Gray Matter Volume as an Intermediate Phenotype for Psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP)
Abstract
Objective
The study examined gray matter volume across psychosis diagnoses organized by dimensional and DSM-IV categories from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) sample.
Method
In total, 351 probands with psychosis (146 with schizophrenia, 90 with schizoaffective disorder, and 115 with psychotic bipolar I disorder), 369 of their first-degree relatives (134 were relatives of individuals with schizophrenia, 106 of individuals with schizoaffective disorder, and 129 of individuals with psychotic bipolar I disorder), and 200 healthy comparison subjects were assessed. Gray matter volumes from 3-T T1-weighted images were analyzed using the VBM8 toolbox for SPM8, and outcomes were determined at a false discovery rate-corrected threshold of p<0.005.
Results
Across the psychosis dimension, probands (N=351) and relatives with psychosis spectrum disorders (N=34) showed substantial overlapping gray matter reductions throughout the neocortex, whereas relatives without psychosis spectrum (N=332) had normal gray matter volumes relative to comparison subjects. Across DSM-IV diagnoses, schizophrenia and schizoaffective probands showed overlapping gray matter reductions in numerous cortical and subcortical regions, whereas psychotic bipolar probands showed limited gray matter reductions localized to the frontotemporal cortex relative to comparison subjects. All relative groups had gray matter volumes that did not differ from comparison subjects.
Conclusions
Across the dimensional psychosis categories, these findings indicate extensive neocortical gray matter reductions in psychosis probands and relatives with psychosis spectrum disorders, possibly reflecting lifetime psychosis burden, but normal gray matter in nonpsychotic relatives. Traditional DSM-IV psychosis grouping revealed partially divergent gray matter phenotypes for probands with schizophrenia or schizoaffective disorder (extensive neocortical or subcortical gray matter reductions) relative to those with psychotic bipolar disorder (smaller reductions were limited to frontotemporal regions). The dimensional conceptualization of psychosis appears useful in defining more homogenous disease categories that may help identify underlying psychosis biomarkers and develop a biologically driven diagnostic system and targeted treatments.
Categorization of serious mental illness remains controversial, with current diagnoses having vague boundaries, weak diagnostic validity, and limited promise for biological significance (1). Current diagnostic categories (e.g., schizophrenia and bipolar disorder) are based predominantly on phenomenological criteria and are not supported by biological definitions. The identification of pathophysiologically relevant disease biomarkers and testing these biomarkers as novel targets for treatment are critical for the field. The Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) is a multisite research collaboration established to test the manifestations and distribution of intermediate phenotypes across schizophrenia-bipolar diagnostic categories and to examine genetic associations of these intermediate phenotypes (2). Given the substantial overlap in clinical, neurophysiological, and molecular characteristics of schizophrenia and bipolar disorder (1), the B-SNIP has focused on testing a dimensional organization of psychosis, a well-defined clinical phenotype that can potentially serve as a useful dimension for exploring underlying disease biomarkers. Testing an intermediate phenotype dimensionally includes pooling all probands with the targeted clinical manifestation (e.g., psychosis) regardless of diagnoses, organizing their biological relatives by the phenotype expression into affected and unaffected, and contrasting all groups with healthy individuals. This approach was undertaken here to test whether psychosis-centered categorization of serious mental illness across schizophrenia-psychotic bipolar I disorder diagnoses would generate groups of probands and relatives with more homogenous disease biomarkers, as measured by whole brain gray matter voxel-based morphometry.
Considerable evidence from previous structural MRI reports supports robust reductions in gray matter volume and/or density throughout cortical and subcortical structures in probands with schizophrenia, with the most substantial deficits in the frontotemporal regions (3–5). In bipolar probands, recent meta-analyses have described gray matter reductions in the prefrontal, anterior cingulate, insular, and temporal cortices, overlapping with those observed in schizophrenia, albeit less extensively (6–8). Small studies focusing on gray matter volume in psychotic bipolar disorder have reported variable results, ranging from schizophrenia-like gray matter reductions (9) to near-normal neocortical volumes (10, 11). Even more controversy surrounds the conceptualization of schizoaffective disorder as a distinct diagnostic entity (12); schizoaffective probands are often intermingled with schizophrenia case subjects and show similar gray matter characteristics (13, 14).
Studies of biological relatives of psychosis probands have found variable gray matter alterations in the basal ganglia, parahippocampal gyrus, and prefrontal cortex in the relatives of schizophrenia probands (15) and in the frontotemporal regions in relatives of bipolar probands (16). Clinical heterogeneity within the relative samples may contribute, with various subgroups of relatives (e.g., affected compared with unaffected relatives) showing distinctive gray matter phenotypes. Characteristically, psychosis spectrum disorders (e.g., schizotypy) have been linked to decreased gray matter volumes in the frontotemporal, parietal, and insular cortices (17–19), whereas no such abnormalities have been observed in unaffected relatives (20). Overall, these findings suggest that probands across schizophrenia-bipolar diagnoses have regionally overlapping gray matter volume reductions (commonly in the frontotemporal neocortex and more extensively in schizophrenia), although reports in bipolar disorder are highly variable. Little is known specifically about gray matter phenotypes in psychotic bipolar disorder. While findings in relatives are inconsistent, relatives with psychosis spectrum disorders tend to show gray matter reductions similar to psychosis probands, suggesting heritability of these structural alterations and their linkage to psychosis. Few studies have contrasted volumetric changes across proband and relative groups defined by the psychosis dimension (20).
