Evaluation of Outcomes With Citalopram for Depression Using Measurement-Based Care in STAR*D: Implications for Clinical Practice
Abstract
OBJECTIVE: Selective serotonin reuptake inhibitors (SSRIs) are widely used to treat depression, but the rates, timing, and baseline predictors of remission in “real world” patients are not established. The authors’ primary objectives in this study were to evaluate the effectiveness of citalopram, an SSRI, using measurement-based care in actual practice, and to identify predictors of symptom remission in outpatients with major depressive disorder. METHOD: This clinical study included outpatients with major depressive disorder who were treated in 23 psychiatric and 18 primary care “real world” settings. The patients received flexible doses of citalopram prescribed by clinicians for up to 14 weeks. The clinicians were assisted by a clinical research coordinator in the application of measurement-based care, which included the routine measurement of symptoms and side effects at each treatment visit and the use of a treatment manual that described when and how to modify medication doses based on these measures. Remission was defined as an exit score of ≤7 on the 17-item Hamilton Depression Rating Scale (HAM-D) (primary outcome) or a score of ≤5 on the 16-item Quick Inventory of Depressive Symptomatology, Self-Report (QIDS-SR) (secondary outcome). Response was defined as a reduction of ≥50% in baseline QIDS-SR score. RESULTS: Nearly 80% of the 2,876 outpatients in the analyzed sample had chronic or recurrent major depression; most also had a number of comorbid general medical and psychiatric conditions. The mean exit citalopram dose was 41.8 mg/day. Remission rates were 28% (HAM-D) and 33% (QIDS-SR). The response rate was 47% (QIDS-SR). Patients in primary and psychiatric care settings did not differ in remission or response rates. A substantial portion of participants who achieved either response or remission at study exit did so at or after 8 weeks of treatment. Participants who were Caucasian, female, employed, or had higher levels of education or income had higher HAM-D remission rates; longer index episodes, more concurrent psychiatric disorders (especially anxiety disorders or drug abuse), more general medical disorders, and lower baseline function and quality of life were associated with lower HAM-D remission rates. CONCLUSIONS: The response and remission rates in this highly generalizable sample with substantial axis I and axis III comorbidity closely resemble those seen in 8-week efficacy trials. The systematic use of easily implemented measurement-based care procedures may have assisted in achieving these results.
Remission, the virtual absence of symptoms, is the aim of depression treatment because it is associated with better function and a better prognosis than is response without remission. Response is typically defined as a clinically meaningful reduction in symptoms (e.g., a reduction of at least 50% in baseline symptom levels). However, response that falls short of remission is suboptimal because it is associated with continued disabling symptoms, negative effects on other axis I and axis III disorders, higher rates of relapse and recurrence, poorer work productivity, more impaired psychosocial functioning, higher levels of health care use, and potentially higher risk for suicide. Remission, on the other hand, is associated with return of normal psychosocial function, higher rates of sustained remission, lower rates of relapse, lower risk of suicide and alcohol/drug abuse, and lack of disabling symptoms (1–3).
Few efficacy studies, even in research settings, have employed remission as an outcome (4–7). Remission rates from research-based, 8-week, randomized, placebo-controlled efficacy trials with depressed, symptomatic volunteers range from 25% to 40% (4), and 12-week efficacy trials with subjects suffering from chronic depression reveal even more modest remission rates of 22%–30% (8, 9).
Results from these efficacy trials lack ecological validity and generalizability to clinical practice (10, 11). Typically, they enroll symptomatic volunteers (often recruited through advertising) with uncomplicated (minimal comorbid general medical or psychiatric conditions), nonchronic, non-substance-abusing, nonsuicidal depression and treat in research clinics as opposed to enrolling patients already seeking health care in typical clinical treatment settings. Unfortunately, no large-scale antidepressant medication trials have evaluated safety, efficacy, and tolerability in “real world” primary or psychiatric care settings with remission as the predefined primary endpoint.
Evidence from practice settings (12) also demonstrates that antidepressant medication treatment is often inadequate in dose and/or duration and that there are unacceptably high dropout rates—all of which likely contribute to lower remission rates. In the available effectiveness trials conducted in real clinical practice settings, even the addition of depression care specialists leads to modest remission rates (15% to 35%) (10, 13, 14).
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study was designed to assess effectiveness of treatments in generalizable samples and ensure the delivery of adequate treatments. The study aimed to define the symptomatic outcomes for outpatients with nonpsychotic major depressive disorder treated initially with citalopram, a prototype of selective serotonin reuptake inhibitors (SSRIs). The primary outcome was remission. Adequate doses of citalopram had to be given for a sufficient time period to ensure that an adequate treatment trial was conducted to assess efficacy in representative practice settings and to ensure that those patients who progressed to the next treatment step in STAR*D were truly treatment resistant. To that end, a systematic but easily implemented approach to treatment, measurement-based care, was developed. Measurement-based care includes the routine measurement of symptoms and side effects at each treatment visit and the use of a treatment manual describing when and how to modify medication doses based on these measures. The manual allows for flexible dosing and was designed to maximize adequate dosing and duration of treatment.
Finally, since most depressed patients do not achieve remission with any initial treatment, baseline features (moderators) that identify who will achieve remission (15, 16) are clinically important. With a rare exception (17), no adequately powered previous studies have searched for baseline features predicting which patients will achieve remission as opposed to those who will respond to treatment. Response moderator studies with small samples have yielded inconsistent correlates of response (18), except for pretreatment depressive symptom severity, which has been associated consistently with lower response rates (19–35). Therefore, STAR*D also aimed to evaluate moderators of symptom remission.
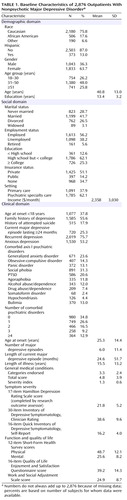
This study defined remission as the a priori primary endpoint and divided baseline moderators into three domains: 1) demographic features (e.g., age, race, ethnicity, and gender), 2) social features (e.g., education, employment status, income, insurance, and marital status), and 3) clinical features (e.g., age at onset of major depressive disorder, length of the current major depressive episode, number of major depressive episodes, length of illness, course of illness [single or recurrent], major depressive disorder subtype [anxious, melancholic, and atypical features], family history of depression, concurrent general medical and axis I psychiatric disorders, symptom severity, and functional status at baseline).
