Amygdala Hyperactivity at Rest in Paranoid Individuals With Schizophrenia
Abstract
Objective:
The amygdala’s role in threat perception suggests that increased activation of this region may be related to paranoid ideation. However, investigations of amygdala function in paranoid individuals with schizophrenia, compared with both healthy individuals and nonparanoid individuals with schizophrenia, have consistently reported reduced task-related activation. The reliance of blood-oxygen-level-dependent functional MRI on a contrast between events and baseline, and the inability to quantitatively measure this baseline, may account for these counterintuitive findings. The present study tested for differences in baseline levels of amygdala activity in paranoid and nonparanoid individuals with schizophrenia using arterial spin labeling perfusion MRI.
Method:
Resting cerebral blood flow (CBF) and task-related activation of the amygdala were measured in 25 healthy individuals, 16 individuals with schizophrenia who were actively paranoid at the time of scanning, and 16 individuals with schizophrenia who were not paranoid.
Results:
Analysis of relative CBF values extracted from the amygdala bilaterally revealed significantly increased activity in the left amygdala in paranoid patient volunteers compared with healthy comparison subjects and nonparanoid patient volunteers. Increased CBF was also evident in the right amygdala but did not reach the level of statistical significance. Paranoid volunteers also showed significantly decreased task-related activation of the amygdala compared with the two other groups.
Conclusions:
These findings suggest that amygdala hyperactivation may underlie paranoia in schizophrenia. Additionally, the reported differences between paranoid and nonparanoid patient volunteers emphasize the importance of considering symptom-based subgroups and baseline levels of activity in future investigations of neural activation in schizophrenia.
Paranoia, defined as the “unfounded fear that others intend to cause you harm” (1), is the most commonly reported delusional belief in individuals diagnosed with schizophrenia spectrum illnesses (2). Such delusions are associated with significant distress and functional impairment (3), often predict hospitalization (4), and significantly predict conversion to psychosis in at-risk samples (5). For these reasons, research has sought to understand the factors that contribute to and maintain paranoid ideation.
Whereas the psychological correlates of paranoia are fairly well understood (2, 6), potential neurobiological bases remain unclear. Given the amygdala’s role in processing salience and threat (7), investigators have postulated a link between amygdala activity and paranoia (8–10), and a review published in 2010 supports this relationship (11). However, instead of seeing increased amygdala activation associated with paranoia as might be predicted, functional neuroimaging studies that have directly compared paranoid to nonparanoid individuals with schizophrenia have consistently reported amygdala hypoactivation in paranoid patients (12–17). In the studies of Williams et al. (16, 17), decreased amygdala responses were paired with increased autonomic arousal (i.e., skin conductance), leading the investigators to hypothesize that a disjunction of arousal and amygdala-prefrontal neural circuits could lead to hypervigilance and maintenance of paranoid ideation. This is a viable explanation; however, an important caveat requires consideration. Each of the studies that have directly compared paranoid and nonparanoid patients has used the blood-oxygenation-level-dependent (BOLD) method. Estimates resulting from BOLD imaging are derived from a contrast between activity occurring during a specific event and that occurring during nonevents, or a baseline. These baselines cannot be directly measured and thus cannot be compared between groups. This raises the possibility that paranoid patients may actually show higher resting, or tonic, levels of amygdala activation and that the reported reductions in activation with task may result from high baseline activity that attenuates the event-evoked change detected by the BOLD method.
In the present study, we sought to test the hypothesis that amygdala hyperactivity would be present in paranoid individuals with schizophrenia by examining resting levels of cerebral blood flow (CBF) using arterial spin labeling imaging. Arterial spin labeling measures CBF using magnetically tagged arterial blood water as an endogenous tracer (18, 19) and provides quantitative measurement of CBF that renders this method optimal for assessing baseline differences between groups. In order to replicate the previous BOLD findings, participants also completed a BOLD functional MRI (fMRI) trustworthiness rating task that we have used in our previous work and that robustly activates the amygdala (13, 14, 20, 21). We predicted that the group of patients showing significant levels of paranoia at the time of scanning would show reduced task-related amygdala activation but greater resting CBF in the amygdala compared with both healthy comparison subjects and nonparanoid individuals with schizophrenia.
Method
Participants
The study included 57 participants from three groups: individuals with schizophrenia or schizoaffective disorder with prominent paranoid symptoms (paranoid schizophrenia, N=16), individuals with schizophrenia or schizoaffective disorder without paranoid symptoms (nonparanoid schizophrenia, N=16), and healthy community comparison subjects (N=25). All participants were between the ages of 18 and 55 years and proficient in English. To be eligible, participants could not report 1) a history of head injury or other medical conditions known to affect brain function (e.g., uncontrolled hypertension, diabetes mellitus, history of seizures), 2) pervasive developmental disorder, or 3) electroconvulsive therapy. Individuals also could not meet criteria for current substance abuse or dependence (except nicotine). The institutional review board of the University of Texas Southwestern Medical School approved the study protocol, and all participants provided written, informed consent.
