Working Memory Deficits and Levels of N-Acetylaspartate in Patients With Schizophreniform Disorder
Abstract
OBJECTIVE: The authors used proton magnetic resonance spectroscopic imaging (1H-MRSI) to assess potential reductions of N-acetylaspartate (a marker of neuronal integrity) in the hippocampal area and dorsolateral prefrontal cortex of patients with schizophreniform disorder. In addition, they assessed the relationship between N-acetylaspartate levels and working memory deficits. METHOD: Twenty-four patients with DSM-IV schizophreniform disorder and 24 healthy subjects were studied. Subjects underwent 1H-MRSI and were given the N-back working memory test. RESULTS: The schizophreniform disorder patients had selective reductions of N-acetylaspartate ratios in the hippocampal area and the dorsolateral prefrontal cortex, and a positive correlation was seen between N-acetylaspartate ratios in the dorsolateral prefrontal cortex and performance during the 2-back working memory condition. CONCLUSIONS: Similar to findings reported in schizophrenia studies, N-acetylaspartate reductions in the hippocampal area and the dorsolateral prefrontal cortex were seen in patients with schizophreniform disorder. Moreover, the results support other evidence that neuronal pathology in the dorsolateral prefrontal cortex accounts for a proportion of working memory deficits already present at illness outset.
The prefrontal cortex is important for performance of cognitive and mnemonic tasks such as working memory (1). Single-cell recording studies in nonhuman primates have demonstrated that neurons in the dorsolateral prefrontal cortex specifically increase their firing during performance of working memory tasks (1). Moreover, temporary inactivation of one or a few neuronal modules results in poorer working memory performance while leaving unaltered sensory and motor processing (1). The key role played by the dorsolateral prefrontal cortex during performance of working memory tasks has been confirmed with numerous functional neuroimaging studies in humans (2, 3).
Working memory deficits are among the constitutive neuropsychological deficits of schizophrenia, and functional imaging studies have consistently indicated dysfunctional activation of the dorsolateral prefrontal cortex during working memory in patients with schizophrenia (4, 5). Proton magnetic resonance spectroscopic imaging (1H-MRSI) of N-acetylaspartate (a marker of neuronal integrity) also has provided evidence of prefrontal neuronal pathology (6–9) (in the latter two studies, data were acquired mostly in prefrontal white matter) as well as pathology in other brain regions, although the results have not been without controversy (10, 11). However, N-acetylaspartate signals derived from 1H-MRSI are sensitive to treatment with antipsychotics (12–15) and also may reflect the functional state of neurons (16–21). Indeed, N-acetylaspartate levels in the dorsolateral prefrontal cortex correlate with physiological activity within the cortical working memory network and have been found to correlate with ratings of negative symptoms in patients with chronic illness (22, 23). The large majority of 1H-MRSI studies have been performed in chronically ill patients and thus are potentially confounded by issues associated with duration of illness and pharmacological treatment. Studying patients with schizophreniform disorder represents a strategy to control for these factors (24).
The purpose of the present study was to measure the level of N-acetylaspartate in the hippocampal area and dorsolateral prefrontal cortex of patients with schizophreniform disorder and to assess its potential relationship with working memory performance.
Method
We studied 24 patients (17 men and seven women; mean age=23.7 years, SD=6.1) with DSM-IV schizophreniform disorder according to the Structured Clinical Interview for DSM-IV Axis I Disorders (25) and 24 age- and sex-matched healthy subjects (mean age=24.0 years, SD=6.1). Exclusion criteria were history of significant drug abuse, active drug use in the past year, head trauma with loss of consciousness, and any significant medical condition. While 20 patients had been receiving antipsychotic drugs for an average of less than 4 weeks (mean days=25.4, SD=32.0), the other four had been drug-naive. Nineteen of the 20 patients were being treated with atypical antipsychotics. The patients were not taking any other psychotropic drug. While five of the patients had a history of sporadic drug use (cannabis), none of the patients had a history of chronic drug abuse. These five patients underwent urinary screening for other major drugs of abuse at admission, and the results were negative. Seventeen of the patients were followed longitudinally and were confirmed as having a diagnosis of DSM-IV schizophrenia at least 6 months after their participation in this study. Seven other patients were lost to follow up. Symptoms were assessed the day of scanning with the Positive and Negative Syndrome Scale. The present study was approved by the local institutional review board, and written informed consent was obtained from all subjects after the procedures had been fully explained.
