Imaging Microglial Activation in Untreated First-Episode Psychosis: A PET Study With [18F]FEPPA
Abstract
Objective:
Neuroinflammation and abnormal immune responses are increasingly implicated in the pathophysiology of schizophrenia. Previous positron emission tomography (PET) studies targeting the translocator protein 18 kDa (TSPO) have been limited by high nonspecific binding of the first-generation radioligand, low-resolution scanners, small sample sizes, and psychotic patients being on antipsychotics or not being in the first episode of their illness. The present study uses the novel second-generation TSPO PET radioligand [18F]FEPPA to evaluate whether microglial activation is elevated in the dorsolateral prefrontal cortex and hippocampus of untreated patients with first-episode psychosis.
Method:
Nineteen untreated patients with first-episode psychosis (14 of them antipsychotic naive) and 20 healthy volunteers underwent a high-resolution [18F]FEPPA PET scan and MRI. Dynamic PET data were analyzed using the validated two-tissue compartment model with arterial plasma input function with total volume of distribution (VT) as outcome measure. All analyses were corrected for TSPO rs6971 polymorphism (which is implicated in differential binding affinity).
Results:
No significant differences were observed between patients and healthy volunteers in microglial activation, as indexed by [18F]FEPPA VT, in either the dorsolateral prefrontal cortex or the hippocampus. There were no significant correlations between [18F]FEPPA VT and duration of illness, clinical presentation, or neuropsychological measures after adjusting for multiple testing.
Conclusions:
The lack of significant differences in [18F]FEPPA VT between groups suggests that microglial activation is not present in first-episode psychosis.
Microglia are a key player in the immune surveillance system of the CNS, where they act as resident macrophages and are the first responders to various types of brain insult (1). As part of the brain inflammatory response, microglia are transformed from a “sentry” state into an “active” state (1, 2). In this process, the microglia change their morphology from branched to globular, with a large increase in cell volume, while migrating to and engulfing the site of insult (3, 4). Activated microglia (for a review, see reference 5) express elevated levels of a protein in their mitochondria known as translocator protein 18 kDa (TSPO) (6). Because TSPO is overexpressed in response to neuroinflammation as compared with normal tissue, it represents an important imaging target for microglial activation.
Over the years, research has focused on the immune response and neuroinflammation as likely contributors to schizophrenia (7–14). Genome-wide association studies (GWAS) have reported links between schizophrenia and genetic variants in the major histocompatibility complex (MHC) genes, supporting the involvement of the immune system in the pathogenesis of schizophrenia (15, 16). Several lines of research support these GWAS findings: 1) in animal models, an induced maternal immune response (administration of synthetic viral analogue polyriboinosinic-polyribocytidillic [poly I:C] acid [17]) results in behavioral, neurochemical (18–20), and structural changes (17) in the brains of the offspring that resemble the immune alterations implicated in schizophrenia; 2) prenatal exposure to a variety of infections has been linked with an increased risk of developing schizophrenia (21–25); and 3) elevated plasma levels of proinflammatory cytokines in schizophrenia provide evidence for the involvement of peripheral inflammatory response (for a review, see reference 26). However, postmortem studies examining microglia abnormality in schizophrenia are inconclusive; increased microglia activation (27), decreased microglial activation (28), and no difference in microglial activation (29) have been reported. Given the limitations of postmortem studies, positron emission tomography (PET) with TSPO radioligands provides an opportunity to study microglial activation in schizophrenia in vivo.