We examined gray matter volumetric phenotypes within the schizophrenia-psychotic bipolar disorder spectrum contrasted by the psychosis dimension or DSM-IV diagnoses. The psychosis dimension was defined by lifetime axis I (in probands) or axis II (cluster A, in relatives) psychosis spectrum diagnoses; the probands as a whole, and relatives with and without cluster A or psychosis spectrum disorders composed the psychosis dimension groups. We tested whether a common gray matter phenotype manifests in probands and relatives across the psychosis dimension and whether gray matter characteristics would discriminate DSM-IV diagnostic groups. Therefore, two sets of analyses were conducted: one contrasting gray matter volumes in probands and relatives across the psychosis dimension and the other contrasting probands and relatives within categorical DSM-IV diagnoses (see Statistical Analyses for details). We hypothesized that 1) across the psychosis dimension, psychosis probands would show reduced gray matter volumes in numerous cortical and subcortical regions, relatives with psychosis spectrum disorders would show milder gray matter deficits regionally overlapping with those in probands, and nonpsychotic relatives would show normal gray matter volumes relative to healthy comparison subjects; and 2) across DSM-IV diagnoses, probands with schizophrenia, probands with schizoaffective disorder, and probands with psychotic bipolar I disorder would show regionally overlapping gray matter reductions that were most extensive in the frontotemporal cortex, with their magnitude being largest in schizophrenia and smallest in bipolar probands, and that their relatives would show overlapping frontotemporal gray matter changes intermediate in magnitude between probands and healthy comparison subjects.
Method
Study Sample
In total, 920 case subjects, including 351 psychosis probands (146 with schizophrenia, 90 with schizoaffective disorder, and 115 with psychotic bipolar I disorder), 369 relatives (134 were relatives of individuals with schizophrenia, 106 of individuals with schizoaffective disorder, and 129 of individuals with psychotic bipolar I disorder), and 200 healthy comparison subjects were recruited into the same dense phenotyping protocol across four B-SNIP sites between January 2008 and February 2011. Detailed characteristics of the B-SNIP clinical population are described elsewhere (2). All case subjects provided written informed consent after the study procedures had been fully explained. The axis I diagnoses for the probands and relatives were based on the Structured Clinical Interview for DSM-IV Axis I Disorders/Patient Edition (SCID-I/P) (21), and relatives’ axis II diagnoses were based on the Structured Interview for DSM-IV Personality (SIDP-IV) (22) (Figure 1). Probands were stable medicated outpatients; relatives with lifetime psychiatric diagnoses were asymptomatic or mildly symptomatic at the time of the imaging acquisition. Relatives who met the criteria for axis I psychotic disorders (41/369 with proband-like psychosis diagnoses [schizophrenia, schizoaffective disorder, or psychotic bipolar I disorder] and 8/369 with other axis I psychoses) were excluded from these analyses. In all, 34 relatives met DSM-IV or SIDP-IV criteria for a cluster A or psychosis spectrum personality disorder and were included. The nonpsychotic relatives group included relatives with no lifetime psychiatric diagnoses (completely unaffected) and those with nonpsychotic axis I or axis II diagnoses (e.g., mood and anxiety disorders or cluster B and C personality disorders). Rates of DSM-IV axis I or axis II diagnoses in relatives are presented in the data supplement that accompanies the online edition of this article.
a Four relatives (two relatives of probands with schizophrenia, one relative of a proband with schizoaffective disorder, and one relative of a proband with psychotic bipolar I disorder) were not included in the psychosis dimension analysis as a result of limited diagnostic information that did not allow for their accurate categorization into either the “relatives with psychosis spectrum personality disorders” or “nonpsychotic relatives” groups.
The demographic and clinical characteristics of the study sample are outlined in Table 1. Between-group differences were observed in age, accounted for by the older age of relatives compared with probands. Schizophrenia probands had a higher proportion of male individuals compared with other groups. Between-group differences were also found in handedness as a result of a higher number of ambidextrous individuals among the schizoaffective relatives compared with the rest of the relative groups and the healthy comparison subjects. While groups did not differ in ethnicity, differences were found in race, with a higher proportion of African Americans among schizophrenia and schizoaffective probands and schizophrenia relatives compared with bipolar probands and healthy comparison subjects. Between-group differences were also found in years of education, where schizophrenia and schizoaffective probands had lower education attainment than all other groups.