1H-MRSI Procedure
1H-MRSI was performed on a conventional GE SIGNA 1.5-T NMR imaging system as previously described (6). The 1H-MRSI pulse sequence acquires four spectroscopic slices (TR=2200 msec, TE=272 msec) involving a 32×32 phase-encoding step over a 240-mm field of view for each slice. Based on full width at half maximum after filtering of k-space, each volume element (“voxel”) has dimensions of 1.4 ml. 1H-MRSI data processing involved locating N-acetylaspartate, choline, and creatine in spectra from each voxel and then displaying the four 32×32 arrays showing spatial variation of the magnitude of each of the signals in each of the slices. Regions of interest were drawn on coplanar MRIs as previously described (6), identifying the hippocampal area, dorsolateral prefrontal cortex, inferior frontal gyrus, superior temporal gyrus, occipital cortex, anterior and posterior cingulate, prefrontal white matter, centrum semiovale, putamen, and thalamus. Metabolites were studied as ratios of the area under each peak: N-acetylaspartate/creatine, N-acetylaspartate/choline, choline/creatine.
Neuropsychological Testing
Working memory was assessed the day of scanning in 14 patients and in 14 healthy subjects with the N-back task. The other subjects were not available for testing because the N-back software was not available yet. In contrast to other versions of the N-back task, our version of the task required subjects to continually update their mental set while responding to previously seen stimuli (numbers) (3). Stimuli were presented on a computer screen, and subjects were asked to respond with the numbers keyboard. “N-back” refers to how far back in the sequence of stimuli that the subject had to recall. The stimuli consisted of numbers (1–4) shown in random sequence and displayed at the points of a diamond-shaped box. There was a nonmemory guided control condition (0-back) that presented the same stimuli but simply required subjects to identify the stimulus currently seen. As memory load increased, the task required the recollection of a stimulus seen one stimulus (1-back) or two stimuli (2-back) earlier while continuing to encode additionally incoming stimuli. Performance data were recorded as the number of correct responses (accuracy).
Statistical Analysis
Differences between patients and comparison subjects were tested separately for each metabolite ratio and for each region of interest by a two-way repeated-measures analysis of variance (ANOVA), with hemisphere (left or right) as the within-group factor and diagnosis as the between-group factor. Post hoc analysis was performed with Tukey’s honestly significant difference test. ANOVA was also used to assess differences in neuropsychological performance between patients and healthy subjects. Correlations were assessed with Pearson’s test.
Results
In the hippocampal area, the patients with schizophreniform disorder and the healthy comparison subjects differed significantly in terms of the N-acetylaspartate/creatine ratio (mean=1.71 [SD=0.34] versus 1.97 [SD=0.29], respectively; F=10.3, df=1, 46, p<0.003) and the N-acetylaspartate/choline ratio (mean=1.06 [SD=0.24] versus 1.18 [SD=0.19]; F=4.06, df=1, 46, p<0.02). Post hoc analysis showed that patients had a significant bilateral reduction of N-acetylaspartate/creatine (p<0.03) (Figure 1) and N-acetylaspartate/choline (p<0.02). No main effect of hemisphere was found for N-acetylaspartate/creatine or N-acetylaspartate/choline. No main effect or interaction was found for choline/creatine.