Healthy comparison subjects were recruited through community advertisements in Dallas County and from other studies in our laboratory. Individuals were screened for personal and family history of psychopathology to ensure that they did not meet criteria for any DSM-IV axis I or II disorder and had no first-degree relatives who met criteria for a psychotic or affective disorder.
Individuals in the schizophrenia groups were recruited from the Southwestern Medical School Division of Translational Neuroscience of Schizophrenia and Metrocare Services, a nonprofit mental health services provider organization in Dallas County. Diagnoses were confirmed with the Structured Clinical Interview for DSM-IV (SCID-P). Severity of symptoms over the last week was assessed with the Positive and Negative Syndrome Scale (PANSS) (22), and ratings on the suspiciousness/persecution item (item P6) were used to divide patients into paranoid and nonparanoid groups. Those scoring ≥4, indicating the presence of clinically significant paranoid ideation on the day of scanning, comprised the paranoid schizophrenia group, and those with a score of 1, indicating the absence of paranoid ideation, comprised the nonparanoid schizophrenia group. These groups did not differ on diagnosis (i.e., schizophrenia compared with schizoaffective; χ2=2.0, p=0.16), medication type (i.e., typical compared with atypical; χ2=0.96, p=0.62), or mean chlorpromazine equivalent dose (t=0.11, df=29, p=0.92) (23). The paranoid schizophrenia group did show greater severity of positive (t=5.10, df=30, p<0.001) and general symptoms (t=3.52, df=30, p=0.001); however, these differences were no longer significant when controlling for the effects of paranoia. Severity of negative symptoms did not differ between groups (t=0.91, df=30, p=0.37). Finally, since previous work suggests that anxiety may be related to amygdala function (24), we also compared groups specifically on level of PANSS-rated anxiety (item G2). The paranoid schizophrenia group (mean=3.94 [SD=1.53]) received higher ratings of anxiety than the nonparanoid group (mean=2.63 [SD=1.78]; t=2.24, df=30, p=0.03).
All three groups did not differ with regard to sex (χ2=1.87, p=0.39), ethnicity (χ2=6.50, p=0.17), age (F=1.34, df=2, 54, p=0.27), education (F=1.39, df=2, 54, p=0.26), or maternal education (F=1.24, df=2, 54, p=0.83). Paternal education significantly differed across groups (F=3.08, df=2, 54, p=0.049) such that the paranoid schizophrenia group showed lower attainment compared with the healthy individuals (p=0.04). Demographic and clinical characteristics of the study sample are presented in Table 1.
| Characteristic | Healthy Comparison Subjects (N=25) | Nonparanoid Schizophrenia Patients (N=16) | Paranoid Schizophrenia Patients (N=16) | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Male | 12 | 48 | 11 | 68.8 | 8 | 50 |
| Ethnicity | ||||||
| Caucasian | 14 | 56 | 8 | 50 | 3 | 18.8 |
| African American | 10 | 40 | 8 | 50 | 12 | 75 |
| Native American | 1 | 4 | 1 | 6.2 | ||
| Diagnosis | ||||||
| Schizophrenia | 10 | 62.5 | 6 | 37.5 | ||
| Schizoaffective Medication typea | 6 | 37.5 | 10 | 62.5 | ||
| Typical | 1 | 6.2 | 1 | 6.2 | ||
| Atypical | 10 | 62.5 | 10 | 62.5 | ||
| Combination | 1 | 6.2 | — | |||
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 33.64 | 12.42 | 38.81 | 13.24 | 38.56 | 7.71 |
| Education (years) | 13.60 | 1.63 | 12.75 | 1.34 | 12.81 | 2.48 |
| Maternal education (years)b | 13.76 | 2.54 | 13.67 | 2.92 | 13.23 | 2.28 |
| Paternal education (years)c | 15.17 | 3.51 | 13.64 | 3.56 | 12.10 | 2.02 |
| Positive and Negative Syndrome Scale score | ||||||
| Positive total | 12.63 | 3.76 | 19.19 | 3.53 | ||
| Item P1-delusions | 2.94 | 1.57 | 4.44 | 0.89 | ||
| Item P2-disorganization | 1.69 | 1.58 | 1.19 | 0.75 | ||
| Item P3-hallucinations | 3.31 | 1.79 | 4.19 | 1.72 | ||
| Item P4-excitement | 1.00 | 0.0 | 1.25 | 0.68 | ||
| Item P5-grandiosity | 1.69 | 1.30 | 1.50 | 1.10 | ||
| Item P6-suspiciousness/persecution | 1.00 | 0.0 | 5.44 | 0.63 | ||
| Item P7-hostility | 1.00 | 0.0 | 1.19 | 0.75 | ||
| Negative total | 11.56 | 4.0 | 13.25 | 6.21 | ||
| General total | 25.19 | 5.58 | 32.0 | 5.37 | ||
| Chlorpromazine equivalent dosaged | 350.52 | 546.75 | 332.22 | 371.26 | ||
TABLE 1. Demographic and Clinical Characteristics of Healthy Comparison Subjects and Nonparanoid and Paranoid Schizophrenia Patients
Imaging Parameters and Procedures
