Prefrontal Broadband Noise, Working Memory, and Genetic Risk for Schizophrenia
Abstract
OBJECTIVE: It has been suggested that increased variability of prefrontal physiological responses may represent a fundamental mechanism underlying frontal lobe deficits in schizophrenia. Increased response variability (“noise”) is thought to result from impaired phase resetting of stimulus-induced dynamic changes of ongoing rhythmic oscillations (field potentials) generated in the apical dendrites of pyramidal neurons. In the present study, the authors explored whether this particular physiological abnormality predicts working memory performance and is related to the genetic risk for schizophrenia. METHOD: Prefrontal response variability of discrete frequency components was investigated across a broad frequency range (0.5–45.0 Hz) during processing of an oddball paradigm in patients with schizophrenia (N=66), their clinically unaffected siblings (N=115), and healthy comparison subjects (N=89). RESULTS: As hypothesized, prefrontal noise was negatively correlated with working memory performance across all subjects. In addition, it was observed that prefrontal noise possesses trait characteristics and is strongly associated with genetic risk for schizophrenia. CONCLUSIONS: Frontal lobe-related cognitive function depends on the ability to synchronize cortical pyramidal neurons, which is in part genetically controlled. Increased prefrontal “noise” is an intermediate phenotype related to genetic susceptibility for schizophrenia.
Deficits of frontal lobe-related information processing are common among schizophrenia patients and are thought to be phenotypic traits related to the genetic risk for schizophrenia (1–7). However, it is unclear what pathophysiological mechanisms account for the neuropsychological impairments. In particular, abnormal prefrontal physiological responses in patients with schizophrenia frequently vary from too little activity to too much, depending on factors such as task conditions and clinical state (8–14). This variable expression pattern suggests the existence of a more fundamental underlying deficit.
There are two lines of evidence that the underlying fundamental physiological trait may be related to increased background noise of prefrontal neuronal activity. The first line of evidence is largely circumstantial and comes from studies of the role of dopamine signaling in prefrontal information processing and evidence of abnormal cortical dopamine innervation in schizophrenia. Weinberger et al. (15) reported that a measure of cortical dopamine turnover (CSF concentrations of the dopamine metabolite homovanillic acid) directly predicted physiological activation of the prefrontal cortex during executive cognition in patients with schizophrenia. Akil et al. (16) described decreased dopaminergic prefrontal cortex innervation in the postmortem brain. Abi-Dargham et al. (17) found increased prefrontal cortex dopamine D1 receptor availability in schizophrenia patients, which strongly predicted working memory performance and was interpreted as a reflection of diminished dopamine innervation. In addition, several functional neuroimaging investigations that were conducted in conjunction with monoaminergic drug administration reported an increase in spatial “focusing” of the prefrontal hemodynamic response during frontal lobe-related tasks (18–23). This hemodynamic cortical response pattern was generally interpreted as an indication of enhanced D1-receptor-mediated signal-to-noise ratio of prefrontal neuronal activity.
These clinical observations are consistent with basic studies implicating dopamine/D1 signaling in modulating cortical signal-to-noise ratio. Single unit recordings of action potentials in the cortex of rats and nonhuman primates have shown that the administration of catecholaminergic drugs or stimulation of D1 receptors abolishes random firing of pyramidal neurons and increases spike rates in selective frequency bands, thereby enhancing the signal-to-noise ratio. These pharmacological effects on single neuron activity are accompanied by improved performance across different task conditions, including choice reaction type and delay type as well as oddball tasks (24–28). The signal-to-noise-ratio increasing effect appears to be mediated via D1 receptor stimulation, which is thought to enhance selective inputs to both pyramidal cells and inhibitory interneurons (29). D1 receptors are found on distal dendrites and spines of pyramidal cells adjacent to asymmetric and presumably glutamatergic synapses (30–33). In addition, tyrosine hydroxylase/dopamine immunopositive terminals in the prefrontal cortex form symmetric synapses on dendrites of GABA immunoreactive neurons in layer VI (34, 35). In vitro whole-cell patch-clamp recordings revealed that dopamine D1 receptor agonists cause large, GABAA-mediated evoked inhibitory postsynaptic currents in pyramidal neurons and also directly enhance postsynaptic N-methyl-d-aspartic acid currents on pyramidal layer V neurons, while a slight reduction of D1-mediated presynaptic glutamate release was observed (36, 37). Gao et al. (38) also described that dopamine reduces glutamate release in layer V pyramidal neurons by D1 activation at presynaptic sites. Recent computational models have incorporated these experimental findings and suggest that dopamine D1-receptor-mediated synaptic inhibition is critical for shaping the selectivity (i.e., signal-to-noise ratio) of neural activity in prefrontal circuits, in the sense that recurrent excitatory-inhibitory synaptic interactions are balanced toward inhibition (39–43). These various experimental and computational observations on the cortical actions of dopamine have supported the notion that diminished mesocortical dopamine signaling and the resulting reduced signal-to-noise ratio of prefrontal neurons might be involved in cognitive and behavioral deficits that are observed in schizophrenia (15, 29, 44, 45).
A second line of evidence suggesting increased noise of cortical neuronal assemblies during information processing in schizophrenia comes from scalp-recorded electrophysiological investigations of single-trial data in patients. These studies described both increased latency jittering of stimulus-evoked brain events (46–48) and a lack of stimulus-induced synchronization (i.e., phase-resetting) over a wide range of frequencies (49). These findings, which have emerged from two parallel approaches to event-related potential analysis, are compatible with each other. The first approach is based on the traditional notion of event-related potential generation, i.e., a fixed-latency and fixed-polarity neural response within circumscribed cortical areas that requires removal by averaging of background EEG and latency jittering as uninformative noise. Therefore, traditional event-related potential studies paid little attention to the increased latency jittering of cortically evoked responses in schizophrenia. The second, frequency-based approach to event-related potential analysis that was previously taken by us (49), however, took into account recent evidence that event-related potentials can arise—at least in part—from stimulus-locked, dynamic changes of the ongoing neural synchrony generating the scalp electromagnetic fields (50–59). These studies have demonstrated phase resetting of EEG oscillations for different poststimulus time windows using techniques such as frequency filtering and decomposition of the frequency spectrum by principle or independent component analysis. This phase resetting of field potentials is related to the latency of the averaged event-related potential (59), suggesting that event-related potential latency-jittering (“noise”) and lack of stimulus-evoked phase resetting of ongoing oscillations describe the same phenomenon. Of note, measurements taken from visual and auditory event-related potential experiments in normal subjects have demonstrated that event-related potential signal amplitude (power) and signal-to-noise ratio vary across conditions and sensory modalities, whereas noise power is highly stable within subjects across experimental conditions (60), possibly reflecting a task-independent, subject-specific physiological trait factor.