Likewise, in the dorsolateral prefrontal cortex, the patients with schizophreniform disorder and the healthy comparison subjects differed significantly in terms of the N-acetylaspartate/creatine ratio (mean=2.05 [SD=0.38] versus 2.32 [SD=0.51], respectively; F=4.44, df=1, 46, p<0.05), and the between-group difference for the N-acetylaspartate/choline ratio approached significance (mean=1.54 [SD=0.25] versus 1.69 [SD=0.45]; F=2.2, df=1, 46, p=0.10). Post hoc analysis revealed that patients had a significant bilateral reduction of N-acetylaspartate/creatine (p<0.05) (Figure 1) and a difference in N-acetylaspartate/choline that approached significance (p=0.10). No main effect of hemisphere was found for either N-acetylaspartate/creatine or N-acetylaspartate/choline. No main effect or interaction was found for choline/creatine. For all metabolite ratios, no main effect or interaction was found in any other region of interest (results for the N-acetylaspartate/creatine ratio are shown in Figure 2). ANOVA revealed sporadic effects of hemisphere and no diagnosis-by-hemisphere interaction. None of the sporadic results, which were not based on a priori hypotheses or previous results, survived an appropriate Bonferroni correction for the number of regions, even at the p=0.10 threshold.
Repeated-measures ANOVA on the N-back data showed a significant effect of diagnosis (F=20.5, df=1, 26, p<0.001), a significant effect of task condition (F=62.7, df=2, 52, p<0.001), and a significant interaction between diagnosis and task condition (F=8.4, df=2, 52, p<0.001). Post hoc analysis showed that patients had lower accuracy than comparison subjects at the 1-back condition (mean=9.26 [SD=3.23] versus 13.7 [SD=0.41], respectively; p<0.01) and the 2-back condition (mean=6.9 [SD=2.4] versus 9.6 [SD=2.8]; p<0.01) but not at the 0-back condition (mean=13.5 [SD=0.9] versus 13.9 [SD=0.1]; p>0.50).
In the patients with schizophreniform disorder, there was a significant correlation between N-acetylaspartate/creatine in the right dorsolateral prefrontal cortex and working memory performance during the 2-back condition (Figure 3). A similar relationship was found for N-acetylaspartate/choline (r=0.39, df=12, p=0.10) but not for choline/creatine (r=0.03, df=12, p=0.90). For the healthy subjects, the correlation between N-acetylaspartate/creatine in the right dorsolateral prefrontal cortex and working memory performance during the 2-back condition was in the same direction but was not significant (r=0.23, df=12, p=0.30). There were no other significant correlations between any 1H-MRSI measures and N-back performance. Consistent with previous reports (14), we found a correlation between N-acetylaspartate/creatine in the right dorsolateral prefrontal cortex and negative symptom ratings from the Positive and Negative Syndrome Scale (r=–0.37, df=22, p=0.04, one-tailed). No other correlation was found between 1H-MRSI measures and clinical parameters (age, length of pharmacological treatment, chlorpromazine equivalents).
Discussion
We found reductions of N-acetylaspartate levels in the hippocampal area and the dorsolateral prefrontal cortex of patients with schizophreniform disorder compared with healthy subjects. We also found a specific correlation between N-acetylaspartate levels in the right dorsolateral prefrontal cortex and performance on the 2-back version of a working memory task in patients with schizophreniform disorder. This correlation is consistent with findings from an earlier schizophrenia study (5) in which correlation between N-acetylaspartate levels in the dorsolateral prefrontal cortex and performance and activation (assessed with functional magnetic resonance imaging [fMRI]) during a 2-back working memory condition was found only on the right. The lack of a statistical correlation between N-acetylaspartate levels in the dorsolateral prefrontal cortex and performance on the 0- and 1-back conditions as well as between N-acetylaspartate levels in the hippocampal area and working memory performance is also consistent with earlier studies and suggests that a threshold of sufficient functional load is necessary to elicit a predictable effect (5).