To date, of five PET studies that have investigated this question in schizophrenia patients on antipsychotic treatment (30–34), three have reported results in line with increased microglial activation in schizophrenia (30–32). The first two of these (30, 31) showed increased binding potential of [11C]PK11195 in schizophrenia compared with healthy volunteers in total gray matter and in the hippocampus, respectively. However, imaging studies using this early radiotracer were hindered by its recognized methodological limitations (35). Using the new generation of TSPO radiotracer [11C]DAA1106, Takano et al. (33) found no difference in binding between schizophrenia patients who had received chronic treatment (N=14) and healthy volunteers (N=14), but they reported significant positive associations between tracer binding, duration of illness, and positive psychotic symptoms. Our recent study evaluating microglial activation in 18 treated patients with schizophrenia (34) using [18F]FEPPA and controlling for TSPO genotype (rs6971 polymorphism) found neither a difference between groups nor any correlation between tracer binding and symptom severity, cognition, or duration of illness. While a recent study using another second-generation TSPO radioligand showed higher [11C]PBR28 distribution volume ratios (DVRs—a different outcome measure reflecting change relative to another brain region) in total gray matter, frontal lobes, and temporal lobes of schizophrenia patients relative to comparison subjects (32), no significant differences were found between the groups when using the validated [11C]PBR28 outcome measure total distribution volume (VT) (36).
Recent studies with new-generation PET radioligands targeting TSPO report significant intersubject variability in binding. On the basis of analyses performed in brain tissue and platelets, three distinct levels of binding affinity have been noted, with participants grouped as high-affinity binders, low-affinity binders, and mixed-affinity binders (37–39). A polymorphism in exon 4 of the TSPO gene (rs6971) has been implicated in this differential binding affinity (40), suggesting that rs6971 gene polymorphism needs to be considered in the quantification of second-generation TSPO radioligands, including [18F]FEPPA (41). Using this methodology, recent PET studies in Alzheimer’s disease and major depressive episode using [18F]FEPPA have demonstrated the ability of this radiotracer to quantify microglia activation in humans (42, 43).
In the present study, we used the validated [18F]FEPPA VT to examine microglial activation in untreated patients in first-episode psychosis with either minimal (less than 4 weeks) or no lifetime exposure to antipsychotics. We explored the association between microglial activation and severity of psychopathology as well as neuropsychological deficits, given reported positive associations between microglial activation and severity of symptoms in schizophrenia (32, 33). We also explored DVRs as a pseudo-reference region method—in this case, the ratio of [18F]FEPPA VT in the region of interest to VT in cerebellum, gray matter, or whole brain (44).
Method
Subjects
Twenty-three untreated patients with psychosis and 20 matched healthy volunteers were initially enrolled and scanned in this study. In the patient group, two who were low-affinity binders and two whose PET images were of insufficient quality were excluded, leaving 19 patients for analysis. All patients were either antipsychotic free with less than 4 weeks of lifetime cumulative exposure (N=5) or antipsychotic naive (N=14). Fourteen of the healthy volunteers were included in our previous cohort (34); the patient populations do not overlap between the studies.
To be eligible for the study, first-episode psychosis patients had to have a diagnosis of schizophreniform disorder, delusional disorder, schizophrenia, or psychosis not otherwise specified, as determined with the Structured Clinical Interview for DSM-IV Axis I Disorders (45), and no concurrent axis I disorders, such as major depressive disorder, which has been shown to be associated with microglial activation (42). Healthy volunteers with any history of psychiatric illness or first-degree relatives with a major mental disorder were excluded. Exclusion criteria for all subjects included a current diagnosis of substance dependence or abuse, pregnancy or current breastfeeding, clinically significant medical illness, and the presence of metal implants precluding MRI.
In the patient group, neurocognitive performance was assessed using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (46). The clinical status of psychosis was assessed with the Positive and Negative Syndrome Scale (PANSS) (47), the Calgary Depression Scale, the Snaith-Hamilton Pleasure Scale, the Scale for the Assessment of Negative Symptoms (SANS), the Global Assessment of Functioning Scale (GAF), and the Apathy Evaluation Scale. Assessments are referenced in the data supplement that accompanies the online edition of this article.
The study was approved by the Research Ethics Board at the Centre for Addiction and Mental Health and the University of Toronto. All participants provided written informed consent after receiving a description of all study procedures.
