Preserved Working Memory and Altered Brain Activation in Persons at Risk for Psychosis
Abstract
Objective
Patients with schizophrenia exhibit impairments in working memory that often appear in attenuated form in persons at high risk for the illness. The authors hypothesized that deviations in task-related brain activation and deactivation would occur in persons with an at-risk mental state performing a working memory task that entailed the maintenance and manipulation of letters.
Method
Participants at ultra high risk for developing psychosis (N=60), identified using the Comprehensive Assessment of At-Risk Mental States, and healthy comparison subjects (N=38) 14 to 29 years of age underwent functional MRI while performing a verbal working memory task. Group differences in brain activation were identified using analysis of covariance.
Results
The two groups did not show significant differences in speed or accuracy of performance, even after accounting for differences in education. Irrespective of task condition, at-risk participants exhibited significantly less activation than healthy comparison subjects in the left anterior insula. During letter manipulation, at-risk persons exhibited greater task-related deactivation within the default-mode network than comparison subjects. Region-of-interest analysis in the at-risk group revealed significantly greater right dorsolateral prefrontal cortex activation during manipulation of letters.
Conclusions
Despite comparable behavioral performance, at-risk participants performing a verbal working memory task exhibited altered brain activation compared with healthy subjects. These findings demonstrate an altered pattern of brain activation in at-risk persons that contains elements of reduced function as well as compensation.
Working memory is engaged for the temporary storage and manipulation of information, and it underpins many higher-order cognitive processes and goal-directed behaviors (1). Deficits in working memory are prominent in schizophrenia (2) and have been consistently observed in functional MRI (fMRI) studies of both established (3–6) and first-episode patients (7–9).
The neurodevelopmental model of schizophrenia posits that cognitive and physiological abnormalities in persons at high risk for schizophrenia may precede the appearance of full-blown symptoms (10–13). Cognitive function in at-risk individuals has been assessed over multiple domains, including fluid intelligence, executive function, working memory, speed of processing, and attention. Relevant to the present study, working memory deficits appear to distinguish persons at risk for psychosis from healthy subjects (14–18).
Functional neuroimaging studies of individuals at risk for psychosis have found that patterns of activation similar to those observed in schizophrenia patients may precede the development of psychosis (19). For example, alterations in prefrontal and parietal activation during working memory tasks have been reported prior to the appearance of significant behavioral change (20, 21). However, there is little agreement regarding the direction of these functional imaging shifts. Some studies have found task-related hyperactivation in the right dorsolateral prefrontal cortex, the left inferior parietal lobule, and the anterior cingulate cortex (22–24). Other studies have found reduced activation in the inferior frontal cortex (including the ventrolateral prefrontal cortex and the anterior insula [25, 26]), the dorsolateral prefrontal cortex, and the parietal cortex (27–29). The lack of agreement between studies may be attributed to the relatively small sample sizes (approximately 20 participants in each group), inclusion of at-risk individuals who are receiving medication, and differences in the task parameters used in working memory studies, as well as variation in behavioral performance between study and comparison samples (30, 31).
To address the inconsistencies in earlier imaging studies, we compared a relatively large sample of individuals with at-risk mental states, identified using the Comprehensive Assessment of At-Risk Mental States (32), with age-matched healthy comparison subjects from the community. Analyses of the imaging data emphasized interrogation of two main networks of regions that have previously shown anomalous activation related to working memory function in persons at risk for psychosis and in schizophrenia patients.
Method
Participants
We studied 69 persons at risk for psychosis and 40 comparison subjects between the ages of 14 and 29 years. Participants were recruited as part of the Longitudinal Youth At-Risk Study, which sought to identify and follow up ultra-high-risk persons over a 2-year period through psychiatric clinics in Singapore at the Institute of Mental Health, the Singapore Armed Forces, and community mental health services.