Our results are consistent with previous studies of chronic patients with schizophrenia that showed N-acetylaspartate reductions in the hippocampal area and dorsolateral prefrontal cortex. Therefore, neuronal pathology in these two brain areas, at least as reflected in diminished N-acetylaspartate measures, is not an epiphenomenon of being chronically ill or of being chronically treated with antipsychotics. It is also of interest that N-acetylaspartate levels in the dorsolateral prefrontal cortex correlated only with performance during the 2-back condition in the schizophreniform disorder patients. The functional neuroimaging literature in schizophrenia has indicated that dorsolateral prefrontal cortex dysfunction becomes consistently manifest if patients are engaged in tasks that depend on prefrontal strategies. The likely complexity and redundancy in prefrontal neuronal processing would be expected to dilute the predictive impact on prefrontal function of N-acetylaspartate measures alone. Our data are consistent with this contention in that the correlation is only found at the higher level of working memory load. Lack of statistical correlation between N-acetylaspartate levels in the hippocampal area and working memory performance is also consistent with this idea and with earlier studies (5).
The correlation between N-acetylaspartate levels in the dorsolateral prefrontal cortex and performance during the 2-back condition of the working memory test did not reach statistical significance in the comparison subjects, even though it was in the same direction as in patients. Earlier 1H-MRSI work in healthy subjects had shown that N-acetylaspartate levels in occipitoparietal white matter correlated positively with an index of general neuropsychological performance (26) and with full-scale IQ measured with the Wechsler Adult Intelligence Scale (27). Both of these studies involved a larger number of subjects (N=43 and N=26, respectively) and a decidedly different brain region. Our data are partially consistent with this earlier work in that no significant correlations were seen in the healthy subjects, even though the direction was the same. Lack of consistency with this earlier work may be ascribed to several reasons. One possibility is that we studied a smaller number of subjects. Another likely explanation is that we investigated a different anatomical region and a more specific neuropsychological function. On the other hand, lack of statistical correlation in healthy subjects suggests the possibility that dorsolateral prefrontal cortex neurons identified by low N-acetylaspartate levels may be critical effectors of the cortical pathophysiology implicated in the working memory deficits of schizophrenia. To the extent that reduced N-acetylaspartate measures are a reflection of impaired functional integrity of neurons, these dorsolateral prefrontal cortex neurons may constitute a rate-limiting factor for working memory performance. Therefore, this putative impairment may constrain the variance in a predictable way, making the correlation in patients more evident than in healthy subjects. This assumption also is consistent with earlier data that showed a correlation between prefrontal N-acetylaspartate measures and activation of the distributed working memory cortical network in patients but not in normal subjects (22). These data were obtained in two groups of patients during performance of the Wisconsin Card Sorting Test and the N-back test. Both data sets showed more pronounced correlations between N-acetylaspartate levels in the right dorsolateral prefrontal cortex and regional cerebral blood flow during performance of the tasks in patients but not in comparison subjects, once again suggesting that the correlation is more robust in patients. Moreover, the selectivity of the correlation between 2-back working memory performance and N-acetylaspartate measures in the right dorsolateral prefrontal cortex is consistent with prior functional imaging studies. The spatial component of the N-back task is important, as numbers flash in four different locations. In fact, the cortical network activated with fMRI by our version of the N-back task is typical for spatial working memory tasks involving the left and right dorsolateral prefrontal cortex as well as bilaterally in the parietal cortex (3, 5). Given that the region that most clearly differentiates between patients and comparison subjects is the right dorsolateral prefrontal cortex (5), it is not surprising that the correlation is found only on that side.