This report addresses the following questions about treatment with citalopram, a representative of the SSRI class of medications:
| 1. | What are the remission and response rates in representative outpatients with nonpsychotic major depressive disorder in primary and psychiatric care settings? | ||||
| 2. | Which citalopram doses, treatment durations, and adverse events characterize patients who do or do not achieve remission? | ||||
| 3. | What pretreatment features in demographic, social, and clinical domains are associated with remission? | ||||
Method
Study Overview and Organization
The rationale, methods, and design of the STAR*D study have been detailed elsewhere (7, 36). Investigators at each of 14 regional centers across the United States oversaw protocol implementation at two to four clinical sites providing primary (N=18) or psychiatric (N=23) care to patients in both the public and private sectors. Clinical research coordinators at each clinical site assisted participants and clinicians in protocol implementation and collection of clinical measures. A central pool of research outcome assessors conducted telephone interviews to obtain primary outcomes.
Participants
All risks, benefits, and adverse events associated with STAR*D participation were explained to subjects, who provided written informed consent before entering the study. The University of Texas Southwestern Medical Center at Dallas and the institutional review boards at each clinical site and regional center and the Data Coordinating Center and the Data Safety and Monitoring Board of the National Institute of Mental Health (NIMH) approved and monitored the protocol.
To maximize generalizability of findings, only patients seeking medical care in routine medical or psychiatric outpatient treatment (as opposed to those recruited through advertisements) were eligible for the study. Minimal exclusion criteria and broad inclusion criteria that allowed a majority of axis I and axis II disorders were used. Outpatients who were 18–75 years of age and had a nonpsychotic major depressive disorder determined by a baseline 17-item Hamilton Depression Rating Scale (HAM-D) (37, 38) score ≥14 were eligible if their clinicians determined that outpatient treatment with an antidepressant medication was both safe and indicated. The initial HAM-D at study entry was administered and scored by the clinical research coordinators. Patients who were pregnant or breast-feeding and those with a primary diagnosis of bipolar, psychotic, obsessive-compulsive, or eating disorders were excluded from the study, as were those with general medical conditions contraindicating the use of protocol medications in the first two treatment steps, substance dependence (only if it required inpatient detoxification), or a clear history of nonresponse or intolerance (in the current major depressive episode) to any protocol antidepressant in the first two treatment steps (7).
Diagnostic and Outcome Measures
The diagnosis of nonpsychotic major depressive disorder, established by treating clinicians, was confirmed by a checklist based on DSM-IV criteria. Previous personal and family histories as well as clinical and demographic information were based on participant self-report. The Psychiatric Diagnostic Screening Questionnaire (39–41) was completed at baseline to estimate the presence of 11 potential concurrent axis I (psychiatric) disorders. Responses to items on the baseline 30-item Inventory of Depressive Symptomatology or HAM-D (37, 38) obtained by research outcome assessors were used to estimate the presence of atypical (42), anxious (43), and melancholic (44) symptom features.
Clinical research coordinators administered an initial HAM-D and the 16-item Quick Inventory of Depressive Symptomatology (QIDS), QIDS Clinician Rating (QIDS-C), and QIDS Self-Report (QIDS-SR) (45–47) to assess depressive symptom severity. The clinical research coordinator also completed the 14-item Cumulative Illness Rating Scale (48, 49) to gauge the severity/morbidity of general medical conditions relevant to different organ systems. Each of the 14 illness categories was scored 0 (no problem) to 4 (extremely severe/immediate treatment required/end organ failure/severe impairment in function). The Cumulative Illness Rating Scale was scored as number of general medical condition categories endorsed (0–13, excluding the psychiatric illness category), severity index (0 to 4) (the average severity of the categories endorsed), and total severity (number of categories times severity).
The primary research outcome was measured by HAM-D score collected by research outcome assessors with telephone-based structured interviews in English or Spanish. Research outcome assessors were not located at any clinical site. The secondary outcomes were based on the QIDS-SR collected at baseline and at each treatment visit.
An automated, telephonic, interactive voice response system (7, 50–52) was used to collect ratings on the 12-item Short-Form Health Survey (53) (perceived physical functioning and mental health functioning), the 16-item Quality of Life Enjoyment and Satisfaction Questionnaire (54), the Work and Social Adjustment Scale (55), and the 5-item Work Productivity and Activity Impairment (56).
Intervention and Measurement-Based Care
Citalopram was selected as a representative SSRI given the absence of discontinuation symptoms, demonstrated safety in elderly and medically fragile patients, once-a-day dosing, few dose adjustment steps, and favorable drug-drug interaction profile (7, 36). The aim of treatment was to achieve symptom remission (defined as QIDS-C score ≤5 collected at each treatment visit for the purposes of clinical decision making). The protocol (7, 36) required a fully adequate dose of citalopram for a sufficient time to ensure that the likelihood of achieving remission was maximized and that those who did not reach remission were truly resistant to the medication.
The treatment protocol was designed to provide an optimal dose of citalopram based on dosing recommendations in a treatment manual (www.star-d.org) that also allowed individualized starting doses and dose adjustments to minimize side effects, maximize safety, and optimize the chances of therapeutic benefit for each patient. Medication management was assisted by ratings of symptoms (QIDS-C completed by the clinical research coordinator) and side effects (ratings of frequency, intensity, and burden) (7) obtained at each treatment visit. Citalopram was started at 20 mg/day and then raised to 40 mg/day by week 4 and to 60 mg/day (final dose) by day 42 (week 6). Dose adjustments were based on how long a subject had received a particular dose, symptom changes, and side effect burden. However, appropriate flexibility was allowed, including initiation of citalopram at <20 mg/day or a slower dose escalation to the optimal target dose of 60 mg/day, so that patients with concomitant general medical disorders, substance abuse/dependence, or other psychiatric disorders could be included safely in the sample.