Participants were assigned to the at-risk group if they met criteria for any of the following three subgroups, based on the Comprehensive Assessment of At-Risk Mental States: 1) a vulnerable subgroup, comprising individuals with a family history of psychosis in a first-degree relative or with a schizotypal disorder; 2) an attenuated psychosis subgroup, comprising individuals with subthreshold psychotic symptoms; and 3) a brief limited intermittent psychotic symptoms subgroup, comprising individuals with a recent history of frank psychotic symptoms that resolved spontaneously within 1 week. At-risk participants had no history of psychiatric, neurological, or serious medical disorders or mental retardation, and they were not receiving any antipsychotic medications at the time of imaging. Persons with current substance abuse were excluded. Imaging data for three at-risk persons were excluded because of excessive head movement (>3 mm across runs). Data for an additional six at-risk participants were excluded because of poor task performance (<75% accuracy on control and maintenance-of-information conditions). Thus, data for 60 at-risk participants were analyzed. Demographic and clinical characteristics for the at-risk group are summarized in Table 1. The small numbers of participants categorized as vulnerable or as having brief limited intermittent psychotic symptoms made subgroup analyses untenable; however, we did investigate differences in brain activity between vulnerable-only participants (subgroup 1) and those exhibiting psychotic-spectrum symptoms (subgroups 2 and 3). For the results of this analysis, see Figure S1 in the data supplement that accompanies the online edition of this article. Six at-risk participants were later diagnosed with a psychotic disorder over a follow-up period ranging from 1 year for those recruited later in the course of the study to 2 years for those examined earlier.
| Characteristic | At-Risk Group (N=60) | Healthy Comparison Group (N=38) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Male | 41 | 68.3 | 21 | 55.3 |
| Handedness | ||||
| Right | 51 | 85.0 | 35 | 92.1 |
| Left | 4 | 6.7 | 1 | 2.6 |
| Mixed | 5 | 8.3 | 2 | 5.3 |
| Ethnicity | ||||
| Chinese | 40 | 66.7 | 23 | 60.5 |
| Malay | 16 | 26.7 | 6 | 15.8 |
| Indian | 3 | 5.0 | 7 | 18.4 |
| Other | 1 | 1.7 | 2 | 5.3 |
| Medication | ||||
| Antidepressantsb | 35 | 58.3 | ||
| Antipsychotic (chlorpromazine)c | 1 | 1.7 | ||
| At-risk subgroupsd | ||||
| Subgroup 1: vulnerable only | 10 | 16.7 | ||
| Subgroup 2: attenuated psychosis | 40 | 66.7 | ||
| Subgroup 3: brief limited intermittent psychotic symptoms | 1 | 1.7 | ||
| Subgroups 1 and 2 | 7 | 11.7 | ||
| Subgroups 2 and 3 | 1 | 1.7 | ||
| All three subgroups | 1 | 1.7 | ||
| Mean | SD | Mean | SD | |
| Age (years) | 21.5 | 2.6 | 22.8 | 4.0 |
| Education (years)e | 11.6 | 2.2 | 13.0 | 2.2 |
| Wechsler Abbreviated Scale of Intelligence, vocabulary scoref | 48.8 | 11.0 | 47.3 | 12.0 |
| Brief Assessment of Cognition in Schizophrenia | ||||
| Digit sequencing score | 20.7 | 4.1 | 20.5 | 3.2 |
| Symbol coding scoree | 59.3 | 11.1 | 66.1 | 9.8 |
| Global Assessment of Functioning scoreg | 54.7 | 10.8 | ||
| Positive and Negative Syndrome Scale | ||||
| Positive subscale score | 11.1 | 3.0 | ||
| Negative subscale score | 12.4 | 3.8 | ||
| General psychopathology subscale score | 26.5 | 7.2 | ||
| Total score | 50.0 | 11.4 | ||
| Comprehensive Assessment of At-Risk Mental State score (total severity plus frequencyh) | 15.9 | 7.7 | ||
| Accuracy (%) | ||||
| LTR condition | 91.3 | 8.5 | 91.5 | 8.3 |
| PLUS condition | 84.5 | 12.4 | 86.3 | 10.8 |
| PLUS2 condition | 78.3 | 15.6 | 81.1 | 14.5 |
| Control condition | 98.3 | 2.2 | 98.2 | 2.7 |
| Reaction time (msec) | ||||
| LTR condition | 811.0 | 105.9 | 835.3 | 116.6 |
| PLUS condition | 873.1 | 150.7 | 885.4 | 168.3 |
| PLUS2 condition | 970.3 | 174.0 | 965.7 | 163.2 |
| Control condition | 610.6 | 82.0 | 605.7 | 103.8 |
In the at-risk group, 35 participants were receiving prescription antidepressants, 24 were not receiving any medications, and one had previously received chlorpromazine at a low dosage but was not receiving any medications at the time of imaging. Aside from the participant who had previously received chlorpromazine, all at-risk participants were antipsychotic naive.