Thus, it is reasonable to hypothesize that prefrontal cortical information processing in schizophrenia is disturbed because of a lack of stimulus-induced synchronous action of prefrontal pyramidal neurons. Even though the physiological and molecular mechanisms are far from clear, it is conceivable that previous observations of reduced phase resetting in schizophrenia patients are not merely a measurement artifact. Nevertheless, validation of these findings is required, which was the main purpose of this study. Specifically, we addressed the following questions: 1) whether stimulus-induced prefrontal field potential variance (i.e., noise estimate) would predict frontal lobe-related cognitive performance, which is commonly deficient in schizophrenia patients; 2) whether prefrontal noise has trait characteristics; and 3) whether increased prefrontal noise would be associated with genetic risk for schizophrenia.
Method
Subjects
Subjects were recruited from national and local sources as part of the “Sibling Study” of the NIMH Clinical Brain Disorders Branch. The details of subject recruitment, evaluation, and potential ascertainment bias are discussed elsewhere (5, 61–63). Briefly, all participants gave written informed consent of an Institutional Review Board-approved protocol. Most families had two eligible full siblings (at least one of whom met DSM-IV criteria for schizophrenia). Participants had to be 18 to 60 years of age, above 70 in premorbid IQ, and able to give informed consent. Applicants with significant medical problems, history of head trauma, and alcohol or drug abuse within the last 6 months were excluded. All subjects were medically screened and interviewed by a research psychiatrist using the Structured Clinical Interview for DSM-IV Axis I Disorders (64).
Task Conditions
Subjects were presented 250 tones of two different pitches by loudspeaker at approximately 80 dB (SPL). Stimulus duration was 50 msec, with 5-msec rise and decay and pseudorandomized interstimulus interval of 1.0–1.5 seconds and order (target: 1500 Hz, probability=20%; nontarget: 1000 Hz, probability=80%). Subjects, who kept eyes closed during the task, were asked to count the number of targets.
As previously outlined (6), a neuropsychological test battery was administered before acquiring the electrophysiological data. The battery included the n-back working memory task (0-back, 1-back, 2-back), which is a test of working memory and executive cognition. The n-back test requires subjects to constantly update their mental set while minimizing interference from incoming stimuli (14). Subjects are told to recall the stimulus (one out of four numbers) seen “n” previously. Stimuli are presented for 1800 msec, with a 2000-msec break at the start of each task epoch. The applied n-back version emphasizes the “executive” aspects of working memory, with continual presentation of incoming stimuli and continual working memory response. For comparison, subjects also had to perform another putative frontal lobe task (Wisconsin Card Sorting Test) as a test of executive function and set shifting. Subjects also received the vigilance and distractibility versions of the Gordon Continuous Performance Test (d-vigilance subscale) as tests for attentional/vigilance processes and the WRAT reading subtest, which is thought to be an estimate of premorbid intellectual levels in schizophrenia patients.
Electrophysiology
Using a Grass model (8-24D) electroencephalograph, event-related EEG was recorded with gold electrodes during the oddball task. Electrode positions were defined according to the international 10/20 system, impedance was kept below 5 kΩ . Eye movements were registered on paper record with an additional channel across electrodes 1 cm lateral and either above or below the corner of the eye. Event-related EEG was measured from 16 electrode positions (Fp1, Fp2, F3, F4, F7, F8, T3, T4, T5, T6, C3, C4, P3, P4, O1, O2). Amplifiers were calibrated by using a 50-μV square pulse. Sampling frequency was 250 Hz with 0.5–70 Hz filters. A relatively high high-pass filter was used, since previous systematic investigations of P300 have suggested that this may improve group contrast between schizophrenia patients and healthy subjects (65, 66). All channels were recorded against separate ears as reference, i.e., left hemispheric electrodes were referenced to the left ear and right hemispheric electrodes were referenced to the right ear. Before data analysis, off-line artifact detection was performed automatically (amplitude criterion >70 μV) and manually repeated afterward by a rater who was blind to diagnosis. Specifically, all EEG segments that contained obvious eye movement, head movement, or muscle artifacts were excluded. Test-retest analyses were performed with a “naturalistic” data set of 15 subjects (three unmedicated, unaffected siblings and 12 schizophrenia inpatients receiving partly changing medication) with a mean duration between sessions of 63.56 days (SD=76.45).
Event-Related Potential/EEG Analysis
Generation of event-related potential grand averages across montage and subsequent topographic EEG magnitude, amplitude, and latency analysis were automatically performed with EEGSYS (67). P300 peak amplitudes against baseline and latencies were determined for the target condition of the event-related potentials by locating the peak within the specified time window: P300 (260–420 msec poststimulus). Baseline was defined as the available 50-msec prestimulus EEG. For quantitative event-related EEG analysis, the recorded signals (–50 msec to 590 msec poststimulus, target condition) were submitted to spectrum analysis using a 256-point fast Fourier transform yielding spectrum values in 1.525-Hz steps. The absolute magnitude in each frequency band was computed expressed in μV (square root of the EEG power). Frequency band partition was: delta=0.5–4.0 Hz, theta=4.5–8.0 Hz, alpha=8.5–12.5 Hz, beta 1=13.0–18.0 Hz, beta 2=18.5–30.0 Hz, gamma=35.0–45.0 Hz. The computation of the noise magnitude, which is subsequently denoted as “noise power,” was calculated following the recommendations of Möcks et al. (68) and Winterer et al. (49, 54). For any given frequency band, the mean magnitude of the single trials is subtracted from the magnitude of the average potential. This way, a quantification of the noise part of the event-related activity is approximated, and “noise” is equivalent with activity that is not time-locked to the stimuli, i.e., spontaneous background activity and jittering of the event-related signal (54).
Statistical Analyses
Statistical analyses were carried out with STATISTICA (69). Comparisons of demographic, clinical, functional, and neuropsychological data were performed with analyses of covariance (ANCOVA), chi-square analyses, regression analyses (Spearman’s r), multiple regression, or Student’s t tests as appropriate.
Exploratory principal component analyses (Varimax rotation) for variable reduction were carried out as described elsewhere (49, 54, 63) across electrode positions for noise power separately for each frequency band as well as P300 amplitudes and were based upon the entire sample of subjects (no major difference of factor solutions between samples). Principal component analysis of the event-related EEG and P300 amplitudes across electrodes revealed a two-factor structure within each frequency band (similar to what has been described before [49, 54]), i.e., one bilateral frontal factor that includes the frontal, central, and anterior temporal electrodes and one bilateral temporoparietal factor that includes mainly the parietal, posterior temporal, and to a lesser extent the occipital electrodes. This factor structure across electrodes was also observed for the P300 amplitudes. However, two notable exceptions were seen. The “frontal” delta factor extended from frontal electrodes, where the maximum was seen, to the posterior, i.e., central, parietal, and occipital electrode positions. The “temporoparietal” delta factor was largely limited to the temporal electrode positions. In the upper frequency bands, we observed a strong trend for a left-right hemispheric factor solution. However, for simplicity, we generally used a two-factor solution calling them “frontal” and “temporoparietal” factors or principal components. The total variance of the temporoparietal noise power factors varied between 8.0% and 19.0%, with eigenvalues in the range between 1.3 and 3.0. The total variance of the frontal noise power factors varied between 45.0% and 75.0%, with eigenvalues in the range between 7.5 and 11.5 (factor loadings available upon request).