Imaging was performed on a 3T Philips Achieva system (Philips Medical Systems, Best, the Netherlands) using the body coil for transmission and an eight-channel head coil for reception. A 4-minute magnetization-prepared, rapid acquisition gradient echo (160 slices, voxel size 1×1×1 mm, matrix=256×204, field of view=256×204, time to repeat/echo time=8.1 ms/3.7 ms) image was first acquired to obtain high-resolution anatomical images for spatial normalization. Pseudo-continuous arterial spin labeling was then used to obtain estimates of resting CBF (25, 26). Forty control and label pairs of images were acquired with the following parameters: labeling duration=1,650 ms, postlabeling delay=1,525 ms, time to repeat/echo time=4,211 ms/14 ms, flip angle=90°, matrix size=80×79, voxel size=3×3×5 mm3, 29 slices, no gap, duration=345 seconds. During scanning, participants were asked to relax, lie still, and keep their eyes open and focused on a small, white cross that was presented in the middle of a black screen.
Following the arterial spin labeling acquisition, participants completed an event-related BOLD imaging task comprised of 60 grayscale frontal images of faces taken from the Trustworthiness/Approachability Task (27). Faces were displayed for 2 seconds and were followed by a variable interstimulus interval of 0.5 seconds–20.5 seconds during which participants fixated on a cross in the middle of a scrambled face. The total task duration was 8.5 minutes. As in previous work (13, 14, 21), participants rated each face as either trustworthy or untrustworthy, and responses were recorded by button press. BOLD fMRI data were acquired during the task with the following parameters: 40 slices, voxel size=3.44×3.44×4 mm, matrix=64×64, field of view=220×220 mm, time to repeat/echo time=2,500 ms/30 ms.
Data Processing
Custom scripts utilizing Statistical Parametric Mapping (SPM2, University College London) were used to calculate CBF. The pseudo-continuous arterial spin labeling image series was initially realigned to the first volume. Pairwise subtraction between the label and control images was then performed to yield 40 difference images that were averaged to create one CBF image per participant using the following perfusion kinetic model:
In this perfusion kinetic model, w is the postlabeling delay time (1,525 ms), λ is the blood-brain partition coefficient (0.9 mL/g), and α is the labeling efficiency (0.86). T1 (1,279 ms) is the averaged T1 value of blood and tissue because the labeled spins spend some time in the blood and some time in the tissue space. M0 is the equilibrium magnetization of the tissue estimated from the control image while accounting for the T1 relaxation of the static spins (28).
To reduce the potential influence of individual physiological differences, the CBF values at each voxel were normalized against the whole-brain gray matter value for each participant (25). Thus, CBF values are provided as relative CBF. These averaged relative CBF images were coregistered to their corresponding structural image and then normalized to Montreal Neurological Institute (MNI) space using the standard template provided by SPM2 and smoothed with an 8-mm full-width at half-maximum three-dimensional isotropic Gaussian kernel.
Preprocessing and analyses of BOLD fMRI data were performed with FEAT (fMRI Expert Analysis Tool), version 6.00 (from FMRIB’s Software Library [FSL]). Images were slice-time corrected, motion-corrected to the median image, high-pass filtered (100 seconds), and spatially smoothed (8-mm full width at half maximum, isotropic). The median functional and anatomical images were coregistered and then transformed into standard space (T1 MNI template) using trilinear interpolation. Subject-level time-series statistical analyses were conducted using FILM (FMRIB’s Improved General Linear Model) with local auto-correlation correction. The events comprising each condition (trustworthy and untrustworthy) were identified according to the individual judgment of each participant, and the two conditions were then modeled with a canonical hemodynamic response function and its temporal derivative. Six rigid body motion parameters were also included as nuisance covariates. The resulting contrast images revealing stimulus-dependent activation relative to fixation baseline were then used in group-level analyses.
Data Analysis
Primary relative CBF analyses.