The protocol recommended treatment visits at 2, 4, 6, 9, and 12 weeks (with an optional week-14 visit if needed). After an optimal trial (based on dose and duration), remitters and responders could enter the 12-month naturalistic follow-up, but all responders who did not achieve remission were encouraged to enter the subsequent randomized trial. Patients could discontinue citalopram before 12 weeks if 1) intolerable side effects required a medication change, 2) an optimal dose increase was not possible because of side effects or participant choice, or 3) significant symptoms (QIDS-C score ≥9) were present after 9 weeks at maximally tolerated doses. Patients could opt to move to the next treatment level if they had intolerable side effects or if the QIDS-C score was >5 after an adequate trial in terms of dose and duration.
A treatment manual (including the treatment protocol and procedures), initial didactic instruction, ongoing support and guidance by the clinical research coordinator, the use of structured evaluation of symptoms and side effects at each visit, and a centralized treatment monitoring and feedback system, together, represented an intensive effort to provide consistent, high-quality care (www.star-d.org) (52). To enhance the quality and consistency of care, physicians used the clinical decision support system that relied on the measurement of symptoms (QIDS-C and QIDS-SR), side effects (ratings of frequency, intensity, and burden), medication adherence (self-report), and clinical judgment based on patient progress. A web-based treatment monitoring system provided feedback to clinical research coordinators regarding the fidelity to the treatment recommendations for each patient. The clinical research coordinators could then help guide physicians in vigorously dosing when inadequate symptom reduction had occurred despite acceptable side effects (7).
Safety Assessments
Side effects were evaluated with the ratings of frequency, intensity, and burden completed by patients at each treatment visit (7). Three 7-point subscales measure the frequency, intensity, and global burden of side effects.
Serious adverse events were monitored with a multitiered approach involving the clinical research coordinators, study clinicians, the interactive voice response system, the clinical manager, safety officers, regional center directors (57), and the NIMH Data Safety and Monitoring Board.
Concomitant Medications
Concomitant treatments for current general medical conditions (as part of ongoing clinical care), for associated symptoms of depression (e.g., sleep, anxiety, and agitation), and for citalopram side effects (e.g., sexual dysfunction) were permitted on the basis of clinical judgment. Stimulants, anticonvulsants, antipsychotics, alprazolam, nonprotocol antidepressants (except trazodone ≤200 mg at bedtime for insomnia), and depression-targeted psychotherapies were proscribed.
Statistical Analysis
Summary statistics of the demographic, social, and clinical characteristics are presented for the analyzable sample of 2,876 patients. Summary statistics of treatment characteristics (e.g., maximum dose achieved, number of treatment visits), serious adverse events, and side effects are presented for the entire sample and by remission status. Logistic regression models assessed the association of the demographic, social, and clinical characteristics with remission, independent of the effect of regional center and baseline depression severity. As a subsequent analysis designed to assess the unique and independent contribution of these variables to remission rates, a stepwise logistic regression model was developed with both the HAM-D and the QIDS-SR. This model identified baseline features associated with remission independent of baseline depression severity and regional center, both within the three domains (demographic, social, and clinical) and across all three domains.
Remission was defined as an exit HAM-D score ≤7 (or last observed QIDS-SR score ≤5). A reduction of ≥50% in baseline QIDS-SR at the last assessment was defined as response. Intolerance was defined a priori as either leaving treatment before 4 weeks or leaving at or after 4 weeks with intolerance as the identified reason. As defined by the original proposal, patients were designated as not achieving remission when their exit HAM-D score was missing. Sensitivity analyses were conducted to determine whether this method of addressing missing data affected study results. Two additional methods also addressed missing data in the analysis of remission based on HAM-D scores: 1) a multiple imputation method and 2) an imputed value generated from an item response theory analysis of the relationship between the HAM-D and the QIDS-C. Statistical significance was defined as a two-sided p value less than 0.05. No adjustments were made for multiple comparisons, so results must be interpreted accordingly.
Results
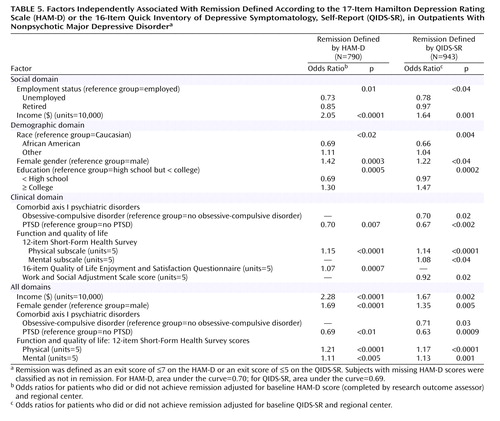
Figure 1 shows the disposition of patients during the course of the study.
Demographic and Clinical Characteristics
Table 1 summarizes the baseline features of the evaluable sample (N=2,876). The patients included in the evaluable sample did not differ from those excluded on any of the characteristics in Table 1 (data not shown). About 62% of the participants were from psychiatric care settings. Minority representation was 24%. Depressive symptoms were moderate to severe (HAM-D >21). More than 75% of the patients met DSM-IV criteria for recurrent or chronic depression. The mean length of illness was 15.5 years (time from onset of first major depressive episode to study entry). At study entry, subjects had an average of 3.3 general medical conditions.
Treatment Features
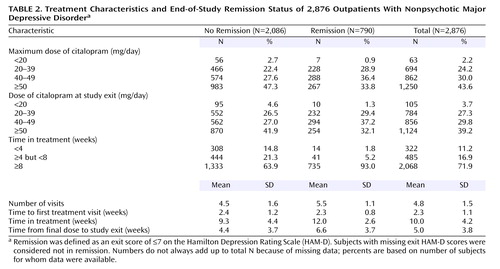
The study protocol recommended five postbaseline visits with an optional sixth visit (for those with meaningful improvement short of remission). Overall, participants averaged 4.8 visits (SD=1.5) (Table 2). Those who met HAM-D remission criteria had 5.5 visits (SD=1.1), and those who did not averaged 4.5 visits (SD=1.6). The time from baseline to the next treatment visit (for both remitters and nonremitters) was slightly over 2 weeks, which was within the recommended visit schedule.