Because we were mainly interested in prefrontal processing, ANCOVA group comparisons were only performed using the frontal factor scores of each individual under inclusion of one randomly selected schizophrenia proband and one randomly selected unaffected sibling from families with multiple sibships. Test-retest stability was also calculated for principal components with intraclass correlation (ICCU) (70). This was achieved by using factor loadings from the principal component analysis as weight factors. These weights were multiplied by the raw magnitude values at each electrode position and subsequently added up for each individual. The resulting individual values for the test and retest set were then subjected to ICC calculations.
For genetic modeling, phenotypic similarity was estimated for sib-pairs (schizophrenia patient and unaffected sibling) using individual factor scores of the electrophysiological variables, treating the electrophysiological measures as quantitative variables, and calculating intraclass correlations (ICCU) (70). Under the assumption of little influence from shared environment with respect to electrophysiological parameters (71), sib-pair intraclass correlations provide indirect information on the heritability of a phenotype. Sib-pairs with one schizophrenia patient and one clinically unaffected sibling per family were selected and matched as far as possible for age and gender, if sibships with multiple unaffected siblings were present. Relative risk (λ) calculations were performed on the entire sample of patients and siblings for the obtained electrophysiological phenotypes based on “qualitative phenotype” definitions. Accordingly, a cutoff for abnormality for the qualitative phenotype was arbitrarily delimited based on the arithmetic mean of healthy comparison subjects and adding one standard deviation or two standard deviations for the noise power principal components. Relative risk ratio (λ) was calculated for these phenotype definitions for unaffected siblings independently of whether their respective schizophrenia sibling showed the electrophysiological phenotype or not (72, 73). Subsequently, the number of affected siblings of index patients, who were themselves affected with the electrophysiological phenotype, was determined; these were concordant pairs for the electrophysiological phenotype. Unaffected siblings of affected patients were defined as nonconcordant. Based on this concordance definition, relative risk (λ′) was calculated in a modified way as (concordant pairs/[concordant pairs plus nonconcordant pairs])/frequency of affected comparison subjects. A standard chi-square statistic was used to test for significance of relative risk values by comparing distributions of affected status in siblings with the comparison group (5). These calculations were performed under inclusion of all unaffected siblings from families with multiple sibships.
Results
Clinical and Demographic Data
Table 1 depicts demographic and clinical data of the participants. No significant age differences were found between healthy comparison subjects and schizophrenia patients (t=1.2, df=153, p<0.24) or unaffected siblings (t=1.6, df=201, p<0.13). Gender differed significantly between comparison subjects and schizophrenia patients (χ2=15.4, df=1, p<0.001) and between comparison subjects and siblings (χ2=5.45, df=1, p=0.02). Healthy subjects had more years of education compared with schizophrenia patients (t=5.18, df=116, p=0.0001) but not compared with unaffected siblings (t=0.33, df=168, p<0.75). Healthy subjects were less frequently smokers compared with schizophrenia patients (χ2=16.3, df=1, p=0.0001) but not compared with siblings (χ2=0.6, df=1, p<0.44). With respect to handedness as measured by the Edinburgh Inventory (74), no significant differences were found between healthy subjects and schizophrenia patients (χ2=0.1, df=1, p<0.79) or siblings (χ2=2.1, df=1, p<0.16). Comparing the percentage of correctly counted targets, healthy subjects did not differ significantly from schizophrenia patients (t=0.53, df=80, p<0.60) or their unaffected siblings (t=–1.02, df=110, p<0.32).
Topographic Noise Pattern
Figure 1 depicts descriptive maps for each group separately (schizophrenia patients, their unaffected siblings, and comparison subjects) of the adjusted mean noise power of the event-related potential fields across montage and selected frequency bands. In the healthy comparison group, noise power in the delta and theta frequency range is highest over the frontal area, whereas in the other frequency bands, most noise power is observed over the frontocentral region (results from topographic ANCOVA group comparisons of noise power are available upon request).
Group Comparisons
Statistical group comparisons of the frontal noise-power principal components are depicted in Figure 2. This figure shows the results of overall two-way multivariate analyses of covariance with diagnosis and gender as the two factors, age and number of EEG trials as covariates, and the electrophysiological principal components as dependent variables. A significant main effect was seen for diagnosis. A marginally significant gender effect was also observed (Rao’s rs=2.83, df=6, 201, p<0.02), as was a tendency for a gender-by-diagnosis interaction (Rao’s rs=1.67, df=12, 402, p<0.08). Women showed lower noise power values than men in the delta-alpha range but higher noise power values in the beta/gamma frequency spectrum. Figure 2 also shows separate ANCOVAs (within the prior multivariate analysis of variance [MANOVA] model) for each frequency band with same factors and covariates as in the MANOVA as well as post hoc comparisons between groups. Schizophrenia patients showed highest noise power values across the entire frequency spectrum; siblings were characterized by intermediate values. Of note, multiple regression analysis across the entire sample revealed that the frontal P300 amplitude principal component was positively correlated with the frontal noise power components (R2=0.41, df=6, 254, p<0.00001).
Prefrontal Information Processing
Neuropsychological performance indices and electrophysiological variables were examined in the delta and alpha-frequency band with rank order correlation analyses (Spearman’s r). Statistically significant negative correlations were observed between frontal noise power in the delta frequency band and 1-back performance (rs=–0.49, N=185, p<0.0001) (Figure 3), 2-back performance (rs=–0.34, N=185, p<0.0002), and 3-back performance (rs=–0.40, N=173, p<0.0001). A significant correlation was also observed with the 0-back condition (rs=–0.16, N=186, p<0.04) and Wisconsin Card Sorting Test perseverative errors (rs=0.22, N=223, p=0.001) as well as with the Continuous Performance Test d-vigilance scores (rs=–0.20, N=224, p=0.003). No significant correlation was observed with WRAT reading scores (rs=0.05, N=225, p<0.49). Separate correlation analyses between the 1-back condition and noise power in the delta frequency band were performed for the schizophrenia patients, their unaffected siblings, and healthy subjects. In all three groups, frontal noise power in the delta frequency band correlated significantly and negatively: schizophrenia patients (rs=–0.43, N=39, p=0.006); siblings (rs=–0.43, N=82, p=0.0006); healthy comparison subjects (rs=–0.31, N=64, p<0.02). Very similar results were obtained when performing correlation analyses between the same neuropsychological task indices and noise power in the alpha frequency band: 1-back performance (rs=–0.39, N=185, p<0.0001), 2-back performance (rs=–0.26, N=185, p<0.0003), 3-back performance (rs=–0.32, N=173, p=0.0002), 0-back performance (rs=–0.09, N=186, p<0.25), Wisconsin Card Sorting Test perseverative errors (rs=0.15, N=223, p<0.03), Continuous Performance Test d-vigilance (rs=–0.12, N=224, p<0.09), and WRAT reading score (rs=0.11, N=225, p<0.10).