| Variablea | Schizophrenia Probands (N=146) | Schizoaffective Disorder Probands (N=90) | Psychotic Bipolar I Probands (N=115) | Relatives of Schizophrenia Probands (N=134) | Relatives of Schizoaffective Disorder Probands (N=106) | Relatives of Psychotic Bipolar I Probands (N=129) | Healthy Comparison Subjects (N=200) | Analysisb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sociodemographic characteristic | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | df | p |
| Age (years) | 35.8 | 12.9 | 36.3 | 11.4 | 35.4 | 12.5 | 43.7 | 15.6 | 43.0 | 15.8 | 40.5 | 16.1 | 39.8 | 12.1 | 7.56 | 6, 913 | <0.001 |
| Education (years) | 12.9 | 2.2 | 13.0 | 2.2 | 14.4 | 2.2 | 14.4 | 2.5 | 14.3 | 3.1 | 14.5 | 2.8 | 15.1 | 2.4 | 15.51 | 6, 906 | <0.001 |
| WRAT IQ estimate | 94.1 | 17.0 | 93.9 | 13.6 | 103.7 | 13.6 | 97.8 | 15.5 | 103.4 | 15.8 | 103.1 | 14.4 | 102.0 | 13.4 | 10.19 | 6, 884 | <0.001 |
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | χ2 | df | p | |
| Male gender | 102 | 69.9 | 37 | 41.1 | 36 | 31.3 | 40 | 29.9 | 31 | 29.2 | 43 | 33.3 | 92 | 46.0 | 72.61 | 6 | <0.001 |
| Handedness | 24.03 | 12 | 0.02 | ||||||||||||||
| Right-handed | 128 | 87.7 | 78 | 86.7 | 95 | 82.6 | 120 | 89.6 | 94 | 88.7 | 109 | 84.5 | 179 | 89.5 | |||
| Left-handed | 17 | 11.6 | 8 | 8.9 | 19 | 16.5 | 14 | 10.4 | 7 | 6.6 | 19 | 14.7 | 19 | 9.5 | |||
| Ambidextrous | 1 | 0.7 | 4 | 4.4 | 1 | 0.9 | 0 | 0.0 | 5 | 4.7 | 1 | 0.8 | 2 | 1.0 | |||
| Ethnicity/Hispanic | 14 | 9.6 | 8 | 8.9 | 8 | 7.0 | 17 | 12.7 | 6 | 5.7 | 11 | 8.5 | 19 | 9.5 | 4.4 | 6 | 0.62 |
| Race | 53.25 | 12 | <0.001 | ||||||||||||||
| Caucasian | 74 | 50.7 | 49 | 54.4 | 86 | 74.8 | 79 | 59.0 | 74 | 69.8 | 109 | 84.5 | 136 | 68.0 | |||
| African-American | 61 | 41.2 | 35 | 38.9 | 21 | 18.3 | 46 | 34.3 | 28 | 26.4 | 16 | 12.4 | 48 | 24.0 | |||
| Other | 11 | 7.5 | 6 | 6.7 | 8 | 7.0 | 9 | 6.7 | 4 | 3.8 | 4 | 3.1 | 16 | 8.0 | |||
| Clinical characteristic | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | df | p |
| Age at illness onset (years) | 21.5 | 8.0 | 19.8 | 8.7 | 20.2 | 8.7 | — | — | — | — | — | — | — | — | 1.37 | 2, 334 | 0.26 |
| Age at first hospitalization (years) | 22.7 | 7.1 | 21.6 | 7.8 | 23.7 | 9.1 | — | — | — | — | — | — | — | — | 1.47 | 2, 296 | 0.23 |
| Lifetime number of hospitalizations | 5.7 | 9.5 | 6.7 | 7.2 | 5.1 | 6.0 | — | — | — | — | — | — | — | — | 0.81 | 2, 271 | 0.45 |
| PANSS scores | |||||||||||||||||
| Total | 65.0 | 17.4 | 68.6 | 15.2 | 56.0 | 13.7 | — | — | — | — | — | — | — | — | 18.08 | 2, 341 | <0.001 |
| Positive subscale | 16.6 | 5.5 | 18.0 | 4.6 | 13.4 | 4.4 | — | — | — | — | — | — | — | — | 23.24 | 2, 341 | <0.001 |
| Negative subscale | 16.9 | 6.4 | 16.0 | 3.9 | 12.9 | 4.3 | — | — | — | — | — | — | — | — | 19.9 | 2, 342 | <0.001 |
| General symptoms subscale | 31.6 | 8.6 | 34.5 | 9.4 | 29.7 | 8.1 | — | — | — | — | — | — | — | — | 7.76 | 2, 342 | <0.001 |
| Young Mania Rating Scale score | 4.9 | 5.2 | 6.4 | 6.2 | 5.7 | 6.3 | — | — | — | — | — | — | — | — | 1.85 | 2, 338 | 0.16 |
| MADRS score | 8.6 | 7.3 | 14.2 | 10.1 | 10.5 | 9.3 | — | — | — | — | — | — | — | — | 11.14 | 2, 336 | <0.001 |
| GAF score | 48.0 | 12.4 | 48.8 | 11.5 | 59.5 | 11.6 | 77.0 | 11.8 | 77.9 | 12.1 | 76.6 | 12.4 | 85.5 | 6.9 | 254.51 | 6, 899 | <0.001 |
| Concomitant medicationc | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |||
| Off psychotropic medications | 5 | 3.4 | 2 | 2.2 | 7 | 6.1 | 61 | 45.5 | 42 | 39.6 | 85 | 65.9 | 200 | 100.0 | |||
| Antipsychotics | 129 | 88.4 | 78 | 86.7 | 82 | 71.3 | 1 | 0.8 | 3 | 2.8 | 5 | 3.9 | 0.0 | 0.0 | |||
| Mood stabilizers | |||||||||||||||||
| Lithium | 8 | 5.5 | 13 | 14.4 | 26 | 22.6 | 0 | 0.0 | 1 | 0.9 | 2 | 1.6 | 0.0 | 0.0 | |||
| Anticonvulsants | 21 | 14.4 | 35 | 38.9 | 72 | 62.6 | 1 | 0.8 | 8 | 7.6 | 8 | 6.2 | 0.0 | 0.0 | |||
| Antidepressants | 57 | 39.0 | 47 | 52.2 | 51 | 44.4 | 19 | 14.2 | 22 | 20.7 | 31 | 24.0 | 0.0 | 0.0 | |||
| Other | 53 | 36.3 | 38 | 42.2 | 48 | 41.7 | 10 | 6.9 | 10 | 9.4 | 15 | 11.6 | 0.0 | 0.0 | |||
| Combined medications | 89 | 61.0 | 75 | 83.3 | 89 | 77.4 | 8 | 6.0 | 12 | 11.3 | 15 | 11.6 | 0.0 | 0.0 | |||