Citalopram treatment averaged 10 weeks (SD=4.2, median=11.6) or 70.2 days (SD=29.2, median=81). Patients who achieved HAM-D remission remained in treatment for a mean of 12 weeks (SD=2.6) (mean=83.8 days, SD=18.1). Almost all (93%) of these patients completed at least 8 weeks, as opposed to only 64% of the patients who did not achieve remission (Table 2).
The mean exit dose of citalopram (41.8 mg/day, SD=16.8) was comparable for patients who did or did not achieve remission. Doses in primary care settings (40.6 mg/day, SD=16.6) and psychiatric care settings (42.5 mg/day, SD=16.8) were comparable.
Symptomatic Outcomes
The overall remission rate was 27.5% (N=790) with the HAM-D definition (primary outcome) and 32.9% (N=943) with the QIDS-SR definition. Remission rates were comparable in primary and psychiatric care for the HAM-D (26.6% versus 28.0%) and the QIDS-SR (32.5% versus 33.1%). The overall QIDS-SR response rate was 47% (N=1,343) (46% primary care, 48% psychiatric care). Figure 2 shows the distribution of the exit QIDS-SR scores. A QIDS-SR score of 10 approximates an HAM-D score of 13 (45).
Figure 3 shows the distribution of the time to first remission and response for those who ultimately did achieve remission and response in this study based on QIDS-SR scores. For those who achieved QIDS-SR remission, the mean time to remission was 6.7 weeks (SD=3.8) and was comparable in primary care (approximately 6 weeks) and psychiatric care (approximately 7 weeks). For those who achieved a QIDS-SR response, the mean time to response was approximately 5.7 weeks (SD=3.5) and was comparable in primary care (mean=5.7 weeks, SD=3.7) and psychiatric specialty care (mean=5.6 weeks, SD=3.5).
For those who achieved remission according to QIDS-SR scores, the mean time in treatment was approximately 12 weeks (SD=3).
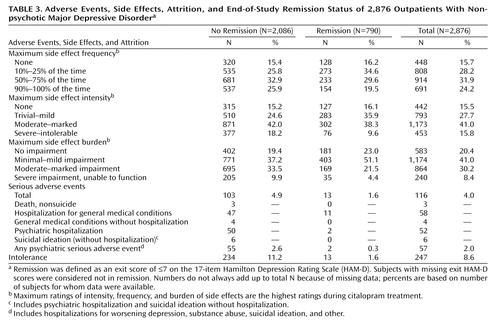
Intolerance and Adverse Events
Only 2% of the patients who achieved HAM-D remission were considered to have discontinued citalopram because of intolerance, compared with 11% of those who did not achieve HAM-D remission (Table 3). Those who achieved HAM-D remission had lower rates of side effect frequency, intensity, and burden at exit and lower rates of serious adverse effects than those who did not achieve HAM-D remission. Overall, 116 participants experienced at least one serious adverse effect; most of these patients (88.8% [N=103]) did not achieve HAM-D remission. There were no suicides in the 2,876 participants in this acute-phase citalopram study.
Pretreatment Correlates of Remission
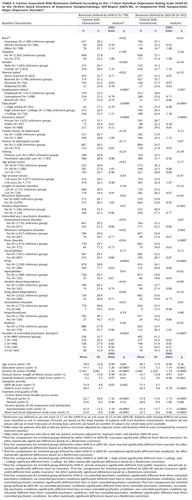
Several pretreatment demographic, social, and clinical features were associated with remission based on either the HAM-D or QIDS-SR following adjustments for baseline symptom severity and regional center (Table 4). Findings were almost identical for the HAM-D and the QIDS-SR except that anxious depression and concurrent generalized anxiety disorder were also associated with lower QIDS-SR remission rates.
Table 5 presents pretreatment features that were nonoverlapping and independently associated with remission after baseline depressive symptom severity and regional center for each domain separately and across all domains were controlled for. Lower remission rates were associated with being unemployed; having a lower income; being non-Caucasian, male, and less educated; and having poorer function and lower quality of life at baseline. Remarkably consistent findings were obtained with the HAM-D and the QIDS-SR.
Discussion
Results of this study should be generalizable to routine clinical practice because this is the largest ecologically valid “real world” study of outpatients with nonpsychotic major depressive disorder treated in psychiatric and primary care settings with diligently followed guidelines. Participants in the study were patients seeking treatment in “real world” clinical practices who had high rates of chronic or recurrent major depressive disorder and concurrent axis I and axis III (general medical conditions) disorders. Since there were very broad inclusion criteria and few exclusion criteria, this study included patients who would have been excluded from most efficacy trials (58–61).
The remission rates (28% for HAM-D; 33% for QIDS-SR) were robust and similar to rates found in uncomplicated, nonchronic symptomatic volunteers enrolled in placebo-controlled, 8-week, randomized, controlled trials with SSRIs (4). These remission rates were better than those found in efficacy studies among patients with chronic depression (22%) (9), possibly because of a number of factors discussed below, including the use of measurement-based care and the clinical research coordinators.
Higher remission rates were found with the QIDS-SR than with the HAM-D because our primary analyses classified patients with missing exit HAM-D as nonremitters a priori. Of the 690 patients with missing exit HAM-D scores, 152 (22.1%) achieved QIDS-SR remission at the last treatment visit.
As described earlier, a sensitivity analysis was conducted to evaluate the methods used to address the missing HAM-D data. Both the multiple imputation approach and the use of values imputed from the observed exit QIDS-C score based on item response theory revealed remarkably similar findings, indicating that the analyses were not affected by the missing data methodology.