Both region-of-interest and exploratory whole-brain analyses were conducted. To examine activation in our a priori region of interest, the right and left amygdala were first anatomically defined using the Wake Forest University PickAtlas. Averaged estimates of relative CBF within the right and left amygdala were then extracted and entered into a repeated-measures analysis of variance (ANOVA) with hemisphere (right versus left) as the within-subject factor and group (healthy comparison compared with nonparanoid schizophrenia compared with paranoid schizophrenia) as the between-subject factor. Age, sex, and race were entered as covariates. The threshold for statistical significance was set at a p value <0.05, and significant group effects were probed with Tukey’s honest significant difference test.
In order to examine regions other than the amygdala in which paranoid and nonparanoid groups may differ in resting relative CBF, exploratory whole-brain voxel-wise analyses were also conducted with a one-way ANOVA (group: healthy comparison compared with nonparanoid schizophrenia compared with paranoid schizophrenia) conforming to random-effects analyses. As above, age, sex, and race were entered as nuisance covariates. Monte Carlo cluster correction (AlphaSim [29]) at a p value <0.05 was used to correct for multiple comparisons. For interpretation, relative CBF values were extracted from clusters showing a main effect of group, and these values were analyzed with a one-way ANOVA with Tukey’s post hoc comparisons.
BOLD analyses.
To examine group differences in task-related activation, mean percent signal change was extracted from the same regions of interest that were applied to the CBF data. These values were entered into a repeated-measures ANOVA with stimulus type (trustworthy versus untrustworthy) and hemisphere (right versus left) as within-subject factors and group (healthy comparison compared with nonparanoid schizophrenia compared with paranoid schizophrenia) as the between-subject factor. Effects with statistical probabilities with a p value <0.05 were considered significant and were followed up with Tukey’s post hoc tests. Behavioral data from the trustworthiness task are presented in the data supplement accompanying the online version of this article.
Supplemental analyses.
Given the link between anxiety and amygdala activation (24) and the difference between patient groups in clinician-rated levels of anxiety on the PANSS, we also repeated the CBF region-of-interest analysis while controlling for this variable. Because healthy comparison subjects did not complete the PANSS, these analyses were restricted to the patient groups. Additionally, to assess the potential effects of morphological differences on the CBF results, a supplemental voxel-based morphometry analysis was also completed. Details of this analysis, including full results, are presented in the data supplement.
Results
Analyses of Resting Relative CBF
The repeated-measures ANOVA on amygdala relative CBF revealed a statistically significant main effect for group (F=3.34, df=2, 51, p=0.04, np2=0.12). Tukey’s post hoc group comparisons demonstrated that individuals with paranoid schizophrenia showed increased resting relative CBF in the left amygdala compared with both nonparanoid schizophrenia patients (p=0.005) and healthy comparison subjects (p<0.05), who did not differ from each other. For the right amygdala, paranoid patients did show higher levels of relative CBF compared with the two other groups; however, these differences did not reach statistical significance (nonparanoid schizophrenia group, p=0.07; healthy comparison group, p=0.12; Figure 1). The main effect of hemisphere was not significant nor was the group-by-hemisphere interaction. The main effect of group also remained significant when controlling for the effect of anxiety within the patient groups (F=8.32, df=1, 26, p=0.008, np2=0.24) and was driven by greater relative CBF in paranoid compared with nonparanoid schizophrenia patients in both the left (p=0.002) and right (p<0.05) amygdala.
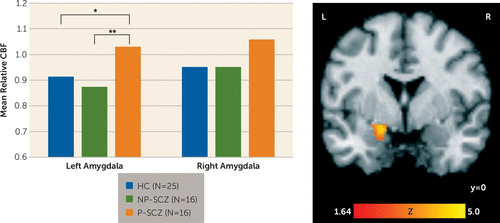
FIGURE 1. Group Differences in Resting Relative Cerebral Blood Flow in the Amygdalaa
a The bar graph shows resting relative cerebral blood flow (CBF) values extracted from the left and right amygdala (left). The image (right) shows the significant main effect of group in the left amygdala from voxel-wise analysis; the image is cluster-level corrected at a p value <0.05 and masked by the amygdala region of interest. HC=healthy comparison; NP-SCZ=nonparanoid schizophrenia; P-SCZ=paranoid schizophrenia.
* p<0.05, ** p<0.01.