The reading subtest scores from Wide-Range Achievement Test (WRAT), used as an estimate of premorbid intellectual functioning, differed across groups, with lower scores in schizophrenia and schizoaffective probands compared with bipolar probands, relatives of schizoaffective and bipolar probands, and comparison subjects. The proband groups did not differ in age at illness onset, age at first psychiatric hospitalization, or lifetime number of hospitalizations. Schizophrenia and schizoaffective probands had higher Positive and Negative Syndrome Scale (PANSS) (23) total scores and positive and negative subscales scores compared with bipolar probands; schizoaffective probands had higher PANSS general subscale scores compared with the rest of proband groups. In addition, schizoaffective probands had higher Montgomery-Åsberg Depression Rating Scale (MADRS) (24) scores compared with schizophrenia and bipolar probands, whereas no between-group differences in Young Mania Rating Scale (25) scores were found. All proband and relative groups scored lower than comparison subjects on the Global Assessment of Functioning, with the lowest scores found in schizophrenia and schizoaffective probands.
Most probands (318/351, 90.6%) were actively treated with various psychotropic medications, including antipsychotics (82.3%), mood stabilizers (49.9%), and antidepressants (44.2%); 253 probands (72.1%) were receiving more than one psychotropic agent. Among relatives, many were unmedicated; antidepressants were the most common medication in the relative groups.
MRI Acquisition and Voxel-Based Morphometry Procedures
Whole-brain structural MRI three-dimensional acquisitions were performed on 3-T scanners (GE Signa, Philips Achieva, Siemens Allegra, and Siemens Trio). All participants at each site were scanned on the same magnet. High resolution isotropic T1-weighted MP-RAGE sequences were obtained following the Alzheimer’s Disease Neuroimaging Initiative (ADNI) protocol (http://www.loni.ucla.edu/ADNI/Research/Cores/). The MP-RAGE sequence parameters were comparable across sites (see details in the online data supplement).
All images were processed by experienced analysts (image preprocessing by ASB and data analyses by EII) who were blind to the participants’ clinical characteristics. T1-weighted MP-RAGE images were prepared for voxel-based morphometry analyses in SPM8/VBM8/MATLAB7 following standardized steps (26): reorientation, high-dimensional nonlinear diffeomorphic anatomical registration using the modulation tool (DARTEL) segmentation and normalization that incorporates precise correction for individual brain size (27), and smoothing. Segmented images were modulated or scaled by the amount of warping to maintain the total amount of gray matter volume (28). Modulated gray matter images were smoothed with a 12-mm isotropic Gaussian kernel and selected for the group-level statistical analyses.
Statistical Analyses
A one-way analysis of variance (ANOVA) with a subsequent post hoc Tukey honestly significant difference test and Yates corrected chi-square test were used, as appropriate, for demographic and clinical variables. To test a priori hypotheses, the primary analyses contrasted gray matter volumes across the psychosis dimension groups (probands, relatives with psychosis spectrum disorders, nonpsychotic relatives, and healthy comparison subjects) and DSM-IV diagnostic groups (schizophrenia, schizoaffective, and psychotic bipolar I probands, their respective relatives, and healthy comparison subjects). These analyses were completed using SPM8 full factorial design. Absolute threshold masking was set at 0.1. All analyses were adjusted for individual brain volume during DARTEL segmentation/normalization step. The distinctive group demographic characteristics that are known to affect brain structure (age, sex, and handedness) (29, 30) as well as site were included as covariates in all statistical models. In addition, all analyses comparing related individuals (i.e., probands and relatives from the same pedigree) were adjusted for random family effects. No diagnosis-by-site interaction (healthy comparison subjects and the three proband groups × four sites) was observed, even at the least stringent threshold (p<0.05, false discovery rate corrected). In addition, between-site comparisons for the healthy comparison subjects and schizophrenia probands revealed similar regional distributions of gray matter reductions across sites, further supporting between-site data comparability (see Figure S1 in the online data supplement). All primary voxel-based morphometry outcomes are reported at a p<0.005, false discovery rate corrected, k=200 contiguous voxels threshold, providing the most informative between-group volumetric differences (see Figure S2 in the online data supplement). Regional outputs were identified using the Group ICA for fMRI Toolbox, GIFT1.3i (31; www.sourceforge.net) checked against a standardized anatomical brain atlas (32).