Healthy comparison subjects were recruited through public advertisements in print and online media. They had no history of psychiatric disorders and were matched for age with at-risk participants. One comparison subject was excluded because of excessive head motion, and one was excluded because of poor performance, leaving 38 healthy subjects for inclusion in the analysis.
Participants underwent a battery of neurocognitive tests assessing a range of functions, including working memory, attention, and vigilance. Additionally, at-risk participants were assessed using the Global Assessment of Functioning Scale (GAF), the Positive and Negative Syndrome Scale (PANSS [42]), the Brief Assessment of Cognition in Schizophrenia scale (43), and the Wechsler Abbreviated Scale of Intelligence (44). Because left-handedness is more common in schizophrenia patients, we did not attempt to balance handedness in the groups to avoid introducing bias; however, handedness was assessed using an inventory (45) for inclusion as a covariate in imaging interactions.
The study protocol was approved by the National Healthcare Group Domain Specific Review Board (Singapore). Written informed consent was obtained from participants above age 21 or from a parent or guardian for participants under 21 (with the participant’s assent) after they received a complete description of the study. All participants were reimbursed for their participation in the study.
Experimental Design
The working memory experiment (46) was one of four fMRI experimental paradigms administered in the functional neuroimaging battery. The other experiments (to be described in detail in separate reports) assessed associative learning and generalization (47), implicit processing of fearful and neutral male and female faces (48), and anticipation of or motivation to obtain a reward (49). The working memory experiment was always administered third.
The working memory experiment, which we refer to here as the working memory task, comprised three task conditions and one control condition. Briefly, the LTR condition evaluated maintenance of information, while the PLUS and PLUS2 conditions evaluated both maintenance and manipulation of information (Figure 1; see also the online data supplement). Participants were required to achieve an accuracy of at least 75% on both the LTR and control conditions. In the scanner, participants were presented with three runs of alternating blocks of conditions.
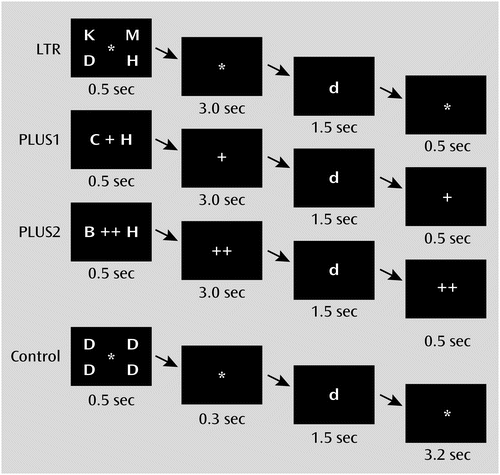
a In the LTR condition, participants were presented with four target letters, maintained them in memory for 3 seconds, and then indicated whether any of the targets matched a probe letter. In the PLUS condition, participants were presented with two target letters, shifted each one forward alphabetically by one position, maintained the new targets in memory for 3 seconds, and then indicated whether either of the new targets matched the probe letter. The PLUS2 condition was identical to the PLUS condition except that participants had to shift each target letter forward by two positions. The control condition was designed to match the perceptual and motor elements of the actual task conditions. Four identical letters were presented, followed by a lowercase probe letter. The proportion of matched probes was 50% in all conditions.
Behavioral performance was assessed using accuracy and response time. These measures were entered in separate four-condition-by-two-group mixed-effects analyses of variance testing for main effects of group and condition for each variable. Significant main effects and interactions were decomposed using t tests.
Image Acquisition and Analysis
Details of the image acquisition parameters, fMRI data preprocessing steps, and statistical analyses are presented in the online data supplement. The functional images were processed and analyzed using BrainVoyager QX, version 1.10.4 (Brain Innovation, Maastricht, the Netherlands), and custom routines were written in MATLAB (MathWorks, Natick, Mass.). The main effect of group and condition-by-group interactions was assessed using a three-condition-by-two-group mixed-effects analysis of covariance (ANCOVA). These analyses used age, sex, education, handedness, ethnicity, and accuracy as covariates. The resulting F-maps were subject to a corrected cluster significance threshold of p<0.05 (initial uncorrected voxel-level threshold, p<0.001).