PET and MRI Data Acquisition and Analysis
Details for PET and MRI data acquisition have been described elsewhere and are summarized below and in the online data supplement. All PET scans were performed using a high-resolution research tomography scanner (Siemens Molecular Imaging, Knoxville, Tenn.) for 125 minutes following an intravenous bolus injection of [18F]FEPPA (mean=182.94 MBq, SD=13.08). Arterial blood samples were collected both automatically (with an automatic blood sampling system: model PBS-101, Veenstra Instruments, Joure, the Netherlands) and manually to measure radioactivity in blood and determine the relative proportion of radiolabeled metabolites. The dispersion- and metabolite-corrected plasma input function was generated as previously described (41).
Image processing and calculation of total distribution volumes (VT).
Time-activity curves were extracted for the dorsolateral prefrontal cortex, the hippocampus, the medial prefrontal cortex, the temporal cortex, total gray matter, and whole brain using a validated in-house imaging pipeline (the ROMI software program) (48). All regions of interests were delineated using proton density MRI in each participant (48). The kinetic parameters of [18F]FEPPA were derived from the time-activity curves using the two-tissue compartment model and plasma input function to obtain the VT for each region of interest, a method that has been validated for [18F]FEPPA quantification and has been described elsewhere (34, 49). PET images were also corrected for partial volume effect using the approach described by Müller-Gärtner et al. (50); the results are presented in the online data supplement (see Figure S1).
Estimation of [18F]FEPPA pseudo-reference-region distribution volume ratio (DVR).
For exploratory purposes, we investigated the difference between the groups using DVR as an outcome measure. DVR is defined as regional VT normalized by VT in the cerebellum, gray matter, or whole brain (DVR=VT_region/VT_k, where k represents cerebellum, gray matter, or whole brain). This method has been used in other TSPO PET studies (32, 51) and is suggested to reduce variability in the data (51).
Voxel-based PET image analysis.
Parametric images of [18F]FEPPA VT were generated using the Logan graphical analysis method to examine voxel-wise group differences of VT. More details are provided in the data supplement.
rs6971 Polymorphism Genotyping
Participants were categorized on the basis of the TSPO rs6971 as high-affinity (C/C), mixed-affinity (C/T), and low-affinity (T/T) binders, as described elsewhere (40, 41). Details of the genotyping procedures are provided in the data supplement.
Statistical Analysis
Demographic measures were examined for any group differences using independent-sample t tests for continuous variables and chi-square tests for categorical variables. Multivariate analysis of variance (MANOVA), with regional VT values and DVRs as the dependent variables, group (patients, healthy volunteers) as the independent variable, and the TSPO genotype (rs6971) as a covariate, were carried out to test for differences in [18F]FEPPA VT and DVR values between clinical groups. Partial correlations were used to examine the association between [18F]FEPPA VT and DVR values and a number of clinical variables, including the duration of untreated psychosis, duration of illness, age at illness onset, number of acute crises, clinical presentation, and neuropsychological measures (controlling for the effects of TSPO rs6971 polymorphism). All statistical analyses were performed using SPSS, version 22.0 (IBM, Armonk, N.Y.), with p values <0.05 considered significant. Bonferroni correction was used to correct for multiple comparisons in regions we set out to test (i.e., the dorsolateral prefrontal cortex and hippocampus only). We also report, for descriptive purposes, differences in the medial prefrontal cortex, the temporal cortex, total gray matter, and whole brain, with VT and DVR data.