Of participants who responded, 56.0% did so only at or after 8 weeks of treatment. Not surprisingly, remission followed response in most cases. Of those who achieved QIDS-SR remission, 40.3% did so only at or after 8 weeks of citalopram.
Results also highlight the feasibility, safety, tolerability, and effectiveness of delivering high-quality care with easy-to-use clinical methods employed at each treatment visit to ensure adequate treatment delivery (measurement-based care approach). The approach may have contributed to the better-than-expected remission rates in this group of patients as well, although a firm conclusion cannot be made without a control group. On the other hand, several controlled studies (10, 14, 62) suggest a clear benefit for a disease management approach in the comprehensive treatment of depression. These studies have emphasized more frequent patient contact as well as more robust psychosocial and educational support to enhance adherence, improve patients’ ability to monitor their own symptoms, and help patients understand the nature of and treatment needs for their depression.
Unlike previous studies (10, 14, 62), this study used pharmacotherapy augmented with diligent measurement-based procedures employing easy-to-use ratings of symptoms and side effect frequency, intensity, and burden, as well as triage points with dosing recommendations that allowed necessary flexibility. This measurement-based care approach represents a paradigm shift to the use of easily employed research tools in clinical practice. Tools used in research settings (e.g., HAM-D or other measures of symptoms, function, or side effects) are not routinely used in practice, which may contribute to the high rates of inadequate treatment with antidepressant medications in routine care (12). Our results also suggest that the use of depressive symptom and side effect ratings (www.ids-qids.org) to guide treatment is feasible in “real world” practices as well as effectiveness trials and can be used to monitor patient progress, to adjust the treatment, and to make clinical decisions. In this study, adequate citalopram doses and treatment duration were achieved with a structured yet flexible dosing schedule.
Several baseline features were associated with higher remission rates, including lower baseline severity; being Caucasian, female, better educated, and more highly paid; and having private insurance, fewer concurrent general medical and psychiatric disorders, better pretreatment physical and mental function (12-item Short-Form Health Survey physical and mental subscales), greater life satisfaction, and a shorter current episode. Taken together, greater illness severity and psychiatric and general medical comorbidity as well as less social support are likely associated with lower remission rates for citalopram. These findings are consistent with some of the previous studies that reported lower response rates to antidepressants in subjects with greater baseline symptom severity and longer current episodes (19, 25, 63–67).
Our sample size was large enough to identify a number of clinically relevant features in developing a model to predict remission for major depressive disorder even after controlling for both severity and treatment settings. These results do not address whether similar or different baseline features would be negatively associated with remission for other antidepressant medications or whether results would differ for psychotherapy or combination(s) of antidepressant treatments.
In our sample, being married or living with someone appeared to have a positive effect on the overall remission rates; married or cohabiting patients met criteria for treatment response with greater frequency than single participants. Although Hagerty and Williams (68) found that patients living alone were more likely to drop out of treatment, our findings indicate that participants who were unmarried or living alone did not drop out early and yet had lower remission rates. Not all studies have found social support to be a significant predictor of treatment outcome (69, 70), but most have suggested social support and, even more specifically, marital status as positive predictors of response.
Study limitations include open treatment design, the use of a single antidepressant agent (citalopram), and the lack of placebo control. Nonspecific treatment effects undoubtedly accounted for some unknown proportion of the acute response or remission rates (71). Additional studies with other antidepressant medications are needed to determine whether the current findings are generalizable to other medications.
These results highlight the need for longer treatment duration and more vigorous medication dosing than is current practice in order to achieve optimal remission rates. Informed triage or critical decision points (i.e., the discontinuation of patients who experience minimal benefit after 6–9 weeks of treatment) allow for extended dosing for those who are benefiting, while curtailing extended treatment for those who experience minimal benefit after a substantial treatment period. The measurement-based care methods used in this study were easily implemented in actual practice. Controlled trials of this approach in practice are recommended.
 |
 |
 |
 |
 |
Received Aug. 1, 2005; revision received Sept. 17, 2005; accepted Sept. 27, 2005. From the Department of Psychiatry, University of Texas Southwestern Medical Center; Epidemiology Data Center, Graduate School of Public Health, University of Pittsburgh, Pittsburgh; Depression Clinical and Research Program, Massachusetts General Hospital, Boston; NIMH, Bethesda, Md.; Department of Psychiatry, University of Mississippi, Jackson; Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh; The Sam and Rose Stein Institute for Research on Aging, University of California, San Diego, School of Medicine; New York State Psychiatric Institute and the Department of Psychiatry, College of Physicians and Surgeons of Columbia University, New York. Address correspondence and reprint requests to Dr. Trivedi, Mood Disorders Program and Clinic, Department of Psychiatry, University of Texas Southwestern Medical Center, Exchange Park Express, American General Tower, 6363 Forest Park Rd., Suite 1300, Dallas, TX 75390-9119; [email protected] (e-mail). The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study is supported by federal funds from NIMH under contract N01 MH-90003 to the University of Texas Southwestern Medical Center at Dallas (A.J. Rush, principal investigator). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. The authors thank Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, King Pharmaceuticals, Organon, Pfizer, and Wyeth for providing medications at no cost for the STAR*D study. Additional information on this study accompanies the online version of the article (ajp.psychiatryonline.org).
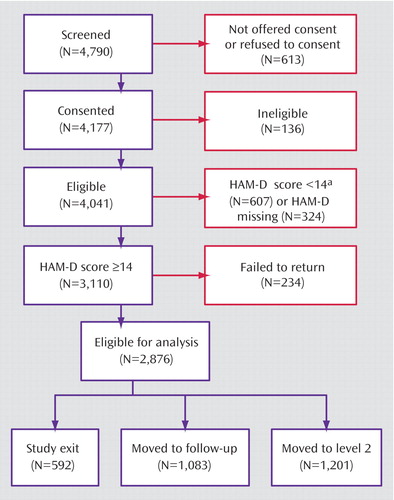
Figure 1. Participant Flow (CONSORT Chart) for the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Studya
aHAM-D=17-item Hamilton Depression Rating Scale.