The physiological role of N-acetylaspartate in neurons has yet to be fully elucidated. In the mature brain (when glial and neuronal cells are completely differentiated), N-acetylaspartate is found almost exclusively in neurons and in highest concentrations in pyramidal glutamatergic neurons (28). A recent in vitro study has also reported that oligodendrocytes express small concentrations of N-acetylaspartate (29). N-Acetylaspartate synthesis takes place in the mitochondria (30), is ADP dependent (30), and is tightly coupled to glucose metabolism (31). The synthetic reaction is a transamination catalyzed by l-aspartate-N-acetyl transferase that uses glutamate (source for aspartate) and either pyruvate or 3-hydroxybutyrate (source of Acetyl CoA) as substrates (30). Moreover, N-acetylaspartate acts via the glutamatergic NMDA receptor to elevate intracellular calcium (32); its concentrations are reduced by pharmacological inhibition of mitochondrial energy metabolism; and it correlates highly with the relative reduction of ATP and O2 consumption (16). All these findings are consistent with a recent proposal that N-acetylaspartate serves as an osmolyte used by molecular water pumps to expel metabolic water out of neurons (33). In this model, it has been tentatively calculated that 1 mol of N-acetylaspartate is produced for 40 mol of glucose or glucose equivalent oxidized in the brain (33), once again stressing the relationship between N-acetylaspartate and glucose metabolism. This basic science literature is paralleled by a series of studies in humans that have demonstrated the reversibility of N-acetylaspartate reductions, thereby suggesting that N-acetylaspartate is sensitive to pathological processes affecting the functioning of neurons (16–21). Moreover, a recent study in healthy subjects and patients with dementia combining 1H-MRSI and [18F]fluorodeoxyglucose positron emission tomography has demonstrated a positive linear relationship between cerebral metabolic rates for glucose and N-acetylaspartate concentrations in cortical gray matter (20). All these studies suggest that N-acetylaspartate concentrations may vary according to the metabolic status of neurons, especially pyramidal neurons.
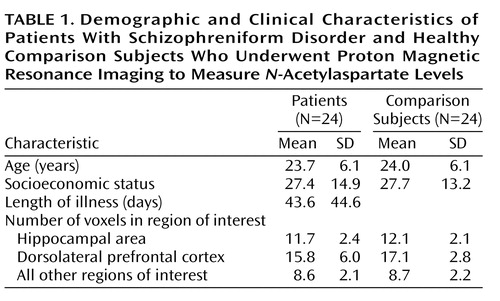
A limitation of the present study is that we did not measure absolute concentrations of N-acetylaspartate. However, the pattern of reductions and of correlations strongly suggests that N-acetylaspartate levels are accounting for the changes and the correlations (34). Another limitation is that we cannot address the gray or white matter origin of these N-acetylaspartate effects because of lack of tissue segmentation. This is important because a 1.4-ml voxel placed in the cortex certainly contains sulcal CSF (no N-acetylaspartate) and white matter (lower N-acetylaspartate than gray matter—as shown by Lim et al. [35]). Therefore, a systematic difference in voxel placement/selection could result in different tissue composition in the voxels between groups and account for differences in the measured spectra. Also, use of ratios only partially addresses this limitation, since there is evidence that metabolites can vary independently (36). To try to address the potential confounder addressed by segmentation correction, i.e., biased voxel placement or anatomical variation in tissue compartments across groups, we performed further analyses. CSF, white matter, and gray matter have different T1 relaxation, thus providing different signal intensities. On the basis of this principle, we reasoned that if the dorsolateral prefrontal cortex and the hippocampal area contained systematically different quantities of CSF, white matter, and gray matter in patients and comparison subjects, some difference in signal intensity in those regions of interest should be measurable between the two groups. Therefore, we superimposed the spectroscopic regions of interest from the dorsolateral prefrontal cortex and the hippocampal area onto the T1-weighted images and measured the signal intensity. Since the signal intensity can be influenced by several factors in each individual, we then normalized the signal intensity from the region of interest to the signal intensity of the whole MRI slice. Statistical comparison between patients and comparison subjects on the normalized signal intensity from the dorsolateral prefrontal cortex and the hippocampal area did not reveal any significant difference (all t<1.4, all p>0.20). Also, the number of voxels included in regions of interest did not systematically differ between patients and comparison subjects (all t<1, all p>0.20) (Table 1).