Results
Participant Characteristics and Injection Parameters
Participants’ demographic and clinical characteristics are presented in Table 1. Among the 19 psychotic subjects, 14 were antipsychotic naive, and 15 were within 5 years of the first episode of their illness at the time of scanning. Except for amount injected, which was significantly higher in the first-episode psychosis group (F=7.43, p=0.01), PET radiotracer injection parameters did not differ between the patient and healthy groups.
| Measure | Healthy Volunteers (N=20) | First-Episode Psychosis Patients (N=19) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Male | 9 | 45.0 | 12 | 63.2 |
| Genotypea | ||||
| High-affinity binder | 14 | 70.0 | 14 | 73.7 |
| Mixed-affinity binder | 6 | 30.0 | 5 | 26.3 |
| Mean | SD | Mean | SD | |
| Age (years) | 27.75 | 8.77 | 27.53 | 6.7 |
| PET measures ([18F]FEPPA) | ||||
| Amount injectedb (mCi) | 4.80 | 0.35 | 5.09 | 0.20 |
| Specific activity (mCi/µmol) | 4659.7 | 4130.2 | 2859.4 | 2797.2 |
| Mass injected (µg) | 0.93 | 0.95 | 1.02 | 0.50 |
| Age at illness onset (years) | 24.0 | 8.0 | ||
| Number of episodes | 1.4 | 1.2 | ||
| Duration of illness (months) | 33.6 | 40.1 | ||
| Calgary Depression Scalec | 3.7 | 3.3 | ||
| Apathy Evaluation Scale | 34.3 | 10.2 | ||
| Snaith-Hamilton Pleasure Scale | 2.1 | 3.2 | ||
| Positive and Negative Syndrome Scale | ||||
| Total score | 68.6 | 13.0 | ||
| Positive score | 19.2 | 3.8 | ||
| Negative score | 16.1 | 6.1 | ||
| General psychopathology score | 33.4 | 7.3 | ||
| Repeatable Battery for the Assessment of Neuropsychological Statusc | ||||
| Total score | 80.9 | 17.2 | ||
| Immediate memory score | 83.2 | 20.0 | ||
| Visuospatial memory score | 83.9 | 16.8 | ||
| Language score | 82.0 | 20.9 | ||
| Attention score | 88.4 | 22.3 | ||
| Delayed memory score | 85.4 | 18.6 | ||
TABLE 1. Participants’ Demographic and Clinical Characteristics and Radioligand Injection Parameters in a Positron Emission Tomography (PET) Study of Microglial Activation in Schizophrenia
Group Differences in [18F]FEPPA VT
After controlling for the rs6971 polymorphism, no significant effect of group (healthy volunteers versus patients) was observed on [18F]FEPPA VT values (Figure 1). The lack of a group effect was observed after controlling for age and/or tobacco use and also with the correction for partial volume effects (see Figure S1 in the data supplement). The results were consistent with other exploratory regions of interest and remained so after excluding the four patients with more than 5 years since their first episode (see the Supplemental Results section and Table S1 in the data supplement).
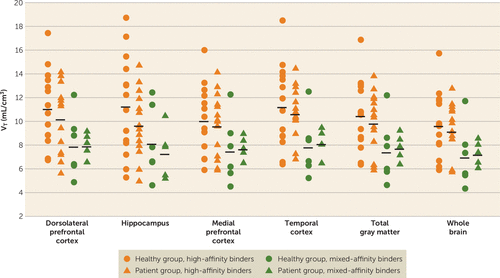
FIGURE 1. Total Distribution Volume (VT) of [18F]FEPPA in Patients With First-Episode Psychosis and Healthy Volunteers Across Different Regions of Interesta
a Horizontal black lines represent mean values.
Exploratory Analysis of DVRs With Cerebellum, Whole Brain, or Gray Matter as Denominator
First, there was no significant group effect on [18F]FEPPA VT in the cerebellum, whole brain, or gray matter before or after correction for partial volume effects (see Tables S2–S4 in the data supplement).
We found no significant effect of clinical group with any of the DVR methods used. While not statistically significant, higher DVR values were observed in healthy volunteers in the hippocampus and dorsolateral prefrontal cortex using all the DVR methods. Results obtained before and after correction for partial volume effect and also for other regions of interest are reported in Tables S2–S4 in the data supplement.
Voxel-Based Analyses
Congruent with results of the region-of-interest analyses, we did not find any group differences using the region-of-interest independent voxel-based analysis, confirming the lack of difference in [18F]FEPPA VT between groups (see Figure S2 in the data supplement).