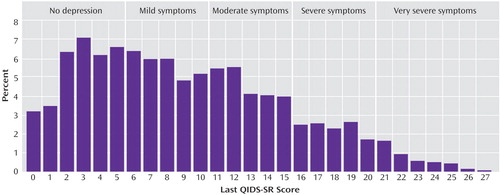
Figure 2. Total Exit Scores on the 16-Item Quick Inventory of Depressive Symptomatology, Self-Report (QIDS-SR), of 2,876 Outpatients With Nonpsychotic Major Depressive Disorder
Figure 3. Percent of 2,876 Outpatients With Nonpsychotic Major Depressive Disorder Who Achieved Response or Remission Defined by 16-Item Quick Inventory of Depressive Symptomatology, Self-Report (QIDS-SR), Scores by Week of Treatmenta
aResponse was defined as improvement of ≥50% in QIDS-SR score from baseline. Remission was defined as a QIDS-SR score of ≤5 at endpoint.
1. Judd LL: Major depressive disorder: longitudinal symptomatic structure, relapse and recovery. Acta Psychiatr Scand 2001; 104:81–83Crossref, Medline, Google Scholar
2. Nierenberg AA, Keefe BR, Leslie VC, Alpert JE, Pava JA, Worthington JJ, Fava M: Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry 1999; 60:221–225Crossref, Medline, Google Scholar
3. Paykel ES: Remission and residual symptomatology in major depression. Psychopathology 1998; 31:5–14Crossref, Medline, Google Scholar
4. Depression Guideline Panel: Clinical Practice Guideline 5: Depression in Primary Care, vol 2: Treatment of Major Depression. Rockville, Md, US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research, 1993Google Scholar
5. Koran LM, Gelenberg AJ, Kornstein SG, Howland RH, Friedman RA, DeBattista C, Klein D, Kocsis JH, Schatzberg AF, Thase ME, Rush AJ, Hirschfeld RM, LaVange LM, Keller MB: Sertraline versus imipramine to prevent relapse in chronic depression. J Affect Disord 2001; 65:27–36Crossref, Medline, Google Scholar
6. Paykel ES, Scott J, Teasdale JD, Johnson AL, Garland A, Moore R, Jenaway A, Cornwall PL, Hayhurst H, Abbott R, Pope M: Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Arch Gen Psychiatry 1999; 56:829–835Crossref, Medline, Google Scholar
7. Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, Thase ME, Nierenberg AA, Quitkin FM, Kashner TM, Kupfer DJ, Rosenbaum JF, Alpert J, Stewart JW, McGrath PJ, Biggs MM, Shores-Wilson K, Lebowitz BD, Ritz L, Niederehe G: Sequenced Treatment Alternatives to Relieve Depression (STAR*D): rationale and design. Control Clin Trials 2004; 25:119–142Crossref, Medline, Google Scholar
8. Keller MB, Gelenberg AJ, Hirschfeld RM, Rush AJ, Thase ME, Kocsis JH, Markowitz JC, Fawcett JA, Koran LM, Klein DN, Russell JM, Kornstein SG, McCullough JP, Davis SM, Harrison WM: The treatment of chronic depression, part 2: a double-blind, randomized trial of sertraline and imipramine. J Clin Psychiatry 1998; 59:598–607Crossref, Medline, Google Scholar
9. Keller MB, McCullough JP, Klein DN, Arnow B, Dunner DL, Gelenberg AJ, Markowitz JC, Nemeroff CB, Russell JM, Thase ME, Trivedi MH, Zajecka J: A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med 2000; 342:1462–1470Crossref, Medline, Google Scholar
10. Unützer J, Katon W, Callahan CM, Williams JW Jr, Hunkeler E, Harpole L, Hoffing M, Della Penna RD, Noel PH, Lin EH, Arean PA, Hegel MT, Tang L, Belin TR, Oishi S, Langston C: Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA 2002; 288:2836–2845Crossref, Medline, Google Scholar
11. Rush AJ, Prien RF: From scientific knowledge to the clinical practice of psychopharmacology: can the gap be bridged? Psychopharmacol Bull 1995; 31:7–20Medline, Google Scholar
12. Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS: The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003; 289:3095–3105Crossref, Medline, Google Scholar
13. Katon WJ, Von Korff M, Lin EH, Simon G, Ludman E, Russo J, Ciechanowski P, Walker E, Bush T: The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry 2004; 61:1042–1049Crossref, Medline, Google Scholar
14. Trivedi MH, Rush AJ, Crismon ML, Kashner TM, Toprac MG, Carmody TJ, Key T, Biggs MM, Shores-Wilson K, Witte B, Suppes T, Miller AL, Altshuler KZ, Shon SP: Clinical results for patients with major depressive disorder in the Texas Medication Algorithm Project. Arch Gen Psychiatry 2004; 61:669–680Crossref, Medline, Google Scholar
15. Baron RM, Kenny DA: The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986; 51:1173–1182Crossref, Medline, Google Scholar
16. Kraemer HC, Wilson T, Fairburn CG, Agras WS: Mediators and moderators of treatment effects in randomized controlled trials. Arch Gen Psychiatry 2002; 59:877–883Crossref, Medline, Google Scholar
17. Bosworth HB, McQuoid DR, George LK, Steffens DC: Time-to-remission from geriatric depression: psychosocial and clinical factors. Am J Geriatr Psychiatry 2002; 10:551–559Crossref, Medline, Google Scholar
18. Nierenberg AA: Predictors of response to antidepressants general principles and clinical implications. Psychiatr Clin North Am 2003; 26:345–352, viiiCrossref, Medline, Google Scholar
19. Bielski RJ, Friedel RO: Prediction of tricyclic antidepressant response: a critical review. Arch Gen Psychiatry 1976; 33:1479–1489Crossref, Medline, Google Scholar
20. Croughan JL, Secunda SK, Katz MM, Robins E, Mendels J, Swann A, Harris-Larkin B: Sociodemographic and prior clinical course characteristics associated with treatment response in depressed patients. J Psychiatr Res 1988; 22:227–237Crossref, Medline, Google Scholar
21. Greenhouse JB, Kupfer DJ, Frank E, Jarrett DB, Rejman KA: Analysis of time to stabilization in the treatment of depression: biological and clinical correlates. J Affect Disord 1987; 13:259–266Crossref, Medline, Google Scholar