Whole-brain voxel-wise comparisons also revealed main effects of group in several focal brain regions encompassing both gray and white matter (Table 2, Figure 2). Healthy comparison subjects showed greater relative CBF than both patient groups in occipital regions, including the middle occipital gyrus extending into the fusiform gyrus, bilateral cuneus, and inferior frontal gyrus, and healthy comparison subjects showed greater relative CBF than paranoid schizophrenia patients in the lateral precentral gyrus (Brodmann’s areas [BAs] 44 and 6). It is noteworthy that nonparanoid schizophrenia patients also showed less relative CBF in this region compared with healthy comparison subjects, but the difference did not reach statistical significance. Both patient groups had higher relative CBF than healthy individuals in the right superior precentral gyrus (BA 4) extending to the paracentral lobule, and paranoid schizophrenia patients showed higher relative CBF compared with healthy comparison subjects in the culmen and ventral cerebellum.
| Cluster Sizea | Anatomical Label and Brodmann’s Area (BA)b | Talairach Coordinates (x, y, z) | Peak Z Score |
|---|---|---|---|
| Healthy comparison>schizophrenia | |||
| 6400 | Right middle occipital gyrus (BA 18) | 36, –90, 2 | 4.72 |
| Right inferior semilunar lobule | 25, –77, –37 | 3.98 | |
| Right fusiform gyrus (BA 18) | 23, –96, –13 | 3.75 | |
| 613 | Left cuneus (BA 19) | –20, –88, 24 | 3.52 |
| Left superior occipital gyrus (BA 19) | –37, –82, 23 | 2.62 | |
| 324 | Left inferior frontal gyrus white matter | –47, 40, 4 | 3.28 |
| 324 | Right middle occipital gyrus (BA 18) | 11, –95, 15 | 2.88 |
| Right cuneus (BA 18) | 2, –79, 29 | 2.86 | |
| Right cuneus (BA 19) | 11, 90, 25 | 2.27 | |
| Healthy comparison>paranoid schizophrenia | |||
| 557 | Left precentral gyrus (BA 44) | –58, 5, 9 | 3.47 |
| Left precentral gyrus (BA 6) | –57, 3, 32 | 2.55 | |
| Schizophrenia>healthy comparison | |||
| 968 | Right precentral gyrus (BA 4) | 22, –24, 58 | 3.53 |
| Left precentral white matter | –7, –32, 64 | 3.27 | |
| Right paracentral lobule (BA 4) | 4, –34, 64 | 3.02 | |
| Nonparanoid schizophrenia>healthy comparison and paranoid schizophrenia | |||
| 1705 | Right culmen | 6, –39, –1 | 3.75 |
| Left splenium of corpus callosum | –1, –31, 12 | 2.98 | |
| Right superior corona radiata | 17, –24, 24 | 2.64 | |
| Paranoid schizophrenia>healthy comparison and nonparanoid schizophrenia | |||
| 3166 | Right anterior limb of internal capsule | 19, 5, 16 | 3.58 |
| Left medial frontal gyrus (BA 25) | –6, 8, –18 | 3.38 | |
| Left genu of corpus callosum | –14, 33, 13 | 3.27 | |
| Paranoid schizophrenia>healthy comparison | |||
| 1215 | Right cerebellum | 34, –42, –59 | 3.26 |
| Right cerebellum | 44, –36, –34 | 3.21 | |
| Right culmen | 26, –32, –36 | 3.03 | |
| Paranoid schizophrenia>nonparanoid schizophrenia | |||
| 329 | Right middle temporal gyrus (BA 21) | 48, 0 –35 | 3.34 |
| Right inferior temporal gyrus white matter | 38, 2, –36 | 3.00 | |
| Right fusiform gyrus white matter | 33, –4, –35 | 2.58 |
TABLE 2. Brain Regions Showing Significant Group Differences in Relative Cerebral Blood Flow
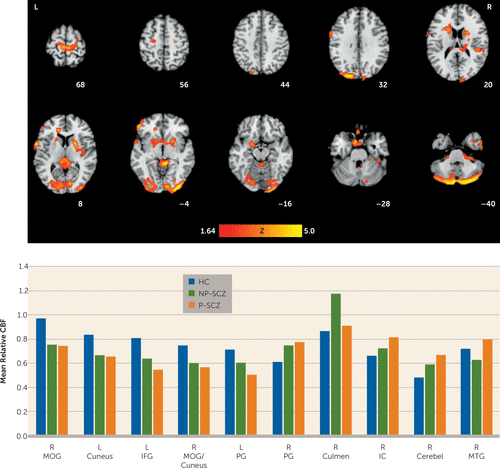
FIGURE 2. Whole-Brain Group Differences in Resting Relative Cerebral Blood Flowa
a The images show neural regions demonstrating a significant main effect of group for resting relative cerebral blood flow (CBF) (top row). Resting relative CBF values were extracted from each cluster to demonstrate the direction of the effect (bottom row). Images are cluster-level corrected at a p value <0.05. Cerebel=cerebellum; HC=healthy comparison; IC=internal capsule; IFG=inferior frontal gyrus; L=left; MOG=middle occipital gyrus; MOG/Cun=middle occipital gyrus/cuneus; MTG=middle temporal gyrus; NP-SCZ=nonparanoid schizophrenia; PG=precentral gyrus; P-SCZ=paranoid schizophrenia; R=right.