In addition, exploratory multiple regression analyses were carried out to examine associations between gray matter volumes and symptom severity (using PANSS, Young Mania Rating Scale, and MADRS scores) and illness duration (adjusted for age, gender, and site) in the proband groups. Associations between gray matter volume and concomitant medication use (use status dichotomized as on or off antipsychotics, mood stabilizers, and antidepressants) were computed in all probands combined (given the small number of participants treated with mood stabilizers and antidepressants) as well as in the bipolar probands alone for current lithium use (i.e., multiple regression analysis with status dichotomized as on or off lithium and direct comparisons of gray matter volume in bipolar probands on [N=26] or off [N=26] lithium). Because the number of relatives on medications was low, especially for antipsychotics and mood stabilizers, we only computed correlations between gray matter volumes and antidepressant use in all relatives combined. All exploratory outcomes were tested at both p<0.005 and p<0.05, false discovery rate corrected, k=200 contiguous voxels thresholds.
Results
Gray Matter Volume Phenotypes Across the Psychosis Dimension
Regional gray matter volumes contrasted along the psychosis dimension (probands, relatives with psychosis spectrum disorders, nonpsychotic relatives, and healthy comparison subjects) revealed a between-group effect (F=2.98, df=5, 903, p<0.005, false discovery rate corrected, k=200) (Figure 2A and see Table S2 in the online data supplement). Subsequent pairwise comparisons revealed diffuse gray matter volume reductions in psychosis probands relative to healthy comparison subjects in the frontal, anterior/posterior cingulate, insular, temporal, parietal, and occipital cortices and in the basal ganglia, thalamus, and cerebellum. Relatives with psychosis spectrum disorders contrasted with healthy comparison subjects showed volume reductions regionally overlapping those found in probands, albeit less extensive. No differences emerged between nonpsychotic relatives and comparison subjects. No differences were observed between the two subgroups within the nonpsychotic relatives group (i.e., completely unaffected and those with nonpsychotic axis I or axis II diagnoses) and healthy comparison subjects. No gray matter volume increases were observed in any psychosis dimension group relative to healthy comparison subjects.
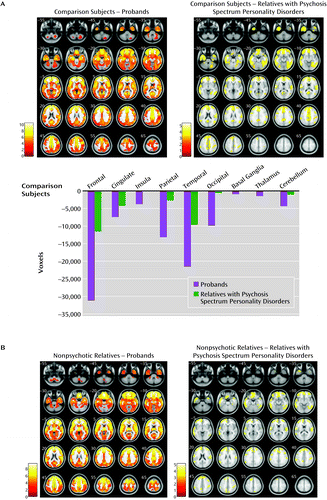
a Panel A shows the regional gray matter volume reductions in the psychosis probands and relatives with psychosis spectrum disorders as compared with healthy comparison subjects. The bar graphs depict cumulative gray matter volume reductions in proband and relatives with psychosis spectrum personality disorders compared with healthy comparison subjects/zero line; voxels of reduction are averaged across the right and the left hemispheres. Panel B shows the regional gray matter volume reductions in probands and relatives with cluster A or psychosis spectrum personality disorders contrasted with nonpsychotic relatives. All imaging contrasts are presented at p<0.005, false discovery rate corrected, k=200 voxels threshold. Images are displayed in neurological convention. Color bars in panel A and panel B indicate t scores.
Pairwise comparisons between the psychosis dimension groups revealed overlapping gray matter volume reductions in the frontotemporal, anterior cingulate, and parietal regions in the psychosis probands and in relatives with psychosis spectrum disorders compared with nonpsychotic relatives (Figure 2B and see Table S2 in the online data supplement). No differences were found between probands and relatives with psychosis spectrum disorders. No differences emerged between completely unaffected relatives compared with relatives with nonpsychotic diagnoses within the nonpsychotic relatives group. No volume increases were found in either the psychosis probands or relatives with psychosis spectrum disorders compared with nonpsychotic relatives.
Gray Matter Volume Phenotypes Across DSM-IV Diagnoses
The proband group was divided by diagnosis (schizophrenia, schizoaffective disorder, and psychotic bipolar I disorder) and assessed against healthy comparison subjects, and a significant between-group effect was observed (F=3.9, df=3, 542, p<0.005, false discovery rate corrected, k=200). Subsequent pairwise comparisons revealed substantial overlapping gray matter reductions in schizophrenia and schizoaffective probands in the frontal, anterior/posterior cingulate, insular, temporal, parietal, and occipital cortices, as well as in the basal ganglia, thalamus, and cerebellum relative to healthy comparison subjects. Probands with psychotic bipolar disorder showed smaller clusters of gray matter reduction in the frontal, anterior/posterior cingulate, insular, temporal, and parietal cortices relative to healthy comparisons subjects, regionally overlapping with those in schizophrenia and schizoaffective probands (Figure 3A and see Table S3 in the online data supplement). No increases in gray matter volume were found in any proband group relative to healthy comparison subjects.
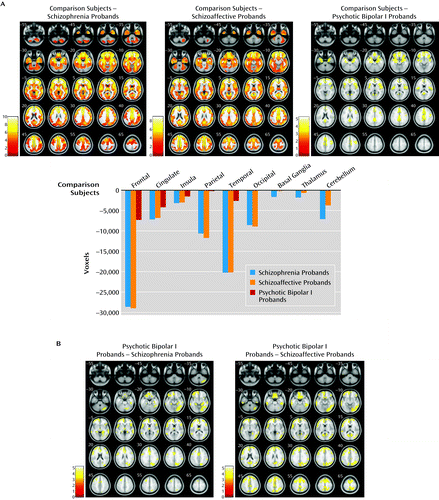
a Panel A shows regional gray matter volume reductions in probands with schizophrenia, schizoaffective disorder, and psychotic bipolar I disorders relative to healthy comparison subjects. The bar graph depicts cumulative gray matter volume reductions in the three proband groups relative to healthy comparison subjects/zero line; voxels of reduction are averaged across the right and the left hemispheres. Panel B shows the regional gray matter volume reductions in probands with schizophrenia and schizoaffective disorder contrasted with bipolar probands. All imaging contrasts are presented at p<0.005, false discovery rate corrected, k=200 voxels threshold. Images are displayed in neurological convention. Color bars in panel A and panel B indicate t scores.