Correlation Between [18F]FEPPA VT and Duration of Illness, Symptom Severity, Clinical Presentation, and Neuropsychological Measures
There were no significant correlations between [18F]FEPPA VT (before and after partial volume error correction) and age at illness onset, number of psychotic episodes, duration of illness, anhedonia as measured by the Snaith-Hamilton Pleasure Scale, general functioning as measured by the GAF, and apathy as measured by the Apathy Evaluation Scale. Interestingly, RBANS total score was significantly associated with [18F]FEPPA VT in the hippocampus (r=0.50, p=0.04; with partial volume effect correction, r=0.51, p=0.04), such that higher [18F]FEPPA VT in the hippocampus was associated with better overall cognitive performance (Figure 2). Follow-up analysis revealed a significant contribution of the RBANS attention subscale (r=0.49, p=0.05). Moreover, we found a negative association between [18F]FEPPA VT in the hippocampus and SANS attention subscore both with and without partial volume correction (r=−0.48, p=0.04) (see Tables S5–S9 in the data supplement). After excluding the four patients with more than 5 years since diagnosis, we found a significant negative correlation between PANSS general psychopathology subscore and [18F]FEPPA VT in gray matter before and after correction for partial volume effects (see Table S10 in the data supplement), such that higher [18F]FEPPA VT was associated with lower PANSS general psychopathology subscore. None of these correlations survived Bonferroni correction.
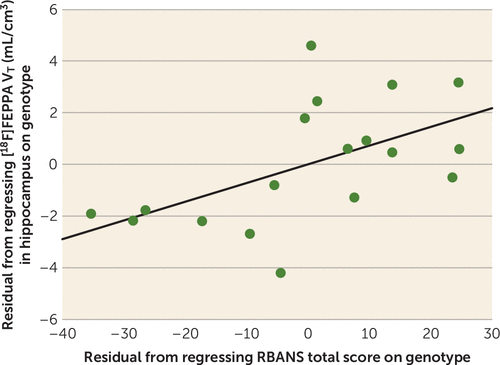
FIGURE 2. Relationship Between [18F]FEPPA VT in Hippocampus and RBANS Total Score in First-Episode Psychosis
Discussion
To the best of our knowledge, this is the first PET study to evaluate microglial activation using a second-generation TSPO radioligand in untreated, mostly antipsychotic-naive patients in first-episode psychosis.
Early PET studies using [11C]PK11195 reported increased tracer binding in the hippocampus of patients with schizophrenia (30) and in the total gray matter of patients with recent-onset schizophrenia (31). Based on previous reports (27, 30) and a postmortem study using second-generation [3H]PBR28 (27), we expected significantly higher [18F]FEPPA binding in the hippocampus and dorsolateral prefrontal cortex of patients with first-episode psychosis compared with healthy volunteers. In the present study, despite the use of a second-generation TSPO radioligand and scanning all untreated (mostly antipsychotic naive) first-episode patients in a high-resolution research tomography scanner, we observed no significant differences in [18F]FEPPA binding between groups. While not statistically significant, [18F]FEPPA uptake was higher in the healthy group than in the patient group in the hippocampus (13.4% higher) and the dorsolateral prefrontal cortex (6% higher). Additionally, we explored [18F]FEPPA binding in the medial prefrontal cortex, the temporal cortex, total gray matter, and whole brain and obtained similar results. Nevertheless, our results are in line with other investigations using second-generation TSPO ligands, such as a study that found no differences in [11C]DAA1106 binding in chronic schizophrenia patients (33) and our previous study using [18F]FEPPA in patients with schizophrenia who had received chronic treatment (34). The present study therefore confirms these findings in a larger sample and in untreated, mostly first-episode patients.