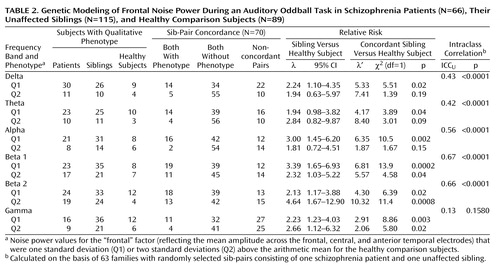
Genetic Modeling
Test-retest intraclass correlations (ICCU) of the frontal noise principal components were high in the theta and alpha frequency band (ICCU=0.85–0.93), intermediate in the delta and beta 1 band (ICCU=0.62–0.69), and low in the beta 2 and gamma frequency band (ICCU=–0.05–0.10). Table 2 shows the between sib-pair intraclass correlations ICCU and relative risk values (λ and λ′) of the frontal noise power principal components for 63 sib-pairs consisting of a schizophrenia patient and an unaffected sibling. On average, intraclass correlations of noise power are high between sib-pairs across frequency bands, with highest values in the alpha and beta frequency range. Relative risk calculations revealed that λ is mostly moderate and that λ′ is high with regard to noise power across the frequency spectrum. It is possible that a somewhat higher risk is conferred by noise power in the medium to upper frequency range (alpha/beta 1).
Discussion
The results of this study suggest that increased, frontally pronounced broadband cortical background noise during cognitive information processing is a characteristic trait pattern of brain activity in schizophrenia patients and to a lesser degree in their clinically unaffected siblings. Moreover, evidence is provided that the amount of frontal background noise predicts poor performance on frontal lobe cognitive tasks as well as genetic risk for schizophrenia.
The negative correlation between frontal background noise and performance on frontal lobe tasks—particularly those involving working memory performance and executive cognition—is intriguing for several reasons. First, it suggests that background noise is not simply an electrical epiphenomenon but a physiological entity with functional implications. Second, background noise is not only inversely related to cognitive performance in schizophrenia patients and siblings but also in healthy comparison subjects. This observation implies that a lack of stimulus-induced synchronization or phase resetting of ongoing field potential oscillations over a wide range of frequencies is generally unfavorable with regard to cortical information processing. Of note, this finding is to some extent in line with a study that has suggested an inverse relationship between parietotemporally generated event-related potential variability and psychometric intelligence (75).
Our findings also suggest that the frontal noise measure qualifies as an interesting quantitative trait for the investigation of susceptibility genes for prefrontal dysfunction in schizophrenia but also in the general population. The prefrontal noise phenotype offers a direct neurophysiological and testable link to theoretical concepts of schizophrenia, e.g., the prefrontal dopamine, glutamate, and GABA hypotheses, which should facilitate candidate gene approaches. From a genetic perspective, it is a particular advantage that the applied prefrontal noise measure manifests excellent test-retest stability as demonstrated in the present study in acutely ill schizophrenia inpatients under changing medication and clinical conditions. In addition, the high intraclass correlation between sib-pairs indirectly suggests high heritability. Other measures of prefrontal function (e.g., behavioral measures of cognitive test performance) have been reported to show considerably lower test-retest stability in patients, especially when symptoms vary (76). Moreover, indices of cognitive ability are generally less heritable (h2=0.4–0.6) (e.g., reference 77) than electrophysiological parameters (h2=0.7–0.9), which show little influence of shared environment (e.g., reference 71). Also, a comparison with previously reported relative risk estimates for deficits in executive cognition (Wisconsin Card Sorting Test performance), which were largely obtained from the same study sample (5, 6), indicates substantially higher λ values of our prefrontal noise measures. Also of considerable interest is that a previously reported electrophysiological genetic risk estimate for schizophrenia (63), i.e., the frontal P300 amplitude, which indirectly measures prefrontal noise, apparently is under less genetic control and also is a less strong predictor of risk for schizophrenia than the present noise estimates. This is broadly consistent with earlier findings of Turetsky et al. (60) on the task-independent intraindividual stability of noise measures as compared with the stability of event-related potentials, and it is also in agreement with investigations on the stability of task-related EEG during oddball conditions (78). These observations, together with the simplicity of the oddball task, should therefore render the frontal noise phenotype a promising candidate for developing electrophysiological animal models that could be used in quantitative trait genetic loci identification. In fact, several electrophysiological oddball-type rat and mouse models have been successfully developed that could be used for this purpose (26, 79–82).
An important methodological question is whether our observations reflect a neurophysiological phenomenon or a measurement artifact that could have resulted from eye movement, blinks, or muscle artifacts. Several findings suggest that the increase of background noise in siblings and schizophrenia patients is of cortical origin. First, frontal noise power predicts working memory performance in schizophrenia patients, their unaffected siblings, and healthy comparison subjects, and the high test-retest intraclass correlations within subjects and high intraclass correlations between sib-pairs suggest a major physiological trait component. In addition, the noise power increase in schizophrenia patients and their siblings was observed relatively consistently across the entire frequency spectrum. With regard to muscle or eye movement artifacts, one would expect a more frequency-selective increase of power. Thus, the topographic noise maximum was found in the central region in several frequency bands (i.e., alpha band) where such artifacts are rarely seen. Moreover, principal component analyses did not provide evidence for “artifact” components with major effects. The applied noise measure obtains further validity from the observed positive correlation between noise and frontal P300 amplitude. A novelty task component—such as during oddball paradigms—results in an orienting reaction with activation of the prefrontal cortex as demonstrated by a number of electrophysiological and functional neuroimaging studies (83–89). Electrophysiologically, this orienting response is expressed as an increase of the frontal P300 amplitude around 300 msec after stimulus presentation. Intracortical EEG recordings have shown that the frontal P300 component does not show a polarity inversion or a clear peak between 220 and 360 msec after the oddball stimulus in the scalp-recorded averaged potential (83–85, 90). As opposed to the classical parietotemporal P300, the frontal P300 following the oddball stimulus thus cannot be regarded as a typical cortical signal but rather as a “noisy” component (for a more detailed discussion see reference 63), which is compatible with the observed positive correlation between frontal P300 amplitude and our frontal noise measure. Nevertheless, it is plausible that nonneurophysiological noise (e.g., eye movements) may have some limited effect, as suggested by the somewhat lower intraclass correlations and lower relative risk values in the very low and high frequency range.
Another issue that needs to be addressed is the question about the underlying neurophysiological mechanism that is responsible for the observed increase of prefrontal noise in schizophrenia patients and their siblings. In principal, there are several possibilities as previously explored and discussed by us (49). Perhaps the most important question is whether increased noise can even be observed independently of stimulus processing during resting condition. Thus, an increase of background power across the entire frequency spectrum in resting EEG theoretically could explain our observation of increased noise. In fact, an increase of power, particularly in the slow frequency range, is a frequent observation in schizophrenia patients. However, our prior work (49), which includes a study of resting EEG in a sample that largely overlapped with the present study sample (61) as well as a schizophrenia twin study of resting EEG (91), suggests that background EEG power is 1) not increased in unaffected family members of schizophrenia patients and 2) is not the main reason for the observed increase of noise in schizophrenia. This prior work, which also included the investigation of EEG coherence changes between pre- and poststimulus EEG, also suggested that it is not simply a reduction of event-related signal amplitude in the sense of less activation or cortical recruitment that accounts for the increase of noise but a lack of event-related EEG synchronization (i.e., stimulus-induced phase resetting of EEG activity).