Pairwise gray matter volume contrasts between proband groups revealed no differences in schizophrenia probands compared with schizoaffective probands in any brain regions (Figure 3B and see Table S3 in the online data supplement). Both schizophrenia and schizoaffective probands had reduced gray matter volumes compared with psychotic bipolar I probands throughout the neocortex and cerebellum; schizophrenia probands showed an additional cluster of gray matter reduction in the left thalamus compared with bipolar probands. No gray matter volume increases characterized either schizophrenia or schizoaffective probands compared with bipolar probands.
The relatives contrast revealed no effect of diagnostic group (schizophrenia, schizoaffective disorder, bipolar disorder, and healthy comparison subject groups) even at the least stringent threshold (p<0.05, false discovery rate corrected).
Associations Between Gray Matter Volume and Lifetime Psychosis Duration, Active Symptoms Severity, and Medication
In probands with schizoaffective disorder, lifetime duration of psychosis correlated inversely with gray matter volumes in the basal ganglia bilaterally, the right temporal lobe, and the thalamus (p=0.05) (see Table S4 in the online data supplement). No significant correlations between gray matter and psychosis duration were found in either schizophrenia or bipolar probands.
Furthermore, in schizoaffective probands, PANSS positive subscale scores correlated inversely with gray matter volumes throughout the neocortex (p=0.005). In schizophrenia probands, PANSS positive subscale scores correlated inversely with gray matter volumes in the right insular and the left parahippocampal gyrus (p=0.05) (see Table S4 in the online data supplement). No other significant correlations emerged between gray matter volume and PANSS, MADRS, or Young Mania Rating Scale scores in any proband group.
No associations between gray matter volume and current use of antipsychotics, mood stabilizers, or antidepressants were found among probands. No association between gray matter volume and active lithium treatment was evident in probands with psychotic bipolar I disorder. Likewise, no between-group differences in gray matter volume were found in bipolar probands on lithium compared with off lithium. No relationship between active antidepressant use and gray matter volume was found in relatives. All exploratory medication outcomes were nonsignificant at p=0.05, false discovery rate corrected, k=200 threshold.
Discussion
This study presents voxel-based morphometry-based gray matter volumetric outcomes contrasted between the psychosis dimension and DSM-IV categorical diagnoses groups from a large multisite psychosis sample (B-SNIP). The psychosis dimension analysis revealed diffuse gray matter reductions in both the psychosis probands and in relatives with psychosis spectrum disorders in overlapping cortical regions (e.g., the frontotemporal, parietal, cingulate, and insular cortices) and the cerebellum relative to healthy comparison subjects. By contrast, relatives unaffected by psychosis, even those with lifetime nonpsychotic axis I or axis II diagnoses, had normal gray matter volume. The DSM-IV diagnosis analysis in probands relative to healthy comparison subjects revealed extensive gray matter reductions in numerous cortical and subcortical regions that were similarly distributed in schizophrenia and schizoaffective probands. Psychotic bipolar I probands had gray matter volume reductions primarily localized to the frontotemporal, cingulate, and insular cortices that were regionally overlapping with those in schizophrenia and schizoaffective probands, albeit less extensive. Probands with schizophrenia and those with schizoaffective disorder showed smaller cortical and subcortical gray matter volumes compared with bipolar probands. First-degree relatives of the psychosis probands, when categorized by DSM-IV diagnoses, had gray matter volumes indistinguishable from comparison subjects, contrary to our a priori prediction (Table 2).
| Gray matter phenotypes within the psychosis dimension | |||
| Probands | Relatives with psychosis spectrum personality disorders | Nonpsychotic relatives | |
| Healthy comparison subjects | ↓↓↓ | ↓↓↓ | = |
| Gray matter phenotypes within the DSM-IV categorical diagnoses | |||
| Probands | |||
| Schizophrenia probands | Schizoaffective disorder probands | Psychotic bipolar I probands | |
| Healthy comparison subjects | ↓↓↓ | ↓↓↓ | ↓ |
| Relatives | |||
| Relatives of schizophrenia probands | Relatives of schizoaffective disorder probands | Relatives of psychotic bipolar I probands | |
| Healthy comparison subjects | = | = | = |
Growing evidence suggests that psychosis as a clinical phenotype may have common biological underpinnings across categorical diagnoses (1). Measurable gray matter changes are observed in individuals near psychosis onset, progress during the initial years of psychosis, and ultimately result in characteristic gray matter alterations found in chronic psychosis samples (33, 34). Data from relatives with psychosis spectrum disorders demonstrate milder but similar gray matter volume reductions (17–19), suggesting a heritable link between psychosis and brain structure phenotypes. Our findings within the psychosis dimension indicate substantial and overlapping gray matter volume reductions in psychosis probands and relatives with psychosis spectrum disorders, in contrast to normal gray matter in nonpsychotic relatives. These gray matter alterations may reflect “psychosis burden,” from extensive reductions in probands where psychosis is fully manifested, to similarly distributed but milder alterations in relatives with mild psychosis spectrum phenotypes, to normal gray matter in relatives without lifetime psychosis spectrum disorders. Remarkably, relatives categorized by traditional schizophrenia or bipolar disorder diagnoses showed no alterations in gray matter volume. Furthermore, a subgroup of relatives with lifetime nonpsychotic axis I or axis II diagnoses had normal gray matter volumes, consistent with previous reports (20). Our findings from analyses of DSM-IV diagnosis in probands, i.e., robust gray matter reductions in probands with schizophrenia and schizoaffective disorder compared with milder overlapping reductions in probands with psychotic bipolar I disorder, may reflect cumulative lifetime psychosis burden in these psychiatric conditions, with psychosis assumed to be more pervasive in schizophrenia and schizoaffective disorder than in bipolar disorder. This is further supported by inverse correlations between lifetime duration of psychosis and gray matter volume in schizoaffective probands in the basal ganglia, the temporal cortex, and the thalamus, the regions long implicated in psychosis in both first-episode and chronic samples (3–5). Inverse correlations between frontotemporal gray matter volumes and PANSS psychosis scores in schizoaffective and schizophrenia probands also support a link between psychosis and gray matter reduction. Overall, these findings suggest that psychosis is a clinical dimension characterized by unique gray matter intermediate phenotypes, namely by reductions primarily localized to the frontotemporal neocortex.