In conclusion, the results of the present study suggest that neuronal pathology in the hippocampal area and the dorsolateral prefrontal cortex may be present even at the onset of schizophrenia and is not the result of chronicity. Moreover, the neuronal changes in the dorsolateral prefrontal cortex monitored with this assay seem to be associated with poor performance on working memory tasks.
 |
Received May 6, 2002; revision received Sept. 23, 2002; accepted Sept. 26, 2002. From the Psychiatric Neuroscience Group, Section on Mental Disorders, Department of Psychiatric and Neurological Sciences, University of Bari, Italy; Clinical Brain Disorders Branch, National Institute of Mental Health, Bethesda, Md.; and the Department of Neuroradiology, IRCCSS “Casa Sollievo della Sofferenza,” San Giovanni Rotondo (FG), Italy. Address reprint requests to Dr. Bertolino, Gruppo di Neuroscienze Psichiatriche, Sezione Clinica Malattie Mentali, Dipartimento di Scienze Neurologiche e Psichiatriche, Universita′ degli Studi di Bari, Piazza Giulio Cesare–9, 70124 Bari, Italy; [email protected] (e-mail). Supported by a grant from the Theodore and Vada Stanley Foundation.
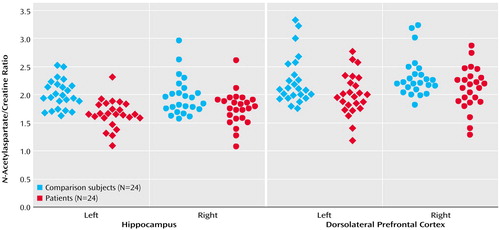
Figure 1. N-Acetylaspartate/Creatine Ratios in the Hippocampus and Dorsolateral Prefrontal Cortex of Patients With Schizophreniform Disorder and Age- and Gender-Matched Healthy Comparison Subjects
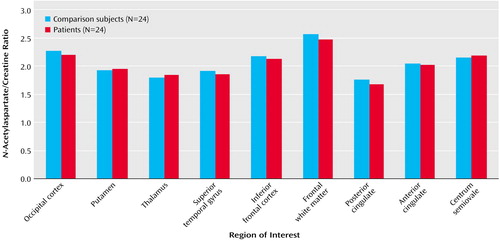
Figure 2. Regional Variations in the N-Acetylaspartate/Creatine Ratio in Patients With Schizophreniform Disorder and Age- and Gender-Matched Healthy Comparison Subjects
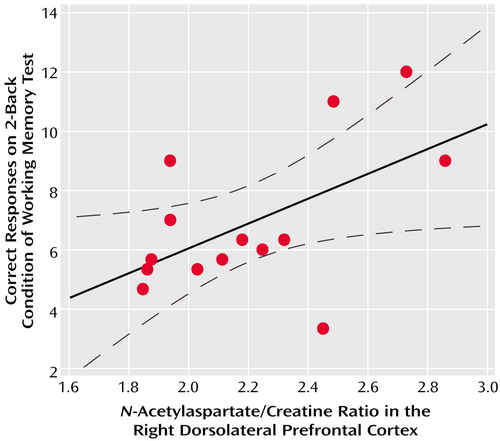
Figure 3. Correlations Between the N-Acetylaspartate/Creatine Ratio in the Right Dorsolateral Prefrontal Cortex and Working Memory Performance in 14 Patients With Schizophreniform Disordera
ar=0.55, df=12, p=0.04.