Until very recently, it was difficult to reconcile findings on the single neuron level with observations on the system level made with functional neuroimaging or scalp-recorded electrophysiology in schizophrenia patients. By extension, one could not infer with certainty that a presumed prefrontal dopamine deficit and reduced neuronal signal-to-noise ratio postulated in schizophrenia was in any way related to “noisier” prefrontal hemodynamic responses or field potentials. The reason for this difficulty was that no correspondence could be demonstrated between single neuron activity (mean spike rate) on one hand and electrical field potentials or hemodynamic responses on the other. Rather, oscillating field potentials or event-related potentials—either measured from the scalp or intracortically—are considered to reflect postsynaptic events, produced by synchronous excitation of apical dendrites of pyramidal cells—a process that is in part under thalamic control (92–100). In a similar way, hemodynamic changes have been shown to be related predominantly to these postsynaptic processes rather than single neuron spiking, and both hemodynamic brain responses and electrical field potentials are correlated with each other (101, 102). However, recent work has revealed a relationship between single neuron spike rhythm and postsynaptically generated field potentials, which has been demonstrated for high-frequency gamma activity in the visual cortex (103, 104), beta and alpha activity in the motor cortex (105), and for slower frequency components in theta frequency range in the hippocampus (106). Comparable findings also have been reported by Lauritzen (102), who investigated the relation of cerebral blood flow (CBF) changes and the spike activity pattern of Purkinje cells. Event correlation analysis showed a random spike firing pattern under baseline conditions but rhythmic oscillations after electrical stimulation while the spike rate remained unchanged and CBF increased. These recent findings on the relation of single neuron spike activity and field potentials and hemodynamic responses could explain two seemingly divergent but likely synonymous effects of prefrontal dopamine: 1) increasing neuronal signal-to-noise ratio by shifting the neuron from a random to a rhythmic firing pattern in a selective frequency band; and 2) increasing and “focusing” the hemodynamic cortical response. However, so far, no study has directly addressed this question on the two-level effects of dopamine in cortical neuronal processing.
 |
 |
Received Dec. 13, 2002; revision received July 3, 2003; accepted July 10, 2003. From the Clinical Brain Disorders Branch, National Institute of Mental Health. Address reprint requests to Dr. Winterer, Unit on Molecular Neuroimaging, Clinical Brain Disorders Branch, National Institute of Mental Health, Bldg. 10, Rm. 4s229A, MSC 1379, Bethesda, MD 20892; [email protected] (e-mail).
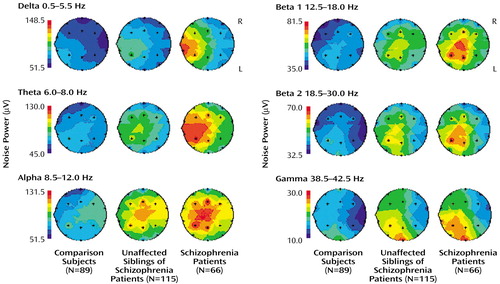
Figure 1. Noise Power Across Selected Frequency Bands During an Auditory Oddball Task in Schizophrenia Patients, Their Unaffected Siblings, and Healthy Comparison Subjectsa
aNoise, or activity not time-locked to the stimuli (i.e., spontaneous background activity and jittering of the event-related signal), was approximated by subtracting the mean magnitude of the single trials from the magnitude of the average potential.
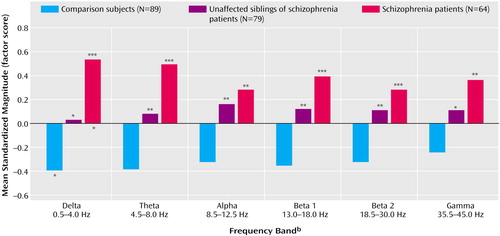
Figure 2. Group Comparisons of Frontal Noise Power Across Selected Frequency Bands During an Auditory Oddball Task in Schizophrenia Patients, Their Unaffected Siblings, and Healthy Comparison Subjectsa
aPrincipal component analysis of event-related EEG and P300 amplitudes revealed a two-factor structure within each frequency band. Noise power values for the “frontal” factor (reflecting the mean amplitude across the frontal, central, and anterior temporal electrodes) are shown in the figure. Asterisks denote post hoc group comparisons (per Tukey’s honestly significant difference test for unequal Ns): those above the x axis are for comparisons with healthy subjects, and those below are for comparisons with unaffected siblings. Data are used from all available healthy subjects. The groups of unaffected siblings and schizophrenia patients consist of one randomly selected schizophrenia patient and one randomly selected sibling per family.
bMANCOVA: Rao’s rs=3.99, df=12, 402, p<0.00001. Within this model, analyses of covariance (df=2, 206) were performed for each frequency band (delta: F=11.09, p=0.00003; theta: F=10.29, p=0.00006; alpha: F=6.84, p=0.001; beta 1: F=8.58, p=0.0003; beta 2: F=5.97, p=0.003; gamma: F=5.46, p=0.005).
c*p<0.05. **p<0.01. ***p<0.001.
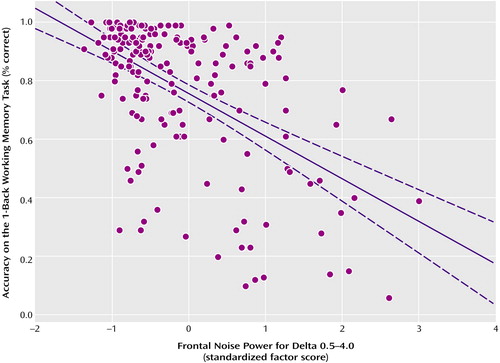
Figure 3. Relationship Between Working Memory and Frontal Noise Power in the Delta Frequency Band During an Auditory Oddball Task in Schizophrenia Patients (N=66), Their Unaffected Siblings (N=115), and Healthy Comparison Subjects (N=89)a
aPrincipal component analysis of event-related EEG and P300 amplitudes revealed a two-factor structure within each frequency band. Noise power values for the “frontal” factor (reflecting the mean amplitude across the frontal, central, and anterior temporal electrodes) for the delta frequency band are shown in the figure.