Our findings across categorical diagnoses indicate partially divergent gray matter volume characteristics for schizophrenia and schizoaffective disorder (with extensive cortical and subcortical gray matter reductions) compared with psychotic bipolar I disorder (with limited gray matter reductions in the frontotemporal and parietal cortex) that are different in the extent and magnitude but that overlap in regional distribution, consistent with previous reports (3–5, 8). The absence of differences between schizophrenia and schizoaffective case subjects raises questions about the biological uniqueness of the schizoaffective disorder construct (12), while the substantive differences between schizophrenia/schizoaffective disorder and psychotic bipolar I disorder provide some support for the schizophrenia-bipolar distinction. The mechanisms underlying these gray matter differences are unknown but can be interpreted in the context of published postmortem findings of gray matter pathology in the neocortex. Histological examination of individuals with schizophrenia indicates no neocortical neuronal loss but altered neuronal cell packing, presumably due to reduced interstitial neuropil, resulting in higher neuronal density and decreased cortical thickness (35). In contrast, analyses from individuals with bipolar disorder reveal decreased neuronal and glial density but normal overall cortical thickness (35). These distinct postmortem findings in schizophrenia compared with bipolar disorder suggest at least partially unique anatomical underpinnings for the two illnesses, providing plausible cellular correlates for the divergent gray matter findings in schizophrenia/schizoaffective probands and psychotic bipolar probands that were observed here. Further structural MRI analyses parsing cortical thickness and surface area may shed light on this issue. Alternatively, it is possible that a relative gray matter volume preservation in bipolar probands could be secondary to a medication effect (i.e., chronic treatment with lithium) and that even if a primary disease-associated loss of neocortical volume exists in these probands, it could be obscured by a volume-enhancing effect of chronic lithium use (36, 37). These two explanations could co-occur. No postmortem studies have contrasted psychotic and nonpsychotic variants of bipolar disorder or tissue from individuals with psychosis spectrum disorders, thus leaving open the question of whether the cellular alterations observed in schizophrenia would generalize to the psychosis dimension.
Previous reports provide support for an effect of psychotropic medications on gray matter volume, with increased gray matter volume or density in the basal ganglia associated with first-generation antipsychotics (38), decreased gray matter volume in the frontotemporal cortex associated with both first- and second-generation antipsychotics (39, 40), and increased gray matter volume or density in diffuse neocortical regions associated with lithium (6, 7, 36, 37). The effect of current medication use was tested here, and the results were negative. However, this outcome could be obscured by the high frequency of mixed medication use (61%−83%) in all proband groups, as well as by the longitudinal effects of both disease and medication on brain structure. Given that the majority of probands reported years of chronic treatment with various psychotropic agents, it is possible that the extensive gray matter deficits in probands with schizophrenia and schizoaffective disorder observed here could be, in part, accounted for by the effect of lifetime antipsychotic use, whereas a relative preservation of gray matter volumes in bipolar probands may be related to chronic lithium treatment, despite our negative findings for active medication use. Disentangling a primary disease effect from a medication confound presents considerable difficulty, especially in a cross-sectional study such as ours, and it cannot be accomplished in probands alone. Nevertheless, with the benefit of data from relatives, we suggest that the characteristic gray matter reductions found in relatives with psychosis spectrum disorders where individuals were largely untreated support at least partial independence of these gray matter alterations from medication and suggest their link to psychosis.
Overall, these results from a large sample of psychosis probands and relatives suggest that the dimensional conceptualization of psychosis is a useful approach for investigations targeting biological markers of serious mental illness. The strengths of our study are the relatively large sample, the study of psychosis probands and their relatives across DSM-IV psychosis categories, and the comprehensive clinical characterization of these individuals that allowed a systematic investigation of the psychosis dimension. Limitations include the cross-sectional nature of the study and the potential confounds related to medications and state of illness. The findings generated by the voxel-based morphometry analyses need to be confirmed and expanded by region-of-interest approaches to morphometric quantification. Future research examining the associations between brain structure and other putative intermediate phenotypes, as well as their molecular underpinnings, may aid in the development of a biologically based classification of serious mental illness to leverage discovery and treatment.