While VT is the gold standard to quantify [18F]FEPPA binding (49), because there is no reference region available, DVR has been proposed as an alternative measure (44). Thus, we also present regional DVR values showing no significant differences between the patient and healthy groups. This finding is inconsistent with the recent study (32) using the second-generation TSPO radioligand [11C]PBR28, which showed higher microglial activation indexed as DVRs in total gray matter and the frontal and temporal lobes of schizophrenia patients compared with healthy volunteers. It should be noted that, using the gold-standard two-tissue compartment model with [11C]PBR28 VT as the outcome measure, the authors of that study did not find any significant difference between schizophrenia patients and healthy volunteers, in line with the present findings and with other studies using second-generation radioligands (33, 34).
The variability of VT in this study, as with other second-generation TSPO ligands, was relatively high even after controlling for the effect of rs6971 polymorphism on binding affinity. However, sample size calculations using the present data suggest that to detect group effects (i.e., higher microglial activation in healthy volunteers than in first-episode patients) in the dorsolateral prefrontal cortex (the observed effect size was 0.22) or the hippocampus (the observed effect was 0.42), we would need 316 or 89 subjects per group, respectively, for a significance level of 0.05 (two-tailed) and 80% power in each brain region.
In patients with first-episode psychosis, we found a trend toward positive correlation between [18F]FEPPA VT in the hippocampus and RBANS total score, suggesting that higher microglial activation in the hippocampus is associated with better cognitive function. Similar trends were present when exploring associations between [18F]FEPPA VT and clinical measures in first-episode psychosis. Although this may be explained by multiple beneficial roles that microglia play in the CNS (52), further studies will clarify the link between psychopathology and neuroinflammation in psychosis.
The results of this study should be interpreted with several limitations in mind. First, a significantly larger amount of [18F]FEPPA was administered to the patient group, on average, than to the healthy group. However, this did not affect our results, as there were no differences between groups in the specific activity or the mass injected. Moreover, we did not find any significant correlations between the amount injected and binding of the radiotracer in any of the regions of interest. Second, studies have shown that microglia are not the only cells that express TSPO, and thus it is possible that a portion of the signal of [18F]FEPPA binding comes from astrocytes (53). Nevertheless, this would not have affected the overall conclusions of our study. TSPO is under neurohormonal control, and there are suggestions that other factors, such as stress and anxiety, can affect TSPO expression (54, 55). Since these factors are commonly seen in psychotic patients, they should be taken into consideration in future studies. We explored a subsample of patients (N=5) for whom a stress scale was available and found a positive association between the state subscale of the State-Trait Anxiety Inventory and [18F]FEPPA binding in the hippocampus (r=0.978, p=0.022) (see Figure S3 in the data supplement). Third, some research groups have reported differences in the arterial input function between schizophrenia patients and comparison subjects (32). While the robust outcome measure VT is inherently designed to be unbiased under such circumstances, using repeated-measures mixed-model analysis, we empirically compared the arterial input function between patients and healthy volunteers at four different time intervals (0–30 minutes, 30–60 minutes, 60–90 minutes, and 90–120 minutes of the PET scan) and found no group differences. Finally, in neurochemical brain imaging studies, relatively small sample sizes constantly represent a potential limitation; however, to our knowledge, this is the largest PET study to date investigating microglial activation in first-episode psychosis, particularly in mostly antipsychotic-naive patients. While similar sample sizes were sufficient to test a difference between patients with Alzheimer’s disease and patients with major depressive episode compared with healthy volunteers using the same radioligand (42, 43), it is possible that microglial activation may have a smaller magnitude in first-episode psychosis and would thus necessitate a larger sample size (as described above).
In conclusion, we found no evidence of increased microglial activation as indexed with [18F]FEPPA binding in the dorsolateral prefrontal cortex and hippocampus in patients with first-episode psychosis compared with healthy volunteers. The lack of significant between-group differences in [18F]FEPPA VT suggests that microglial activation is not present in first-episode psychosis. Neuroinflammatory processes may take place earlier in the course of schizophrenia, such as during the clinical high-risk state of psychosis, or may be present only in a subpopulation of patients.