1. Goldman-Rakic PS: The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry 1999; 46:650-661Crossref, Medline, Google Scholar
2. Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE: Temporal dynamics of brain activation during a working memory task. Nature 1997; 386:604-608Crossref, Medline, Google Scholar
3. Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR: Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex 1999; 9:20-26Crossref, Medline, Google Scholar
4. Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S: Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry 1999; 45:1128-1137Crossref, Medline, Google Scholar
5. Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR: Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 2000; 10:1078-1092Crossref, Medline, Google Scholar
6. Bertolino A, Nawroz S, Mattay VS, Barnett AS, Duyn JH, Moonen CTW, Frank JA, Tedeschi G, Weinberger DR: Regionally specific pattern of neurochemical pathology in schizophrenia as assessed by multislice proton magnetic resonance spectroscopic imaging. Am J Psychiatry 1996; 153:1554-1563Link, Google Scholar
7. Deicken RF, Zhou L, Corwin F, Vinogradov S, Weiner MW: Decreased left frontal lobe N-acetylaspartate in schizophrenia. Am J Psychiatry 1997; 154:688-690Link, Google Scholar
8. Brooks WM, Hodde-Vargas J, Vargas LA, Yeo RA, Ford CC, Hendren RL: Frontal lobe of children with schizophrenia spectrum disorders: a proton magnetic resonance spectroscopic study. Biol Psychiatry 1998; 43:263-269Crossref, Medline, Google Scholar
9. Cecil KM, Lenkinski RE, Gur RE, Gur RC: Proton magnetic resonance spectroscopy in the frontal and temporal lobes of neuroleptic naive patients with schizophrenia. Neuropsychopharmacology 1998; 20:131-140Crossref, Google Scholar
10. Bartha R, al-Semaan YM, Williamson PC, Drost DJ, Malla AK, Carr TJ, Densmore M, Canaran G, Neufeld RW: A short echo proton magnetic resonance spectroscopy study of the left mesial-temporal lobe in first-onset schizophrenic patients. Biol Psychiatry 1999; 45:1403-1411Crossref, Medline, Google Scholar
11. Kegeles LS, Shungu DC, Anjilvel S, Chan S, Ellis SP, Xanthopoulos E, Malaspina D, Gorman JM, Mann JJ, Laruelle M, Kaufmann CA: Hippocampal pathology in schizophrenia: magnetic resonance imaging and spectroscopy studies. Psychiatry Res 2000; 98:163-175Crossref, Medline, Google Scholar
12. Bertolino A, Callicott JH, Mattay VS, Weidenhammer KM, Rakow R, Egan MF, Weinberger DR: The effect of treatment with antipsychotic drugs on brain N-acetylaspartate measures in patients with schizophrenia. Biol Psychiatry 2001; 49:39-46Crossref, Medline, Google Scholar
13. Braus DF, Ende G, Weber-Fahr W, Demirakca T, Henn FA: Favorable effect on neuronal viability in the anterior cingulate gyrus due to long-term treatment with atypical antipsychotics: an MRSI study. Pharmacopsychiatry 2001; 34:251-253Crossref, Medline, Google Scholar
14. Ende G, Braus DF, Walter S, Weber-Fahr W, Soher B, Maudsley AA, Henn FA: Effects of age, medication, and illness duration on the N-acetyl aspartate signal of the anterior cingulate region in schizophrenia. Schizophr Res 2000; 41:389-395Crossref, Medline, Google Scholar
15. Bustillo JR, Lauriello J, Rowland LM, Jung RE, Petropoulos H, Hart BL, Blanchard J, Keith SJ, Brooks WM: Effects of chronic haloperidol and clozapine treatments on frontal and caudate neurochemistry in schizophrenia. Psychiatry Res 2001; 107:135-149Crossref, Medline, Google Scholar
16. Bates TE, Strangward M, Keelan J, Davey GP, Munro PMG, Clark JB: Inhibition of N-acetylaspartate production: implications for 1H MRS studies. Neuroreport 1996; 7:1397-1400Crossref, Medline, Google Scholar
17. De Stefano N, Matthews P, Antel JP, Preul M, Francis G, Arnold DL: Chemical pathology of acute demyelinating lesions and its correlations with disability. Ann Neurol 1995; 38:901-909Crossref, Medline, Google Scholar
18. Hugg JW, Kuzniecky RI, Gilliam FG, Morawetz RB, Fraught RE, Hetherington HP: Normalization of contralateral metabolic function following temporal lobectomy demonstrated by 1H magnetic resonance spectroscopic imaging. Ann Neurol 1996; 40:236-239Crossref, Medline, Google Scholar