The manipulation aspect of working memory was of particular interest, since it involves acting on the contents of short-term memory. We identified activation associated with the manipulation component to investigate group differences in activation within the regions examined. From the conjunction map of each maintenance plus manipulation condition against the maintenance-only condition (PLUS>LTR and PLUS2>LTR), six fronto-parietal regions known to be involved in working memory processes (46, 50) were selected as regions of interest to be considered for between-group analyses. A three-condition-by-two-group mixed-effects ANCOVA was performed for the parameter estimates at each region of interest. A statistical threshold of p<0.008 (corrected for multiple comparisons for six regions of interest) was applied in this second-level analysis to identify significant condition-by-group interactions.
To evaluate the possible modulatory effects of antidepressant medication on brain signal (51–53), we repeated the above analysis with the at-risk group divided into medicated and nonmedicated subgroups.
Finally, in the at-risk group, we explored the correlation between brain activation and clinical symptom severity (using the GAF scores, the subscale and total scores of the PANSS, and the combined intensity and frequency scores of the Comprehensive Assessment of At-Risk Mental States), as well as measures of cognition (using the symbol coding and digit sequencing scores of the Brief Assessment of Cognition in Schizophrenia and the vocabulary score of the Wechsler Abbreviated Scale of Intelligence). These analyses were conducted for brain regions showing group differences in overall task-related activation and regions involved in manipulation of working memory contents. To ensure that only areas activated during the working memory task entered these analyses, brain areas were masked using the functional activation masks derived from each group for the corresponding condition or contrast.
Results
Behavioral Data
There were no significant differences between the 60 at-risk participants and 38 comparison subjects in age, handedness, sex, or ethnicity (Table 1). At-risk participants had less education on average than comparison subjects. However, there were no significant differences in vocabulary scores between the two groups.
There was a main effect of condition on both accuracy (F=151.9, df=3, 288, p<0.001) and response time (F=459.0, df=3, 288, p<0.001) but no effect of group on either measure of behavioral performance. Increasing difficulty elicited fewer correct responses and longer response times in both groups, but there were no significant group differences. The condition-by-group interaction was not significant for either accuracy or response time.
When the analysis was repeated to take into account use of antidepressant medication, the main effect of condition remained significant (accuracy: F=153.3, df=3, 285, p<0.001; response time: F=455.6, df=3, 285, p<0.001). There was no effect of group, nor were there any condition-by-group interactions (see Table S2 in the online data supplement).
Imaging Data
Engagement of verbal working memory across the different task conditions elicited a common network of left and right fronto-parietal regions in both the at-risk and comparison groups. As expected, activation in these regions increased in magnitude and spatial extent with increasing task difficulty (Figure 2).
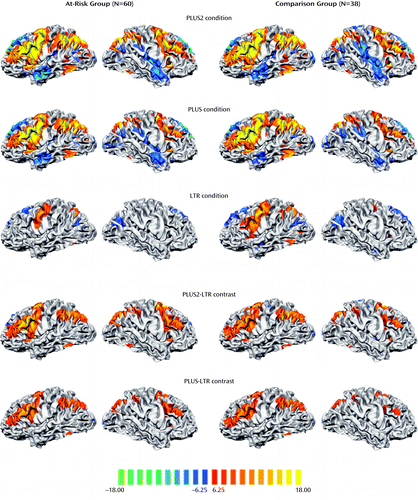
a The rows depict activation maps showing regions where MR signal change was significantly different from the control condition in the at-risk and healthy comparison groups for each of the three task conditions and the two manipulation contrasts. All maps were thresholded at p<0.0001 (Bonferroni corrected).
Activation in the left anterior insula and posterior cingulate cortex showed a significant main effect of group (Figure 3; see also Table S1 in the online data supplement). The at-risk group exhibited reduced anterior insula activation and increased posterior cingulate cortex deactivation relative to the comparison group in each of the three experimental conditions. The effects remained significant when both medicated and nonmedicated at-risk participants were accounted for (see Figure S2 and Table S2 in the data supplement).