Differences also emerged between the patient groups. Nonparanoid individuals showed more relative CBF than both healthy comparison subjects and paranoid individuals in an area including the culmen and posterior corpus callosum. Consistent with the region-of-interest analysis above, the paranoid schizophrenia group had greater relative CBF than the healthy comparison and nonparanoid schizophrenia groups in a large cluster including the right internal capsule extending into the putamen (Talairach coordinates x, y, z: 23, –2, 6; Z=3.23) and the left medial frontal gyrus extending into the amygdala (Talairach coordinates x, y, z: –18, 2, –14; Z=2.72). Additionally, the middle temporal gyrus and white matter regions of the inferior temporal and fusiform gyrus also showed greater relative CBF in paranoid schizophrenia compared with nonparanoid schizophrenia.
There was minimal overlap between regions of group difference in CBF and regions showing morphological disparities. Overlap was seen in the right paracentral lobule and bilateral internal capsule only (see Figure S1 in the online data supplement). For both regions, healthy comparison subjects showed greater tissue volume than both patient groups; however, the two patient groups showed greater CBF.
Analyses of BOLD Signal Change in the Amygdala
The repeated-measures ANOVA on mean percent signal change revealed a main effect of group (F=3.25, df=2, 54, p<0.05, np2=0.11) that was qualified by a significant group-by-hemisphere interaction (F=3.19, df=2, 54, p<0.05, np2=0.11). Post hoc group comparisons demonstrated that paranoid schizophrenia patients showed decreased activation in the left amygdala compared with healthy individuals (p=0.02) but not compared with nonparanoid schizophrenia patients (p=0.29). In the right amygdala, paranoid schizophrenia patients showed less activation than both healthy (p=0.03) and nonparanoid (p=0.01) individuals. Healthy comparison subjects and nonparanoid individuals with schizophrenia did not differ from each other in either the left or right amygdala (Figure 3). No other main effects or interactions were significant.
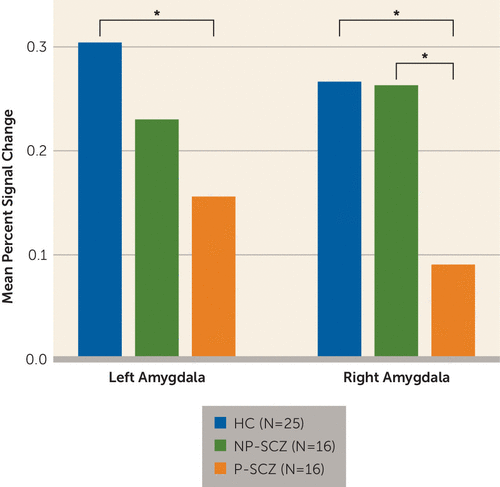
FIGURE 3. Mean Percent Signal Change During Judgments of Trustworthiness Extracted From the Right and Left Amygdalaa
a HC=healthy comparison; NP-SCZ=nonparanoid schizophrenia; P-SCZ=paranoid schizophrenia.
* p<0.05
Discussion
This study used arterial spin labeling imaging to assess potential differences in resting cerebral blood flow between paranoid and nonparanoid individuals with schizophrenia in an effort to clarify the association between amygdala activity and paranoid ideation. Patients who were actively paranoid at the time of scanning showed significantly increased blood flow in the left amygdala compared with both nonparanoid individuals with schizophrenia and healthy comparison subjects. Increases in resting CBF for paranoid individuals were also evident in the right amygdala, although to a lesser degree. Additionally, these same paranoid individuals showed reduced task-related amygdala activity compared with healthy comparison subjects in the bilateral amygdala and compared with nonparanoid patients in the right amygdala. The finding of increased baseline amygdala activity paired with decreased stimulus-driven activity may explain why previous BOLD comparisons of paranoid and nonparanoid patients have consistently, and counterintuitively, shown decreased amygdala activation in paranoid individuals. A higher resting baseline may create a ceiling effect that limits the signal change detectable by the BOLD method. Thus, the present findings suggest that paranoia is not related to reduced amygdala activity but rather that increased tonic activity of the amygdala may serve as a mechanism for paranoid ideation. This interpretation is consistent with the known link between the amygdala and threat perception and the conceptualization of paranoia as the continual perception of environmental threat.