19. Jenkins BG, Klivenyi P, Kustermann E, Andreassen OA, Ferrante RJ, Rosen BR, Beal MF: Nonlinear decrease over time in N-acetyl aspartate levels in the absence of neuronal loss and increases in glutamine and glucose in transgenic Huntington’s disease mice. J Neurochem 2000; 74:2108-2119Crossref, Medline, Google Scholar
20. O’Neill J, Eberling JL, Schuff N, Jagust W, Reed B, Soto G, Ezekiel F, Klein G, Weiner MW: Method to correlate 1H MRSI and 18FDG-PET. Magn Reson Med 2000; 43:244-250Crossref, Medline, Google Scholar
21. Vion-Dury J, Salvan AM, Confort-Gouny S, Dhiver C, Cozzone P: Reversal of brain metabolic alterations with zidovudine detected by proton localised magnetic resonance spectroscopy. Lancet 1995; 345:60-61Crossref, Medline, Google Scholar
22. Bertolino A, Esposito G, Callicott JH, Mattay VS, Van Horn JD, Frank JA, Berman KF, Weinberger DR: Specific relationship between prefrontal neuronal N-acetylaspartate and activation of the working memory cortical network in schizophrenia. Am J Psychiatry 2000; 157:26-33Link, Google Scholar
23. Callicott JH, Bertolino A, Egan MF, Mattay VS, Langheim FJP, Weinberger DR: Selective relationship between prefrontal N-acetylaspartate measures and negative symptoms in schizophrenia. Am J Psychiatry 2000; 157:1646-1651Link, Google Scholar
24. Iancu I, Dannon PN, Ziv R, Lepkifker E: A follow-up study of patients with DSM-IV schizophreniform disorder. Can J Psychiatry 2002; 47:56-60Medline, Google Scholar
25. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders Research Version (SCID-I). New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
26. Jung RE, Yeo RA, Chiulli SJ, Sibbitt WL Jr, Weers DC, Hart BL, Brooks WM: Biochemical markers of cognition: a proton MR spectroscopy study of normal human brain. Neuroreport 1999; 10:3327-3331Crossref, Medline, Google Scholar
27. Jung RE, Brooks WM, Yeo RA, Chiulli SJ, Weers DC, Sibbitt WL Jr: Biochemical markers of intelligence: a proton MR spectroscopy study of normal human brain. Proc R Soc Lond B Biol Sci 1999; 266:1375-1379Crossref, Google Scholar
28. Urenjak J, Williams SR, Gadian DG, Noble M: Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci 1993; 13:981-989Crossref, Medline, Google Scholar
29. Bhakoo KK, Pearce D: In vitro expression of N-acetyl aspartate by oligodendrocytes: implications for proton magnetic resonance spectroscopy in vivo. J Neurochem 2000; 74:254-262Crossref, Medline, Google Scholar
30. Clark JB: N-Acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci 1998; 20:271-276Crossref, Medline, Google Scholar
31. Moreno A, Ross BD, Blüml S: Direct determination of the N-acetyl-l-aspartate synthesis rate in the human brain by 13C MRS and [1-13C]glucose infusion. J Neurochem 2001; 77:347-350Crossref, Medline, Google Scholar
32. Rubin Y, LaPlaca MC, Smith DH, Thibault LE, Lenkinski RE: The effect of N-acetylaspartate on the intracellular calcium concentration in NTera2-neurons. Neurosci Lett 1995; 198:209-212Crossref, Medline, Google Scholar
33. Baslow MH, Kitada K, Suckow RF, Hungund BL, Serikawa T: The effects of lithium chloride and other substances on levels of brain N-acetyl-l-aspartic acid in Canavan disease-like rats. Neurochem Res 2002; 27:403-406Crossref, Medline, Google Scholar
34. Friedman SD, Brooks WM, Jung RE, Hart BL, Yeo RA: Proton MR spectroscopic findings correspond to neuropsychological function in traumatic brain injury. AJNR Am J Neuroradiol 1998; 19:1879-1885Medline, Google Scholar
35. Lim KO, Adalsteinsson E, Spielman D, Sullivan EV, Rosenbloom MJ, Pfefferbaum A: Proton magnetic resonance spectroscopic imaging of cortical gray and white matter in schizophrenia. Arch Gen Psychiatry 1998; 55:346-352Crossref, Medline, Google Scholar
36. Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan EV, Lim KO: In vivo spectroscopic quantification of the N-acetyl moiety, creatine, and choline from large volumes of brain gray and white matter: effects of normal aging. Magn Reson Med 1999; 41:276-284Crossref, Medline, Google Scholar