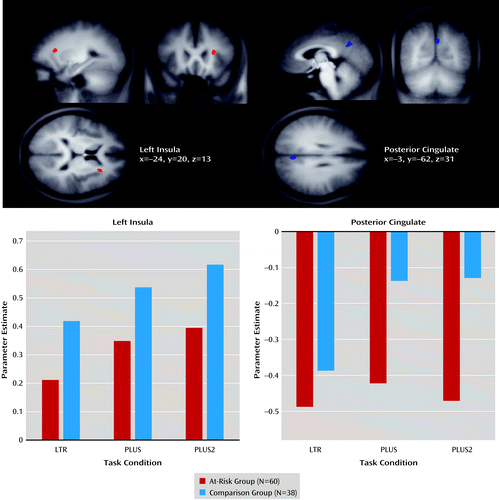
a The images depict the left insula and posterior cingulate, and the bar graphs show the MR signal change across task condition and group in these regions.
Condition-by-group interactions were identified from a 3×2 ANCOVA. Regions showing significant interaction effects included the right inferior frontal gyrus, right frontal eye field, right insula, precuneus, left precentral gyrus, posterior cingulate cortex, medial prefrontal cortex, left middle frontal gyrus, and left anterior middle frontal gyrus (see Table S1 in the data supplement). The posterior cingulate cortex, medial prefrontal cortex, left middle frontal gyrus, and left anterior middle frontal gyrus revealed task-related deactivation, while all other regions revealed task-related activation. In the regions where task-related activation was observed, the at-risk group exhibited a greater increase in activation during the manipulation conditions than the comparison group. In the regions where task-related deactivation was observed, the at-risk group exhibited relatively more pronounced deactivation during the manipulation conditions than the comparison group.
Activation associated with manipulation was assessed using a conjunction of the manipulation-related contrasts (PLUS–LTR and PLUS2–LTR). Both the PLUS and PLUS2 conditions elicited greater fronto-parietal activation than the LTR condition in both the at-risk and comparison groups (Figure 2). From the maps showing manipulation-related activity, we analyzed six regions of interest that were centered on the locus of peak signals in the frontal and parietal lobes (see Table S1 in the data supplement).
The only region of interest that showed significant condition-by-group interaction was the right posterior dorsolateral prefrontal cortex (F=5.94, df=2, 192, p=0.004) (Figure 4). The at-risk group exhibited greater increase in manipulation-related activity than the comparison group. Both medicated and nonmedicated at-risk participants exhibited greater MR manipulation-related activity than comparison subjects, although the between-group differences were less pronounced (F=3.20, df=4, 190, p=0.02) (see Figure S2 and Table S3 in the data supplement).
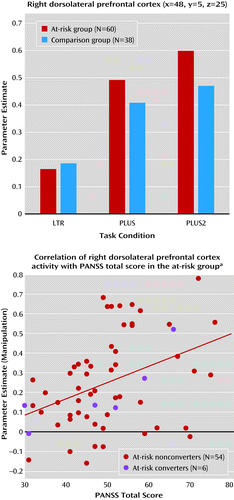
a The bar graph shows the MR signal change across task condition and group in the right dorsolateral prefrontal cortex region of interest. The scatterplot shows the correlation between the MR signal change associated with manipulation in the right dorsolateral prefrontal cortex and Positive and Negative Syndrome Scale (PANSS) total score in the at-risk group. There was a significant correlation between right dorsolateral prefrontal cortex activity and PANSS total score (r=0.40, df=58, p=0.001). The blue circles indicate at-risk participants who later converted to psychosis.
Association Between Brain Activation and Symptom Severity
There was a significant correlation between manipulation-related activation and PANSS total scores (r=0.40, df=58, p=0.001) in the right dorsolateral prefrontal cortex (Figure 4). Correlations with PANSS positive, negative, and general psychopathology scores in the right dorsolateral prefrontal cortex were positive but nonsignificant. None of the other symptom scales or measures of cognition were correlated with MR signal in contrasts of interest.
Discussion
This study represents one of the largest functional imaging studies comparing persons at high risk for developing psychosis with healthy comparison subjects. We observed altered patterns of activation in the at-risk group as they maintained and manipulated letters in working memory. These group differences remained significant after accounting for differences in education and antidepressant use (see the online data supplement).