22. Hooley JM, Teasdale JD: Predictors of relapse in unipolar depressives: expressed emotion, marital distress, and perceived criticism. J Abnorm Psychol 1989; 98:229–235Crossref, Medline, Google Scholar
23. Joyce PR, Paykel ES: Predictors of drug response in depression. Arch Gen Psychiatry 1989; 46:89–99Crossref, Medline, Google Scholar
24. Katz MM, Koslow SH, Maas JW, Frazer A, Bowden CL, Casper R, Croughan J, Kocsis J, Redmond EJ: The timing, specificity and clinical prediction of tricyclic drug effects in depression. Psychol Med 1987; 17:297–309Crossref, Medline, Google Scholar
25. Keller MB, Lavori PW, Mueller TI, Endicott J, Coryell W, Hirschfeld RM, Shea T: Time to recovery, chronicity, and levels of psychopathology in major depression: a 5-year prospective follow-up of 431 subjects. Arch Gen Psychiatry 1992; 49:809–816Crossref, Medline, Google Scholar
26. Kocsis JH, Mason BJ, Frances AJ, Sweeney J, Mann JJ, Marin D: Prediction of response of chronic depression to imipramine. J Affect Disord 1989; 17:255–260Crossref, Medline, Google Scholar
27. Vallejo J, Gasto C, Catalan R, Bulbena A, Menchon JM: Predictors of antidepressant treatment outcome in melancholia: psychosocial, clinical and biological indicators. J Affect Disord 1991; 21:151–162Crossref, Medline, Google Scholar
28. Kocsis JH: New issues in the prediction of antidepressant response. Psychopharmacol Bull 1990; 26:49–53Medline, Google Scholar
29. Brugha TS, Bebbington PE, MacCarthy B, Sturt E, Wykes T, Potter J: Gender, social support and recovery from depressive disorders: a prospective clinical study. Psychol Med 1990; 20:147–156Crossref, Medline, Google Scholar
30. Goodwin FK: Predictors of antidepressant response. Bull Menninger Clin 1993; 57:146–160Medline, Google Scholar
31. Hoencamp E, Haffmans PM, Duivenvoorden H, Knegtering H, Dijken WA: Predictors of (non-) response in depressed outpatients treated with a three-phase sequential medication strategy. J Affect Disord 1994; 31:235–246Crossref, Medline, Google Scholar
32. Friedman RA, Parides M, Baff R, Moran M, Kocsis JH: Predictors of response to desipramine in dysthymia. J Clin Psychopharmacol 1995; 15:280–283Crossref, Medline, Google Scholar
33. Cohn CK, Robinson DS, Roberts DL, Schwiderski UE, O’Brien K, Ieni JR: Responders to antidepressant drug treatment: a study comparing nefazodone, imipramine, and placebo in patients with major depression. J Clin Psychiatry 1996; 57(suppl 2):15-18Google Scholar
34. Esposito K, Goodnick P: Predictors of response in depression. Psychiatr Clin North Am 2003; 26:353–365Crossref, Medline, Google Scholar
35. Aliapoulous J, Zisook S: Tricyclic antidepressant medications, in Predictors of Treatment Response in Mood Disorders. Edited by Goodnick PJ. Washington, DC, American Psychiatric Press, 1996, pp 1-36Google Scholar
36. Fava M, Rush AJ, Trivedi MH, Nierenberg AA, Thase ME, Sackeim HA, Quitkin FM, Wisniewski S, Lavori PW, Rosenbaum JF, Kupfer DJ: Background and rationale for the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. Psychiatr Clin North Am 2003; 26:457–494Crossref, Medline, Google Scholar
37. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
38. Hamilton M: Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6:278–296Crossref, Medline, Google Scholar
39. Zimmerman M, Mattia JI: A self-report scale to help make psychiatric diagnoses: the Psychiatric Diagnostic Screening Questionnaire. Arch Gen Psychiatry 2001; 58:787–794Crossref, Medline, Google Scholar
40. Zimmerman M, Mattia JI: The Psychiatric Diagnostic Screening Questionnaire: development, reliability and validity. Compr Psychiatry 2001; 42:175–189Crossref, Medline, Google Scholar
41. Rush AJ, Zimmerman M, Wisniewski SR, Fava M, Hollon SD, Warden D, Biggs MM, Shores-Wilson K, Shelton RC, Luther JF, Thomas B, Trivedi MH: Comorbid psychiatric disorders in depressed outpatients: demographic and clinical features. J Affect Disord 2005; 87:43–55Crossref, Medline, Google Scholar
42. Novick JS, Stewart JW, Wisniewski SR, Cook IA, Manev R, Nierenberg AA, Rosenbaum JF, Shores-Wilson K, Balasubramani GK, Biggs MM, Zisook S, Rush AJ (STAR*D investigators): Clinical and demographic features of atypical depression in outpatients with major depression: preliminary findings from STAR*D. J Clin Psychiatry 2005; 66:1002–1011Crossref, Medline, Google Scholar
43. Fava M, Alpert JE, Carmin CN, Wisniewski SR, Trivedi MH, Biggs MM, Shores-Wilson K, Morgan D, Schwartz T, Balasubramani GK, Rush AJ: Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D. Psychol Med 2004; 34:1299–1308Crossref, Medline, Google Scholar
44. Khan AY, Carrithers J, Preskorn SH, Wisniewski SR, Lear R, Rush AJ, Stegman D, Kelley C, Kreiner K, Nierenberg AA, Fava M: Clinical and demographic factors associated with DSM-IV melancholic depression. Ann Clin Psychiatry (in press)Google Scholar
45. Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB: The 16-item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C), and Self-Report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003; 54:573–583Crossref, Medline, Google Scholar
46. Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, Witte B, Kashner TM: The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med 2004; 34:73–82Crossref, Medline, Google Scholar