1. Goldberg TE, Gold JM: Neurocognitive deficits in schizophrenia, in Schizophrenia. Edited by Hirsch SR, Weinberger DR. Oxford, UK, Blackwell Science, 1995, pp 146–162Google Scholar
2. Park S, Holzman PS, Goldman-Rakic PS: Spatial working memory deficits in the relatives of schizophrenic patients. Arch Gen Psychiatry 1995; 52:821–828Crossref, Medline, Google Scholar
3. Cannon TD, Huttunen MO, Lonnqvist J, Tuulio-Henriksson A, Pirkola T, Glahn D, Finkelstein J, Hietanen M, Kaprio J, Koskenvuo M: The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet 2000; 67:369–382Crossref, Medline, Google Scholar
4. Faraone SV, Seidman LJ, Kremen WS, Toomey R, Pepple JR, Tsuang MT: Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: the effect of genetic loading. Biol Psychiatry 2000; 48:120–126Crossref, Medline, Google Scholar
5. Egan MF, Goldberg TE, Gscheidle T, Weirich M, Bigelow LB, Weinberger DR: Relative risk of attention deficits in siblings of patients with schizophrenia. Am J Psychiatry 2000; 157:1309–1316Link, Google Scholar
6. Egan MF, Goldberg TE, Gscheidle T, Weirich M, Rawlings R, Hyde TM, Bigelow L, Weinberger DR: Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry 2001; 50:98–107Crossref, Medline, Google Scholar
7. Weinberger DR, Egan MF, Bertolino A, Calicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE: Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry 2001; 50:825–844Crossref, Medline, Google Scholar
8. Wolkin A, Jaeger J, Brodie JD, Wolf AP, Fowler J, Rotrosen J, Gomez-Mont F, Cancro R: Persistence of cerebral metabolic abnormalities in chronic schizophrenia as determined by positron emission tomography. Am J Psychiatry 1985; 142:564–571Link, Google Scholar
9. Weinberger DR, Berman KF, Zec RF: Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia, I: regional cerebral blood flow evidence. Arch Gen Psychiatry 1986; 43:114–124Crossref, Medline, Google Scholar
10. Turetsky B, Colbath EA, Gur RE: P300 subcomponent abnormalities in schizophrenia: longitudinal stability and relationship to symptom change. Biol Psychiatry 1998; 43:31–39Crossref, Medline, Google Scholar
11. Stevens AA, Goldman-Rakic PS, Gore JC, Fulbright RK, Wexler BE: Cortical dysfunction in schizophrenia during auditory word and tone working memory demonstrated by functional magnetic resonance imaging. Arch Gen Psychiatry 1998; 55:1097–1103Crossref, Medline, Google Scholar
12. Mathalon DH, Ford JM, Pfefferbaum A: Trait and state aspects of P300 amplitude reduction in schizophrenia: a retrospective longitudinal study. Biol Psychiatry 2000; 47:434–449Crossref, Medline, Google Scholar
13. Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S: Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry 1999; 45:1128–1137Crossref, Medline, Google Scholar
14. Callicott JH, Bertolino A, Mattay VS, Langheim FJP, Duyn J, Coppola R, Goldberg TE, Weinberger DR: Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 2000; 10:1078–1092Crossref, Medline, Google Scholar
15. Weinberger DR, Berman KF, Illowsky BP: Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia, III: a new cohort and evidence for a monoaminergic mechanism. Arch Gen Psychiatry 1988; 45:609–615Crossref, Medline, Google Scholar
16. Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA: Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry 1999; 156:1580–1589Link, Google Scholar
17. Abi-Dargham A, Mawalawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M: Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 2002; 22:3708–3719Crossref, Medline, Google Scholar
18. Daniel DG, Berman F, Weinberger DR: The effect of apomorphine on regional blood flow in schizophrenia. J Neuropsychiatry 1989; 1:377–384Crossref, Medline, Google Scholar
19. Daniel DG, Weinberger DR, Jones DW, Zigun JR, Coppola R, Handel S, Bigelow LB, Goldberg TE, Berman KF, Kleinman JE: The effect of amphetamine on regional cerebral blood flow during cognitive activation in schizophrenia. J Neurosci 1991; 11:1907–1917Crossref, Medline, Google Scholar
20. Friston KJ, Grasby PM, Bench CJ, Froth CD, Cowen PJ, Liddle PF, Frackowiak RSJ, Dolan RJ: Measuring the neuromodulatory effects of drugs in man with positron emission tomography. Neurosci Lett 1992; 141:106–110Crossref, Medline, Google Scholar
21. Dolan RJ, Fletcher P, Frith CD, Friston KJ, Frackowiak RSJ, Grasby PM: Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature 1995; 378:180–182Crossref, Medline, Google Scholar
22. Mattay VS, Berman KF, Ostrem JL, Esposito G, Van Horn JD, Bigelow LB, Weinberger DR: Dextroamphetamine enhances “neural network-specific” physiological signals: a positron-emission tomography rCBF study. J Neurosci 1996; 16:4816–4822Crossref, Medline, Google Scholar
23. Mattay V, Callicott JH, Bertolino A, Heaton I, Frank JA, Coppola R, Berman KF, Goldberg TE, Weinberger DR: Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage 2000; 12:268–275Crossref, Medline, Google Scholar
24. Sawaguchi T: Catecholamine sensitivities of neurons related to a visual reaction time task in the monkey prefrontal cortex. J Neurophysiol 1987; 58:1100–1122Crossref, Medline, Google Scholar
25. Sawaguchi T, Matsumura M, Kubota K: Dopamine enhances the neuronal activity of spatial short-term memory task in the primate prefrontal cortex. Neurosci Res 1988; 5:465–473Crossref, Medline, Google Scholar
26. Robledo P, Kaneko WM, Ehlers CL: The effects of acute cocaine administration on auditory event-related potentials in rats. Neurosci Lett 1993; 160:4–8Crossref, Medline, Google Scholar
27. Williams GV, Goldman-Rakic PS: Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 1995; 376:572–575Crossref, Medline, Google Scholar
28. Sawaguchi T: The effects of dopamine and its antagonists on directional delay-period activity of prefrontal neurons in monkeys during an oculomotor delayed-response task. Neurosci Res 2001; 41:115–128Crossref, Medline, Google Scholar
29. Goldman-Rakic PS, Muly EC, Williams GV: D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev 2000; 31:295–301Crossref, Medline, Google Scholar
30. Colonnier M: Synaptic patterns on different cell types in the different laminae of the cat visual cortex: an electron microscope study. Brain Res 1968; 9:268–287Crossref, Medline, Google Scholar
31. DeFelipe J, Conti F, Van Eyck SL, Manzoni T: Demonstration of glutamate-positive axon terminals forming asymmetric synapses in cat neocortex. Brain Res 1988; 455:162–165Crossref, Medline, Google Scholar
32. Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS: D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci USA 1994; 91:5720–5724Crossref, Medline, Google Scholar
33. Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS: Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci 1995; 15:7821–7836Crossref, Medline, Google Scholar