Relating our findings to the broader literature on amygdala function in schizophrenia requires caution given that the vast majority of studies have not differentiated patients based on the presence of paranoia. However, one previous arterial spin labeling study did report hyperperfusion in the left amygdala of nonmedicated individuals with schizophrenia (30), and positron emission tomography studies, which are not constrained by the same limitations as BOLD imaging studies, also provide support for increased amygdala activation at baseline in schizophrenia patients. Specifically, both Taylor et al. (31) and Fernandez-Egea et al. (32) reported hyperactivation of the amygdala in schizophrenia patients compared with healthy individuals. A meta-analysis of primarily BOLD studies also suggests that schizophrenia patients may show more amygdala activation than healthy individuals when processing neutral stimuli (33), but how increased responding to neutral stimuli and increased baseline activity may fit together and how these relationships may differ between paranoid and nonparanoid patients requires further investigation. Liu et al. (34) demonstrated that baseline CBF does influence the BOLD signal and that including estimates of baseline CBF can increase the sensitivity of BOLD analyses. Future investigations of amygdala function in schizophrenia may therefore benefit from concurrently measuring CBF and blood oxygenation.
Results of the whole-brain analysis on CBF largely replicate previous arterial spin labeling studies that have compared schizophrenia patients with healthy individuals. As reported in the present study, increased CBF for healthy individuals compared with schizophrenia patients has previously been found in the middle occipital gyrus (35, 36), fusiform gyrus (37, 38), cuneus (30, 37), and inferior frontal gyrus (35, 36). Similarly, increased CBF for schizophrenia patients was noted in the precentral gyrus, cerebellum, and corpus callosum, all of which are consistent with previous reports (30, 36). The whole-brain direct comparison between paranoid and nonparanoid patients is novel to the present study and extends the literature by highlighting the potential importance of white matter function for this symptom dimension. Paranoid patients, compared with nonparanoid patients, showed greater white matter CBF in the internal capsule, corpus callosum, and white matter portions of the inferior temporal and fusiform gyri. Collectively, these findings suggest that increased neuronal signaling at rest, particularly from sensory areas to frontal regions, may be associated with paranoid ideation. This interpretation is also consistent with our previous work showing a positive correlation between CBF in the corona radiata and the severity of positive symptoms (36).
While our study establishes a link between increased amygdala activation and paranoia, some limitations should be considered. First, the results are correlational and do not provide a temporal ordering between amygdala activity and paranoid thinking. They also do not address the question of whether increased amygdala CBF is a state or trait characteristic of paranoid patients (i.e., does resting CBF fluctuate with paranoia or would it be evident in individuals with a history of paranoia but who are not currently paranoid). Longitudinal studies that can track CBF and symptom changes over time are needed. Second, the paranoid schizophrenia group reported higher levels of anxiety than the nonparanoid schizophrenia group. It is possible that increased anxiety may have contributed to the present results; however, this seems unlikely given that the difference between the patient groups in amygdala CBF was strengthened when statistically controlling for levels of anxiety. Nevertheless, anxiety has been shown to be a strong correlate of paranoia (as it was in our study [r=0.41 across all patients]) that could serve as a contributing factor or as a consequence (6), suggesting that the potential role of anxiety should not be dismissed completely. Third, it could be argued that CBF differences between groups resulted from volumetric reductions in the patient groups. The limited overlap between our voxel-based morphometry and CBF results suggests that this is not the case. One possible exception could be the findings in the internal capsule. However, in this region, volumetric reductions were evident for both patient groups compared with healthy comparison subjects, whereas CBF differences were limited to the paranoid group.
Notwithstanding these limitations, the present findings identify increased resting cerebral blood flow in the amygdala in paranoid, compared with nonparanoid, individuals with schizophrenia and suggest that amygdala hyperactivity may subserve paranoid ideation. Given the negative effect of paranoia on the social functioning of individuals with schizophrenia, attenuating amygdala activation, through pharmacology for example, may improve quality of life. These results also highlight the need to consider symptom-based subgroups in future investigations of amygdala functioning in schizophrenia.