47. Rush AJ, Bernstein IH, Trivedi MH, Carmody TJ, Wisniewski S, Mundt JC, Shores-Wilson K, Biggs MM, Woo A, Nierenberg AA, Fava M: An evaluation of the Quick Inventory of Depressive Symptomatology and the Hamilton Rating Scale for Depression: a Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial report. Biol Psychiatry (in press)Google Scholar
48. Linn BS, Linn MW, Gurel L: Cumulative Illness Rating Scale. J Am Geriatr Soc 1968; 16:622–626Crossref, Medline, Google Scholar
49. Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF III: Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res 1992; 41:237–248Crossref, Medline, Google Scholar
50. Kobak KA, Greist JH, Jefferson JW, Mundt JC, Katzelnick DJ: Computerized assessment of depression and anxiety over the telephone using interactive voice response. MD Comput 1999; 16:63–68Google Scholar
51. Mundt JC: Interactive voice response systems in clinical research and treatment. Psychiatr Serv 1997; 48:611–612Link, Google Scholar
52. Trivedi MH, Rush AJ, Wisniewski SR, Warden D, McKinney W, Downing M, Berman SR, Farabaugh A, Luther J, Nierenberg AA, Lis J, Sackeim H: Factors associated with health-related quality of life among outpatients with major depressive disorder: a STAR*D report. J Clin Psychiatry (in press)Google Scholar
53. Ware J Jr, Kosinski M, Keller SD: A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34:220–233Crossref, Medline, Google Scholar
54. Endicott J, Nee J, Harrison W, Blumenthal R: Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull 1993; 29:321–326Medline, Google Scholar
55. Mundt JC, Marks IM, Shear MK, Greist JH: The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry 2002; 180:461–464Crossref, Medline, Google Scholar
56. Reilly MC, Zbrozek AS, Dukes EM: The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993; 4:353–365Crossref, Medline, Google Scholar
57. Nierenberg AA, Trivedi MH, Ritz L, Burroughs D, Greist J, Sackeim H, Kornstein S, Schwartz T, Stegman D, Fava M, Wisniewski SR: Suicide risk management for the Sequenced Treatment Alternatives to Relieve Depression study: applied NIMH guidelines. J Psychiatr Res 2004; 38:583–589Crossref, Medline, Google Scholar
58. Posternak MA, Zimmerman M, Keitner GI, Miller IW: A reevaluation of the exclusion criteria used in antidepressant efficacy trials. Am J Psychiatry 2002; 159:191–200Link, Google Scholar
59. Rapaport MH, Pollack M, Wolkow R, Mardekian J, Clary C: Is placebo response the same as drug response in panic disorder? Am J Psychiatry 2000; 157:1014–1016Link, Google Scholar
60. Zimmerman M, Chelminski I, Posternak MA: Exclusion criteria used in antidepressant efficacy trials: consistency across studies and representativeness of samples included. J Nerv Ment Dis 2004; 192:87–94Crossref, Medline, Google Scholar
61. Zimmerman M, Chelminski I, Posternak MA: Generalizability of antidepressant efficacy trials: differences between depressed psychiatric outpatients who would or would not qualify for an efficacy trial. Am J Psychiatry 2005; 162:1370–1372Link, Google Scholar
62. Hunkeler EM, Meresman JF, Hargreaves WA, Fireman B, Berman WH, Kirsch AJ, Groebe J, Hurt SW, Braden P, Getzell M, Feigenbaum PA, Peng T, Salzer M: Efficacy of nurse telehealth care and peer support in augmenting treatment of depression in primary care. Arch Fam Med 2000; 9:700–708Crossref, Medline, Google Scholar
63. Hirschfeld RM, Montgomery SA, Aguglia E, Amore M, Delgado PL, Gastpar M, Hawley C, Kasper S, Linden M, Massana J, Mendlewicz J, Moller HJ, Nemeroff CB, Saiz J, Such P, Torta R, Versiani M: Partial response and nonresponse to antidepressant therapy: current approaches and treatment options. J Clin Psychiatry 2002; 63:826–837Crossref, Medline, Google Scholar
64. Keller MB, Klerman GL, Lavori PW, Coryell W, Endicott J, Taylor J: Long-term outcome of episodes of major depression. clinical and public health significance. JAMA 1984; 252:788–792Crossref, Medline, Google Scholar
65. Rush AJ, Roffwarg HP, Giles DE, Schlesser MA, Fairchild C, Tarell J: Psychobiological predictors of antidepressant drug response. Pharmacopsychiatria 1983; 16:192–194Crossref, Medline, Google Scholar
66. Fava M, Uebelacker LA, Alpert JE, Nierenberg AA, Pava JA, Rosenbaum JF: Major depressive subtypes and treatment response. Biol Psychiatry 1997; 42:568–576Crossref, Medline, Google Scholar
67. Iosifescu DV, Nierenberg AA, Alpert JE, Smith M, Bitran S, Dording C, Fava M: The impact of medical comorbidity on acute treatment in major depressive disorder. Am J Psychiatry 2003; 160:2122–2127Link, Google Scholar
68. Hagerty BM, Williams RA: The effects of sense of belonging, social support, conflict, and loneliness on depression. Nurs Res 1999; 48:215–219Crossref, Medline, Google Scholar
69. George LK, Blazer DG, Hughes DC, Fowler N: Social support and the outcome of major depression. Br J Psychiatry 1989; 154:478–485Crossref, Medline, Google Scholar
70. Hirschfeld RM, Klerman GL, Andreasen NC, Clayton PJ, Keller MB: Psycho-social predictors of chronicity in depressed patients. Br J Psychiatry 1986; 148:648–654Crossref, Medline, Google Scholar
71. Khan A, Leventhal RM, Khan SR, Brown WA: Severity of depression and response to antidepressants and placebo: an analysis of the Food and Drug Administration database. J Clin Psychopharmacol 2002; 22:40–45Crossref, Medline, Google Scholar