34. Sesack SR, Snyder CL, Lewis DA: Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA-immunoreactive dendrites in rat and monkey cortex. J Comp Neurol 1995; 363:264–280Crossref, Medline, Google Scholar
35. Sesack SR, Hawrylak VA, Melchitzky DS, Lewis DA: Dopamine innervation of a subclass of local circuit neurons in monkey prefrontal cortex: ultrastructural analysis of tyrosine hydroxylase and parvalbumin immunoreactive structures. Cereb Cortex 1998; 8:614–622Crossref, Medline, Google Scholar
36. Seamans JK, Gorelova N, Durstewitz D, Yang CR: Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci 2001; 21:3628–3638Crossref, Medline, Google Scholar
37. Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ: Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci USA 2001; 98:301–306Crossref, Medline, Google Scholar
38. Gao WJ, Krimer LS, Goldman-Rakic PS: Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci USA 2001; 98:295–300Crossref, Medline, Google Scholar
39. Amit DJ, Brunel N: Model of global spontaneous activity and local structured activity during delay periods in the cerebral cortex. Cereb Cortex 1997; 7:237–252Crossref, Medline, Google Scholar
40. Compte A, Brunel N, Goldman-Rakic PS, Wang XJ: Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex 2000; 10:910–923Crossref, Medline, Google Scholar
41. Durstewitz D, Kelc M, Gunturkun O: A neurocomputational theory of the dopaminergic modulation of working memory functions. J Neurosci 1999; 19:2807–2822Crossref, Medline, Google Scholar
42. Durstewitz D, Seamans JK, Sejnowski TJ: Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol 2000; 83:1733–1750Crossref, Medline, Google Scholar
43. Brunel N, Wang XJ: Effects of neuromodulation in a cortical network model of object working memory dominated by recurrent inhibition. J Comput Neurosci 2001; 11:63–85Crossref, Medline, Google Scholar
44. Weinberger DR: Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Crossref, Medline, Google Scholar
45. Goldman-Rakic PS: Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci 1994; 6:348–357Crossref, Medline, Google Scholar
46. Donchin E, Callaway E, Jones RT: Auditory evoked potential variability in schizophrenia, II: the application of discriminant analysis. Electroencephalogr Clin Neurophysiol 1970; 29:429–440Crossref, Medline, Google Scholar
47. Callaway E, Jones RT, Donchin E: Auditory evoked potential variability in schizophrenia. Electroencephalogr Clin Neurophysiol 1970; 29:421–428Crossref, Medline, Google Scholar
48. Zouridakis G, Boutros NN, Jansen BH: A fuzzy clustering approach to study the auditory P50 component in schizophrenia. Psychiatry Res 1997; 69:169–181Crossref, Medline, Google Scholar
49. Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Wuebben Y, Herrmann WM, Coppola R: Schizophrenia: reduced signal-to-noise ratio and impaired phase-locking during information processing. Clin Neurophysiol 2000; 111:837–849Crossref, Medline, Google Scholar
50. Sayers BM, Beagley HA, Henshall WR: The mechanism of auditory evoked EEG responses. Nature 1974; 247:481–483Crossref, Medline, Google Scholar
51. Basar E: EEG-Brain Dynamics: Relation Between EEG and Brain Evoked Potentials. New York, Elsevier, 1980Google Scholar
52. Brandt ME, Jansen BH, Carbonari JP: Pre-stimulus spectral EEG patterns and the visual evoked response. Electroencephalogr Clin Neurophysiol 1991; 80:16–20Crossref, Medline, Google Scholar
53. Bressler SL, Coppola R, Nakamura R: Episodic multiregional cortical coherence at multiple frequencies during visual task performance. Nature 1993; 366:153–156Crossref, Medline, Google Scholar
54. Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Dahhan N, Herrmann WM, Coppola R: Cortical activation, signal-to-noise ratio and stochastic resonance during information processing in man. Clin Neurophysiol 1999; 110:1193–1203Crossref, Medline, Google Scholar
55. Friston KJ: The labile brain, I: neuronal transients and nonlinear coupling. Philos Trans R Soc Lond B Biol Sci 2000; 355:215–236Crossref, Medline, Google Scholar
56. Varela F, Lachaux JP, Rodriguez E, Martinerie J: The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci 2001; 2:229–239Crossref, Medline, Google Scholar
57. Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ: Perception’s shadow: long-distance synchronization of human brain activity. Nature 1999; 397:430–433Crossref, Medline, Google Scholar
58. Salinas E, Sejnowski TJ: Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci 2001; 2:539–550Crossref, Medline, Google Scholar
59. Makeig S, Westerfield M, Jung T-P, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ: Dynamic brain sources of visual evoked responses. Science 2002; 295:690–694Crossref, Medline, Google Scholar
60. Turetsky BI, Raz J, Fein G: Noise and signal power and their effects on evoked potential estimation. Electroencephalogr Clin Neurophysiol 1988; 71:310–318Crossref, Medline, Google Scholar
61. Winterer G, Egan F, Raedler T, Hyde T, Coppola R, Weinberger DR: An association between reduced interhemispheric EEG coherence in the temporal lobe and genetic risk for schizophrenia. Schizophr Res 2001; 49:129–143Crossref, Medline, Google Scholar
62. Winterer G, Egan MF, Raedler T, Coppola R, Weinberger DR: Event-related potentials and genetic risk for schizophrenia. Biol Psychiatry 2001; 50:407–417Crossref, Medline, Google Scholar
63. Winterer G, Egan MF, Raedler T, Sanchez CE, Jones DW, Coppola R, Weinberger DR: P300 and genetic risk for schizophrenia. Arch Gen Psychiatry 2003; 60:1158–1167Crossref, Medline, Google Scholar
64. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders Research Version (SCID-I). New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
65. Ebmaier KP, Glabus M, Potter DD, Salzen EA: The effects of different high-pass filter settings on peak latencies in the event-related potentials of schizophrenics, patients with Parkinson’s disease and control subjects. Electroencephalogr Clin Neurophysiol 1992; 84:280–287Crossref, Medline, Google Scholar
66. Glabus MF, Blackwood DHR, Ebmaier KP, Souza V, Walker MT, Sharp CW, Dunan JT, Muir W: Methodological considerations in measurement of the P300 component of the auditory oddball in schizophrenia. Electroencephalogr Clin Neurophysiol 1994; 90:123–134Crossref, Medline, Google Scholar
67. Hartwell J: EEGSYS Version 5.7 Users’ Guide. Baltimore, Friends Medical Science Research Center, 1998Google Scholar
68. Möcks J, Gasser T, Köhler W: Basic statistical parameters of event-related potentials. J Psychophysiol 1988; 2:61–70Google Scholar
69. STATISTICA for Windows. Tulsa, Okla, Statsoft, 1998Google Scholar
70. Bartko JJ, Carpenter WT: On the methods and theory of reliability. J Nerv Ment Dis 1976; 163:307–316Crossref, Medline, Google Scholar
71. Van Beijsterveldt CE, Boomsma DI: Genetics of the human electroencephalogram (EEG) and event-related brain potentials (ERPs): a review. Hum Genet 1994; 94:319–330Crossref, Medline, Google Scholar