1 : Microglia: a sensor for pathological events in the CNS. Trends Neurosci 1996; 19:312–318Crossref, Medline, Google Scholar
2 : Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev 1995; 20:269–287Crossref, Medline, Google Scholar
3 : Migration of perilesional microglia after focal brain injury and modulation by CC chemokine receptor 5: an in situ time-lapse confocal imaging study. J Neurosci 2005; 25:7040–7047Crossref, Medline, Google Scholar
4 : Caspase signalling controls microglia activation and neurotoxicity. Nature 2011; 472:319–324Crossref, Medline, Google Scholar
5 : Imaging microglial activation during neuroinflammation and Alzheimer’s disease. J Neuroimmune Pharmacol 2009; 4:227–243Crossref, Medline, Google Scholar
6 : High densities of benzodiazepine receptors in human cortical areas. Nature 1977; 269:702–704Crossref, Medline, Google Scholar
7 : Investigation of serum cytokine levels and cytokine production in whole blood cultures of paranoid schizophrenic patients. Arch Immunol Ther Exp (Warsz) 2001; 49:439–445Medline, Google Scholar
8 : Recent progress in animal modeling of immune inflammatory processes in schizophrenia: implication of specific cytokines. Neurosci Res 2006; 56:2–13Crossref, Medline, Google Scholar
9 : Cytokine effects on cortical neuron MAP-2 immunoreactivity: implications for schizophrenia. Biol Psychiatry 2001; 50:743–749Crossref, Medline, Google Scholar
10 : Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry 2007; 62:711–721Crossref, Medline, Google Scholar
11 : Interleukin-12 plasma levels in drug-naïve patients with a first episode of psychosis: effects of antipsychotic drugs. Psychiatry Res 2008; 158:206–216Crossref, Medline, Google Scholar
12 : Lower plasma CC16, a natural anti-inflammatory protein, and increased plasma interleukin-1 receptor antagonist in schizophrenia: effects of antipsychotic drugs. Schizophr Res 1996; 21:39–50Crossref, Medline, Google Scholar
13 : Changes of plasma concentrations of interleukin-1 alpha and interleukin-6 with neuroleptic treatment for schizophrenia. Br J Psychiatry 1994; 164:251–253Crossref, Medline, Google Scholar
14 : Cytokines and schizophrenia: microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci 2009; 63:257–265Crossref, Medline, Google Scholar
15 : Common variants conferring risk of schizophrenia. Nature 2009; 460:744–747Crossref, Medline, Google Scholar
16 : Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 2009; 460:753–757Crossref, Medline, Google Scholar
17 : Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology 2003; 28:1778–1789Crossref, Medline, Google Scholar
18 : Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology 2008; 33:441–456Crossref, Medline, Google Scholar
19 : Dopamine and serotonin levels following prenatal viral infection in mouse: implications for psychiatric disorders such as schizophrenia and autism. Eur Neuropsychopharmacol 2008; 18:712–716Crossref, Medline, Google Scholar
20 : Prenatal exposure to infection: a primary mechanism for abnormal dopaminergic development in schizophrenia. Psychopharmacology (Berl) 2009; 206:587–602Crossref, Medline, Google Scholar
21 : Maternal infection and schizophrenia: implications for prevention. Schizophr Bull 2011; 37:284–290Crossref, Medline, Google Scholar
22 : Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 2010; 167:261–280Link, Google Scholar
23 : Association between prenatal exposure to poliovirus infection and adult schizophrenia. Am J Psychiatry 1999; 156:1100–1102Abstract, Google Scholar
24 : Prenatal infection as a risk factor for schizophrenia. Schizophr Bull 2006; 32:200–202Crossref, Medline, Google Scholar
25 : Association between prenatal exposure to bacterial infection and risk of schizophrenia. Schizophr Bull 2009; 35:631–637Crossref, Medline, Google Scholar
26 : Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther 2011; 132:96–110Crossref, Medline, Google Scholar
27 : A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab 2013; 33:53–58Crossref, Medline, Google Scholar
