Association Between Prenatal Exposure to Poliovirus Infection and Adult Schizophrenia
Abstract
OBJECTIVE: The authors’ goal was to determine whether there is an association between prenatal exposure to poliovirus infection and later development of schizophrenia. METHOD: All Finnish patients born between 1951 and 1969 with discharge diagnoses of schizophrenia (N=13,559) were identified from the Finnish Hospital Discharge Register. Information on the monthly number of cases of paralytic poliomyelitis was obtained for each province in Finland. The authors analyzed the incidence of births of individuals who later developed schizophrenia by using a Poisson regression model with year and place of birth, age, sex, season of birth, and smoothed incidence of poliomyelitis in different gestational periods as explanatory variables. RESULTS: An association between the incidence of poliomyelitis and the incidence of births 5 months later of individuals who later developed schizophrenia was observed. Without controlling for seasonality, the effect was significant throughout the second trimester. CONCLUSIONS: Second-trimester exposure to poliovirus infection may increase the risk for the later development of schizophrenia.
It has been suggested that prenatal infection with poliovirus, an enterovirus, contributes to the development of schizophrenia because a decline in the incidence of schizophrenia occurred in many countries after the introduction of polio vaccination (1, 2). Poliovirus epidemics peak in late summer and early autumn, and this fits with the season-of-birth effect seen in schizophrenia, if the effect is caused by an infection in the second trimester of gestation (1, 3). There is also a similar geographic variation in the seasonality of poliomyelitis epidemics (3) and the seasonality of births of individuals who later developed schizophrenia (4). Further support for the hypothesis comes from the latency of effect of poliovirus (new symptoms can emerge decades after initial infection) and the partial regional correlation of brain lesions in postpolio syndrome and schizophrenia (5, 6).
Two previous studies (7, 8) compared the incidence of paralytic poliomyelitis and the number of births of individuals who later developed schizophrenia. The first (7) detected no association, but the second (8) found a significant correlation between poliomyelitis and births of individuals who later developed schizophrenia. This finding is difficult to explain because poliomyelitis preceded the births of individuals who later developed schizophrenia by 18 months. In neither of these studies was the patients’ place of birth known. A third study (9) found no relationship between the number of deaths from polio and births of individuals who later developed schizophrenia; however, death is a rare outcome of poliovirus infection.
We report an ecological study of the association between the incidence of poliomyelitis and births of individuals who later developed schizophrenia, which, compared with previous studies, benefits from more accurate information concerning both patients and the incidence of poliomyelitis.
METHOD
All patients born between 1951 and 1969 who were given hospital discharge diagnoses of schizophrenia (ICD-8 and ICD-9 diagnostic category 295) (N=13,559) were identified from the Finnish Hospital Discharge Register. Place of birth of all of these patients was obtained from the National Population Register. Information from both registers was available until 1992.
The monthly number of new cases of paralytic poliomyelitis from the three largest towns and all of the provinces in Finland (N=10–12 between 1950 and 1969) was obtained from the National Research and Development Center for Welfare and Health.
The Population Register Center provided sex-specific, monthly number of births in each municipality from 1950 to 1969, the number of persons alive on Dec. 31, 1969, and the number of deaths per year from 1970 to 1991. This information enabled us to calculate exact person-years.
We used a Poisson regression model with the number of births of individuals who later developed schizophrenia as a response variable and geographic area, birth cohort, age, sex, month of birth, and incidence of paralytic poliomyelitis as explanatory variables. Circular transformation (10) was applied to the month of birth to analyze seasonal variation. Population size in each cell was used as a weight to obtain correct estimates.
High variability in the monthly incidence of poliomyelitis by province caused by small numbers in most cells was smoothed by using moving averages with a 3-month window. Incidence of poliomyelitis was then dichotomized to an indicator variable (0=no cases of poliomyelitis, 1=any cases of poliomyelitis). In another analysis, the incidence of poliomyelitis was divided into deciles; the first six deciles were pooled because the incidence was zero.
Incidence of births of individuals who later developed schizophrenia was modeled without and with the incidence of polio. Improvement in model fit was tested by using a chi-square likelihood ratio test. Analyses were performed with the statistical software S-PLUS, version 3.3 (11).
RESULTS
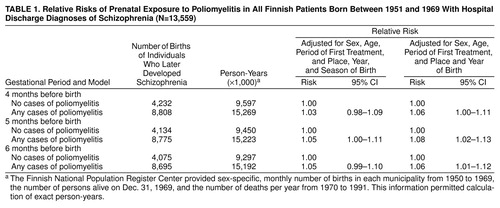
In the smoothed model, the occurrence of poliomyelitis was associated with a higher rate of births 5 months later of individuals who later developed schizophrenia (χ2=3.75, df=1, p=0.05) (Table 1). The effect was more significant without smoothing and strongest in the seventh (relative risk=1.13, 95% CI=1.07–1.20) and eighth (relative risk=1.05, 95% CI=1.00–1.11) deciles. Without adjusting for seasonality, we found that the effect of polio was significant throughout the second trimester (Table 1).