72. Gardner MJ, Altman DG: Statistics With Confidence. London, BMJ Publications, 1994Google Scholar
73. Khoury MJ, Beaty TH, Cohen BH: Fundamentals of Genetic Epidemiology. Oxford, UK, Oxford University Press, 1993Google Scholar
74. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97–113Crossref, Medline, Google Scholar
75. Barrett PT, Eysenck HJ: The relationship between evoked potential component amplitude, latency, contour length, variability, zero-crossings, and psychometric intelligence. Pers Individ Diff 1994; 16:3–32Crossref, Google Scholar
76. Ingram F, Greve KW, Ingram PT, Soukup VM: Temporal stability of the Wisconsin Card Sorting Test in an untreated patient sample. Br J Clin Psychol 1999; 38:209–211Crossref, Medline, Google Scholar
77. Luciano M, Wright MJ, Smith GA, Geffen GM, Geffen LB, Martin NG: Genetic covariance among measures of information processing speed, working memory, and IQ. Behav Genet 2001; 31:581–592Crossref, Medline, Google Scholar
78. Salinsky MC, Oken BS, Morehead L: Test-retest reliability in EEG frequency analysis. Electroencephalogr Clin Neurophysiol 1991; 79:383–392Crossref, Google Scholar
79. Ruusuvirta T, Penttonen M, Korhonen T: Auditory cortical event-related potentials to pitch deviances in rats. Neurosci Lett 1998; 248:45–48Crossref, Medline, Google Scholar
80. Shinba T: Neuronal firing activity in the dorsal hippocampus during the auditory discrimination oddball task in awake rats: relation to event-related potential generation. Brain Res Cogn Brain Res 1999; 8:241–250Crossref, Medline, Google Scholar
81. Umbricht DS, Latanov A, Vissotksi D, Nitsch R, Lipp H-P: Development of a mouse model of deficits in preattentive auditory processing in schizophrenia. Biol Psychiatry 2002; 64S:1002Google Scholar
82. Connolly P, Liang Y, Turetsky BI, Gur RE, Kanes SJ, Bilker W, Lenox RH, Siegel SJ: Strain dependent expression of mismatch negativity in mice (abstract). Biol Psychiatry 2002; 51(suppl):55SGoogle Scholar
83. Baudena P, Halgren E, Heit G, Clarke JM: Intracerebral potentials to rare target and distractor auditory and visual stimuli, III: frontal cortex. Electroencephalogr Clin Neurophysiol 1995; 94:251–264Crossref, Medline, Google Scholar
84. Halgren E, Baudena P, Clarke JM, Heit G, Liegeois C, Chauvel P, Musolino A: Intracerebral potentials to rare target and distractor auditory and visual stimuli, I: superior temporal plane and parietal lobe. Electroencephalogr Clin Neurophysiol 1995; 94:191–220Crossref, Medline, Google Scholar
85. Halgren E, Baudena P, Clarke JM, Heit G, Marinkovic K, Devaux B, Vignal J-P, Biraben A: Intracerebral potentials to rare target and distractor auditory and visual stimuli, II: medial, lateral and posterior temporal lobe. Electroencephalogr Clin Neurophysiol 1995; 94:229–250Crossref, Medline, Google Scholar
86. Knight RT: Contribution of human hippocampal region to novelty detection. Nature 1996; 383:256–259Crossref, Medline, Google Scholar
87. Tulving E, Kroll N: Novelty assessment in the brain and long-term memory encoding. Psychon Bull Rev 1995; 2:387–390Crossref, Medline, Google Scholar
88. Haxby JV, Ungerleider L, Horwitz B, Maisog J, Rappaport S, Grady C: Face encoding and recognition in the human brain. Proc Natl Acad Sci USA 1996; 93:922–927Crossref, Medline, Google Scholar
89. Constable RT, Carpentier A, Pugh K, Westerveld M, Oszunar Y, Spencer DD: Investigation of the human hippocampal formation using a randomized event-related paradigm and Z-shimmed functional MRI. Neuroimage 2000; 12:55–62Crossref, Medline, Google Scholar
90. Frodl T, Juckel G, Gallinat J, Bottlender R, Riedel M, Preuss U, Moller HG, Hegerl U: Dipole localization of P300 and normal aging. Brain Topogr 2000; 13:3–9Crossref, Medline, Google Scholar
91. Stassen HH, Coppola R, Gottesman II, Torrey EF, Kuny S, Rickler KC, Hell D: EEG differences in monozygotic twins discordant and concordant for schizophrenia. Psychophysiology 1999; 36:109–117Crossref, Medline, Google Scholar
92. Bremer F: Cerebral and cerebellar potentials. Physiol Rev 1958; 38:357–388Crossref, Medline, Google Scholar
93. Klee MR, Offenloch K, Tigges J: Cross-correlation analysis of electroencephalographic potentials and slow membrane transients. Science 1965; 147:519–521Crossref, Medline, Google Scholar
94. Creutzfeldt OD, Watanabe S, Lux HD: Relations between EEG phenomena and potentials of single cortical cells, I: evoked responses after thalamic and epicortical stimulation. Electroencephalogr Clin Neurophysiol 1966; 20:1–18Crossref, Medline, Google Scholar
95. Creutzfeldt OD, Watanabe S, Lux HD: Relations between EEG phenomena and potentials of single cortical cells, II: spontaneous and convulsoid activity. Electroencephalogr Clin Neurophysiol 1966; 20:19–37Crossref, Medline, Google Scholar
96. Nicholson C: Theoretical analysis of field potentials in anisotropic ensembles of neuronal elements. IEEE Trans Biomed Eng 1973; 20:278–288Crossref, Medline, Google Scholar
97. Steriade M, McCormick DA, Sejnowski TJ: Thalamocortical oscillations in the sleeping and aroused brain. Science 1993; 262:679–685Crossref, Medline, Google Scholar
98. Contreras D, Steriade M: Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci 1995; 15:604–622Crossref, Medline, Google Scholar
99. Steriade M: Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. Cereb Cortex 1997; 7:583–604Crossref, Medline, Google Scholar
100. Mahon S, Deniau JM, Charpier S: Relationship between EEG potentials and intracellular activity of striatal and cortico-striatal neurons: an in vivo study under different anesthetics. Cereb Cortex 2001; 11:360–373Crossref, Medline, Google Scholar
101. Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A: Neurophysiological investigation of the basis of the fMRI signal. Nature 2001; 412:150–157Crossref, Medline, Google Scholar
102. Lauritzen M: Relationship of spikes, synaptic activity, and local changes of cerebral blood flow. J Cereb Blood Flow Metab 2001; 21:1367–1383Crossref, Medline, Google Scholar
103. Engel AK, Fries P, Singer W: Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci 2001; 2:704–716Crossref, Medline, Google Scholar
104. Fries P, Schroder J-H, Roelfsema PR, Singer W, Engel AK: Oscillatory neuronal synchronization in primary visual cortex as a correlate of stimulus selection. J Neurosci 2002; 22:3739–3754Crossref, Medline, Google Scholar
105. Jackson A, Spinks RL, Freeman TCB, Wolpert DM, Lemon RN: Rhythm generation in monkey motor cortex explored using pyramidal tract stimulation. J Psychophysiol 2002; 541:685–699Google Scholar
106. Bilkey DK, Heinemann U: Intrinsic theta-frequency membrane potential oscillations in layer III/V perirhinal cortex neurons of the rat. Hippocampus 1999; 9:510–518Crossref, Medline, Google Scholar