28 : Decreases in peripheral-type benzodiazepine receptors in postmortem brains of chronic schizophrenics. J Neural Transm (Vienna) 1997; 104:1361–1370Crossref, Medline, Google Scholar
29 : Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol 2006; 112:305–316Crossref, Medline, Google Scholar
30 : Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med 2009; 50:1801–1807Crossref, Medline, Google Scholar
31 : Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry 2008; 64:820–822Crossref, Medline, Google Scholar
32 : Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [11C] PBR28 PET brain imaging study. Am J Psychiatry 2016; 173:44–52Link, Google Scholar
33 : Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int J Neuropsychopharmacol 2010; 13:943–950Crossref, Medline, Google Scholar
34 : Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. Schizophr Bull 2015; 41:85–93Crossref, Medline, Google Scholar
35 : Imaging microglial activation with TSPO PET: lighting up neurological diseases? J Nucl Med 2015; 57:165–168Crossref, Medline, Google Scholar
36 : Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. Neuroimage 2008; 40:43–52Crossref, Medline, Google Scholar
37 : Comparison of [(11)C]-(R)-PK 11195 and [(11)C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: implications for positron emission tomographic imaging of this inflammation biomarker. Neuroimage 2010; 49:2924–2932Crossref, Medline, Google Scholar
38 : Two binding sites for [3H]PBR28 in human brain: implications for TSPO PET imaging of neuroinflammation. J Cereb Blood Flow Metab 2010; 30:1608–1618Crossref, Medline, Google Scholar
39 : Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J Nucl Med 2011; 52:24–32Crossref, Medline, Google Scholar
40 : An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab 2012; 32:1–5Crossref, Medline, Google Scholar
41 : Translocator protein (18 kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [(18)F]-FEPPA. J Cereb Blood Flow Metab 2012; 32:968–972Crossref, Medline, Google Scholar
42 : Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 2015; 72:268–275Crossref, Medline, Google Scholar
43 : In-vivo imaging of grey and white matter neuroinflammation in Alzheimer’s disease: a positron emission tomography study with a novel radioligand, [18F]-FEPPA. Mol Psychiatry 2015; 20:1579–1587Crossref, Medline, Google Scholar
44 : Cerebellum can serve as a pseudo-reference region in Alzheimer disease to detect neuroinflammation measured with PET radioligand binding to translocator protein. J Nucl Med 2015; 56:701–706Crossref, Medline, Google Scholar
45 : Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCIDI/P), Version 2.0. New York, New York State Psychiatric Institute, Biometric Research, 1995Google Scholar
46 : Repeatable Battery for the Assessment of Neuropsychological Status. San Antonio, Tex, Psychological Corp, 1998Google Scholar
47 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
48 : An automated method for the extraction of regional data from PET images. Psychiatry Res 2006; 147:79–89Crossref, Medline, Google Scholar
49 : Quantitation of translocator protein binding in human brain with the novel radioligand [18F]-FEPPA and positron emission tomography. J Cereb Blood Flow Metab 2011; 31:1807–1816Crossref, Medline, Google Scholar
50 : Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab 1992; 12:571–583Crossref, Medline, Google Scholar
51 : Regional brain distribution of translocator protein using [(11)C]DPA-713 PET in individuals infected with HIV. J Neurovirol 2014; 20:219–232Crossref, Medline, Google Scholar
52 : Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry 2015; 2:258–270Crossref, Medline, Google Scholar
53 : Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J Neurosci 2012; 32:10809–10818Crossref, Medline, Google Scholar
54 : Peripheral benzodiazepine receptors: molecular pharmacology to possible physiological significance in stress-induced hypertension. Clin Neuropharmacol 1996; 19:475–496Crossref, Medline, Google Scholar
55 : Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov 2010; 9:971–988Crossref, Medline, Google Scholar