1 : Virtual reality study of paranoid thinking in the general population. Br J Psychiatry 2008; 192:258–263Crossref, Medline, Google Scholar
2 : The cognitive and affective structure of paranoid delusions: a transdiagnostic investigation of patients with schizophrenia spectrum disorders and depression. Arch Gen Psychiatry 2009; 66:236–247Crossref, Medline, Google Scholar
3 : Psychotic experiences in the general population: a twenty-year prospective community study. Schizophr Res 2007; 92:1–14Crossref, Medline, Google Scholar
4 : Which patients with non-affective functional psychosis are not admitted at first psychiatric contact? Br J Psychiatry 1994; 165:101–106Crossref, Medline, Google Scholar
5 : Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry 2008; 65:28–37Crossref, Medline, Google Scholar
6 : Suspicious minds: the psychology of persecutory delusions. Clin Psychol Rev 2007; 27:425–457Crossref, Medline, Google Scholar
7 : What does the amygdala contribute to social cognition? Ann N Y Acad Sci 2010; 1191:42–61Crossref, Medline, Google Scholar
8 : Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog Neurobiol 2005; 77:283–298Medline, Google Scholar
9 : Social threat perception and the evolution of paranoia. Neurosci Biobehav Rev 2004; 28:333–342Crossref, Medline, Google Scholar
10 : Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry 2003; 54:515–528Crossref, Medline, Google Scholar
11 : The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci Biobehav Rev 2010; 34:468–486Crossref, Medline, Google Scholar
12 : A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Res 1999; 92:11–31Crossref, Medline, Google Scholar
13 : Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res 2008; 99:164–175Crossref, Medline, Google Scholar
14 : An investigation of the relationship between activation of a social cognitive neural network and social functioning. Schizophr Bull 2008; 34:688–697Crossref, Medline, Google Scholar
15 : Neural responses to dynamic expressions of fear in schizophrenia. Neuropsychologia 2007; 45:107–123Crossref, Medline, Google Scholar
16 : Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am J Psychiatry 2004; 161:480–489Link, Google Scholar
17 : Fronto-limbic and autonomic disjunctions to negative emotion distinguish schizophrenia subtypes. Psychiatry Res 2007; 155:29–44Crossref, Medline, Google Scholar
18 : Perfusion imaging. Magn Reson Med 1992; 23:37–45Crossref, Medline, Google Scholar
19 : Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci USA 1992; 89:212–216Crossref, Medline, Google Scholar
20 : Implicit trustworthiness decisions: automatic coding of face properties in the human amygdala. J Cogn Neurosci 2007; 19:1508–1519Crossref, Medline, Google Scholar
21 : Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci 2002; 5:277–283Crossref, Medline, Google Scholar
22 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia, 1987; 13:261–276Google Scholar
23 : Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 2003; 64:663–667Crossref, Medline, Google Scholar
24 : Distributed circuits underlying anxiety. Front Behav Neurosci 2014; 8:112Crossref, Medline, Google Scholar
25 Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magnetic Reson Med 2010; 63:765–771Crossref, Medline, Google Scholar
26 : Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magnetic Reson Med 2008; 60:1488–1497Crossref, Medline, Google Scholar
27 : The human amygdala in social judgment. Nature 1998; 393:470–474Crossref, Medline, Google Scholar
28 : Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J Magn Reson Imaging 2013; 38:1177–1183Crossref, Medline, Google Scholar
29 Ward BD: Simultaneous inference for fMRI data, 2000. http://afninimhnihgov/pub/dist/doc/manual/AlphaSimpdfGoogle Scholar
30 : Resting-state perfusion in nonmedicated schizophrenic patients: a continuous arterial spin-labeling 3.0-T MR study. Radiology 2010; 256:253–260Crossref, Medline, Google Scholar
31 : Neural response to emotional salience in schizophrenia. Neuropsychopharmacology 2005; 30:984–995Crossref, Medline, Google Scholar
32 : 18FDG PET study of amygdalar activity during facial emotion recognition in schizophrenia. Eur Arch Psychiatry Clin Neurosci 2010; 260:69–76Crossref, Medline, Google Scholar
33 : Amygdala recruitment in schizophrenia in response to aversive emotional material: a meta-analysis of neuroimaging studies. Schizophr Bull 2012; 38:608–621Crossref, Medline, Google Scholar
34 : Does baseline cerebral blood flow affect task-related blood oxygenation level dependent response in schizophrenia? Schizophr Res 2012; 140:143–148Crossref, Medline, Google Scholar
35 : Pseudo-continuous arterial spin labeling MRI study of schizophrenic patients. Schizophr Res 2014; 154:113–118Crossref, Medline, Google Scholar
36 : Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Res 2011; 194:64–72Crossref, Medline, Google Scholar
37 : Static and dynamic characteristics of cerebral blood flow during the resting state in schizophrenia. Schizophr Bull 2013; 41:163-170Crossref, Medline, Google Scholar
38 : Resting state cerebral blood flow and objective motor activity reveal basal ganglia dysfunction in schizophrenia. Psychiatry Res 2011; 192:117–124Crossref, Medline, Google Scholar
39 : Co-planer Stereotaxic Atlas of the Human Brain. New York, Thieme Medical Publishers, 1988Google Scholar
40 : Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants. Neuroimage 2009; 46:486–499Crossref, Medline, Google Scholar