1 : Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull 2008; 34:760–773Crossref, Medline, Google Scholar
2 : Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry 2013; 170:1263–1275Link, Google Scholar
3 : Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry 2005; 162:2233–2245Link, Google Scholar
4 : The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry 2008; 165:1015–1023Link, Google Scholar
5 : Are bipolar disorder and schizophrenia neuroanatomically distinct? an anatomical likelihood meta-analysis. Front Hum Neurosci 2010; 4:189Crossref, Medline, Google Scholar
6 : Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry 2008; 65:1017–1032Crossref, Medline, Google Scholar
7 : Structural magnetic resonance imaging in bipolar disorder: an international collaborative mega-analysis of individual adult patient data. Biol Psychiatry 2011; 69:326–335Crossref, Medline, Google Scholar
8 : Grey matter differences in bipolar disorder: a meta-analysis of voxel-based morphometry studies. Bipolar Disord 2012; 14:135–145Crossref, Medline, Google Scholar
9 : Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biol Psychiatry 2005; 57:633–639Crossref, Medline, Google Scholar
10 : Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. Br J Psychiatry 2005; 186:369–377Crossref, Medline, Google Scholar
11 : Brain gray matter phenotypes across the psychosis dimension. Psychiatry Res 2012; 204:13–24Crossref, Medline, Google Scholar
12 : Is schizoaffective disorder a distinct categorical diagnosis? a critical review of the literature. Neuropsychiatr Dis Treat 2008; 4:1089–1109Crossref, Medline, Google Scholar
13 : Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry 1998; 55:1084–1091Crossref, Medline, Google Scholar
14 : Comparison of hippocampal volumes in schizophrenia, schizoaffective and bipolar disorder. Coll Antropol 2011; 35(suppl 1):249–252Medline, Google Scholar
15 : The neuroanatomy of psychotic diathesis: a meta-analytic review. J Psychiatr Res 2012; 46:1249–1256Crossref, Medline, Google Scholar
16 : Brain structural signature of familial predisposition for bipolar disorder: replicable evidence for involvement of the right inferior frontal gyrus. Biol Psychiatry 2013; 73:144–152Crossref, Medline, Google Scholar
17 : Schizotypal personality disorder and MRI abnormalities of temporal lobe gray matter. Biol Psychiatry 1999; 45:1393–1402Crossref, Medline, Google Scholar
18 : Structural brain differences in patients with schizophrenia and schizotypal disorder demonstrated by voxel-based morphometry. Eur Arch Psychiatry Clin Neurosci 2004; 254:406–414Crossref, Medline, Google Scholar
19 : Cortical gray and white matter volume in unmedicated schizotypal and schizophrenia patients. Schizophr Res 2008; 101:111–123Crossref, Medline, Google Scholar
20 : Is gray matter volume an intermediate phenotype for schizophrenia? a voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry 2008; 63:465–474Crossref, Medline, Google Scholar
21 : Structured Clinical Interview for DSM-IV Axis I Disorders/Patient Edition (SCID-I/P). New York, New York State Psychiatric Institute, Biometrics Research Department, 1996Google Scholar
22 : Structured Interview for DSM-IV Personality. Washington DC, American Psychiatric Press, 1997Google Scholar
23 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
24 : A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
25 : A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
26 : Voxel-based morphometry: the methods. Neuroimage 2000; 11:805–821Crossref, Medline, Google Scholar
27 : A fast diffeomorphic image registration algorithm. Neuroimage 2007; 38:95–113Crossref, Medline, Google Scholar
28 : A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001; 14:21–36Crossref, Medline, Google Scholar
29 : Regional specificity of sex effects on subcortical volumes across the lifespan in healthy aging. Hum Brain Mapp (Epub ahead of print, Sept 21, 2012)Google Scholar
30 : Cerebral asymmetry: a quantitative, multifactorial, and plastic brain phenotype. Twin Res Hum Genet 2012; 15:401–413Crossref, Medline, Google Scholar
31 : A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 2001; 14:140–151Crossref, Medline, Google Scholar
32 : The Human Brain: Surface, Blood Supply, and Three-Dimensional Sectional Anatomy, 2nd ed. New York, Springer Wien, 1999Crossref, Google Scholar
33 : Structural brain alterations at different stages of schizophrenia: a voxel-based morphometric study. Schizophr Res 2008; 104:44–60Crossref, Medline, Google Scholar
34 : Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry 2011; 70:672–679Crossref, Medline, Google Scholar
35 : Cellular pathology in the dorsolateral prefrontal cortex distinguishes schizophrenia from bipolar disorder. Curr Mol Med 2003; 3:427–436Crossref, Medline, Google Scholar
36 : Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol Psychiatry 2007; 62:7–16Crossref, Medline, Google Scholar
37 : A longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment-responsive bipolar disorder patients. J Clin Psychiatry 2009; 70:699–705Crossref, Medline, Google Scholar
38 ;
39 : The effects of antipsychotics on the brain: what have we learnt from structural imaging of schizophrenia? a systematic review. Curr Pharm Des 2009; 15:2535–2549Crossref, Medline, Google Scholar
40 : Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 2011; 68:128–137Crossref, Medline, Google Scholar