DISCUSSION
This study found that exposure to poliovirus infection 5 months before birth increased the risk for the later development of schizophrenia. Without seasonality in the model, the effect was more significant and covered the whole second trimester, which suggests that exposure to poliovirus infection may explain some of the seasonality in births of individuals who later develop schizophrenia. The relatively modest effect of the exposure is unsurprising given that genetic factors are most important in the etiology of schizophrenia (12) and that other environmental risk factors have already been suggested (13). The timing is in accordance with previous findings of a neurodevelopmental insult during the second trimester of fetal life (13). The association was not strongest when the incidence of paralytic poliomyelitis was highest, but the latter is only an approximation of the population exposure to polioviruses. Paralytic symptoms develop in less than 1% of those infected and after an incubation period up to 1 month (3). In addition, the case-infection ratio depends on age, level of immunity in the population, and type of poliovirus (3).
The strengths of this study are that the exact time and place of birth of both patients and the general population and regional statistics concerning poliomyelitis were obtained. Even the most severe polio epidemics in the 1950s spread slowly from one province to another, and none spread across the whole country. The limitations are that it is unknown whether a person actually was exposed and that the number of cases of paralytic polio is not a direct measurement of the incidence of poliovirus infection among pregnant women.
An association between exposure to poliovirus infection during the second trimester of fetal development and adult schizophrenia could explain some of the observed decline in the incidence of schizophrenia. Rantakallio et al. (14) found that neonatal meningitis caused by Coxsackie B5, another enterovirus, was associated with a greater risk of adult schizophrenia. These results indicate the need for further studies of the association between enteroviruses and schizophrenia, ideally, prospective follow-up studies of individuals who are known to have been exposed to enterovirus infections during gestation.
Received Sept. 8, 1998; revision received Dec. 7, 1998; accepted Dec. 16, 1998. From the National Public Health Institute, Department of Mental Health and Alcohol Research, Department of Nutrition, and Department of Virology, Helsinki. Address reprint requests to Dr. Suvisaari, National Public Health Institute, Department of Mental Health and Alcohol Research, Mannerheimintie 166, FIN-00300 Helsinki, Finland; [email protected] (e-mail). Supported by the Academy of Finland, the Jalmari and Rauha Ahokas Foundation, and the Foundation for Psychiatric Research. The authors thank Mrs. Pipsa Pellinen for her assistance in locating the monthly reports of infectious diseases and Dr. Mary Cannon for her comments.
 |
1. Eagles JM: Are polioviruses a cause of schizophrenia? Br J Psychiatry 1992; 160:598–600Google Scholar
2. Squires RF: How a poliovirus might cause schizophrenia: a commentary on Eagles’ hypothesis. Neurochem Res 1997; 22:647–656Crossref, Medline, Google Scholar
3. Nathanson N, Martin JR: The epidemiology of poliomyelitis: enigmas surrounding its appearance, epidemicity, and disappearance. Am J Epidemiol 1979; 110:672–692Crossref, Medline, Google Scholar
4. Torrey EF, Torrey BB, Peterson MR: Seasonality of schizophrenic births in the United States. Arch Gen Psychiatry 1977; 34:1065–1070Google Scholar
5. Bruno RL, Cohen JM, Galski T, Frick NM: The neuroanatomy of post-polio fatigue. Arch Phys Med Rehabil 1994; 75:498–504Medline, Google Scholar
6. Heckers S: Neuropathology of schizophrenia: cortex, thalamus, basal ganglia, and neurotransmitter-specific projection systems. Schizophr Bull 1997; 23:403–421Crossref, Medline, Google Scholar
7. Watson CG, Kucala T, Tilleskjor C, Jacobs L: Schizophrenic birth seasonality in relation to the incidence of infectious diseases and temperature extremes. Arch Gen Psychiatry 1984; 41:85–90Crossref, Medline, Google Scholar
8. Torrey EF, Rawlings R, Waldman IN: Schizophrenic births and viral diseases in two states. Schizophr Res 1988; 1:73–77Crossref, Medline, Google Scholar
9. O’Callaghan EO, Sham PC, Takei N, Murray G, Glover G, Hare EH, Murray RM: The relationship of schizophrenic births to 16 infectious diseases. Br J Psychiatry 1994; 165:353–356Crossref, Medline, Google Scholar
10. Batschelet E: Circular Statistics in Biology. London, Academic Press, 1981Google Scholar
11. S-Plus Guide to Statistical and Mathematical Analysis, version 3.3. Seattle, StatSci, MathsSoft, 1995Google Scholar
12. Cannon TD, Kaprio J, Lönnqvist J, Huttunen M, Koskenvuo M: The genetic epidemiology of schizophrenia in a Finnish twin cohort. Arch Gen Psychiatry 1998; 55:67–74Crossref, Medline, Google Scholar
13. Huttunen MO, Machon RA, Mednick SA: Prenatal factors in the pathogenesis of schizophrenia. Br J Psychiatry 1994; 164:15–19Crossref, Google Scholar
14. Rantakallio P, Jones P, Moring J, von Wendt L: Association between central nervous system infections during childhood and adult onset schizophrenia and other psychosis: a 28-year follow-up. Int J Epidemiol 1997; 26:837–843Crossref, Medline, Google Scholar



