and language-impaired children. Brain Lang 1992; 43:623–641Crossref, Medline, Google Scholar
68. Benton AL, Hamsher K: Multilingual Aphasia Examination. Iowa City, Iowa, AJA Associates, 1989Google Scholar
69. Wechsler D: Wechsler Adult Intelligence Scale—Revised. San Antonio, Tex, Psychological Corp, 1981Google Scholar
70. Kaplan E, Goodglass H, Weintraub S: The Boston Naming Test, 2nd ed. Philadelphia, Lea & Febiger, 1983Google Scholar
71. Caplan D, Hildebrandt N: Modified Battery for the Assessment of Syntactic Comprehension. Boston, Massachusetts General Hospital, Department of Neurology, Neuropsychology Laboratory, 1992Google Scholar
72. Cnaan A, Laird NM, Slasor P: Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med 1997; 16:2349–2380Crossref, Medline, Google Scholar
73. Haas GL, Sweeney JA, Hien DA, Goldman D, Deck M: Gender differences in schizophrenia (abstract). Schizophr Res 1991; 4:277Crossref, Google Scholar
74. Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, Bilker WB, Gur RC: Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry 2000; 57:761–768Crossref, Medline, Google Scholar
75. Hoff AL, Riordan H, O’Donnell D, Stritzke P, Neale C, Bovvio A, Annand AK, DeLisi LE: Anomalous lateral sulcus asymmetry and cognitive function in first-episode schizophrenia. Schizophr Bull 1992; 18:257–272Crossref, Medline, Google Scholar
76. Goldstein JM, Jerram M, Poldrack RA, Anagnoson R, Breiter HC, Makris N, Goodman JM, Tsuang MT, Seidman LJ: Sex differences in prefrontal cortical brain activity during fMRI of auditory verbal working memory. Neuropsychology 2005; 19:509–519Crossref, Medline, Google Scholar
77. Buckner RL, Raichle ME, Petersen SE: Dissociation of human prefrontal cortical areas across different speech production tasks and gender groups. J Neurophysiol 1995; 74:2163–2173Crossref, Medline, Google Scholar
78. Sommer IE, Aleman A, Bouma A, Kahn RS: Do women really have more bilateral language representation than men? a meta-analysis of functional imaging studies. Brain 2004; 127(part 8):1845-1852Google Scholar
79. Sommer IE, Ramsey NF, Kahn RS: Language lateralization in schizophrenia, an fMRI study. Schizophr Res 2001; 52:57–67Crossref, Medline, Google Scholar
80. Weiss EM, Hofer A, Golaszewski S, Siedentopf C, Brinkhoff C, Kremser C, Felber S, Fleischhacker WW: Brain activation patterns during a verbal fluency test: a functional MRI study in healthy volunteers and patients with schizophrenia. Schizophr Res 2004; 70:287–291Crossref, Medline, Google Scholar
81. Sommer IE, Ramsey NF, Mandl RC, Kahn RS: Language lateralization in female patients with schizophrenia: an fMRI study. Schizophr Res 2003; 60:183–190Crossref, Medline, Google Scholar
82. Kansaku K, Kitazawa S: Imaging studies on sex differences in the lateralization of language. Neurosci Res 2001; 41:333–337Crossref, Medline, Google Scholar