At-risk participants exhibited reduced activation in the left anterior insula compared with comparison subjects in all task conditions. When manipulating letters, the at-risk group displayed more pronounced task-related deactivation of the default-mode network than the comparison group. A region-of-interest analysis of MR signal in regions known to be involved in working memory found greater manipulation-related activation in the right dorsolateral prefrontal cortex. In the at-risk group, the magnitude of right dorsolateral prefrontal cortex signal during working memory manipulation correlated with PANSS symptom scores.
Absence of Significant Behavioral Differences Between Groups
The equivalence of behavior between the at-risk and comparison groups, despite differences in education, across all three conditions allowed us to attribute the altered patterns of activation to the at-risk mental state (23, 29, 31, 54), as opposed to poorer performance in at-risk participants. Additionally, the comparability of performance across medicated and nonmedicated participants reassures us that the imaging findings were unlikely to be a result of antidepressant therapy. The large size of our sample allowed us to perform subgroup analysis with reasonable power.
Reduced Insula Activation in At-Risk Participants
Reduced anterior insula activity was observed across all task conditions in the at-risk group and was our most robust finding. The anterior insula and anterior cingulate cortex are part of a salience network (55) that supports high-level executive processes.
Greater Task-Related Deactivation in At-Risk Participants
There have been conflicting reports regarding the direction of altered task-related deactivation within the default-mode network in patients with schizophrenia or their first-degree relatives (63–65). For example, decreased task-related deactivation accompanied by increased resting-state connectivity within the default-mode network has been reported in patients and first-degree relatives (38). Reduced task-related deactivation corresponding with lowered cognitive performance occurs in other settings, such as in Alzheimer’s disease (66) and following sleep deprivation (46), and could be expected in the at-risk group. On the other hand, some patients with schizophrenia exhibit increased task-related deactivation within the default-mode network in association with changes in resting-state connectivity (39). In our at-risk group, increased task-related deactivation during task performance was observed in the default-mode network nodes, including the medial prefrontal cortex, posterior cingulate cortex, and left middle frontal gyrus. While this could reflect the need for greater effort in suppressing self-directed thought in at-risk persons but not in schizophrenia patients, further studies are necessary to clarify this issue.
Elevated Prefrontal Cortex Activation During Manipulation in At-Risk Participants
It has been suggested that persons with a schizophrenia spectrum disorder exhibit increases in prefrontal activation as a compensatory change because of deficits in other cortical regions (67). Greater activation in the right dorsolateral prefrontal cortex, a region implicated in executive processes within working memory, could be compensatory for altered recruitment of the anterior insula. Within the prefrontal cortex, the dorsolateral prefrontal cortex contributes relatively more to manipulation of short-term memory, while the ventrolateral prefrontal cortex contributes primarily to information maintenance (68–70). Previous neuroimaging studies suggest that executive functions in working memory, associated with parietal and dorsolateral prefrontal cortex function, may contribute more to working memory impairment in schizophrenia (6, 7, 71) than the impaired maintenance of information.
Most fMRI studies of working memory involving patients with an established diagnosis of schizophrenia and some studies of first-episode schizophrenia have reported reduced performance accuracy. However, the associated functional imaging findings have been mixed. Some studies have reported reduced activation in prefrontal and parietal regions in patients compared with comparison subjects (5, 8, 29), while other studies have reported increased activation in these regions (4, 35, 72).
In a study of patients with schizophrenia, there was a greater increase in activity with elevated cognitive load in patients with good task performance compared with those with poor performance (73). This suggests that in high-performing patients, reallocation of processing resources to prefrontal areas may allow behavioral performance to be maintained at a level comparable to that of comparison subjects. By contrast, in lower-performing patients, this capacity to adaptively recruit additional brain regions has been found to be exceeded and reflected in decreased prefrontal activation (30, 74, 75). This pattern of neuroimaging findings resembles adaptation to cognitive aging (76, 77), in which participants who perform slower exhibit greater activation in the dorsolateral prefrontal cortex. In the present study, our finding of hyperactivation in the right dorsolateral prefrontal cortex in the at-risk group, together with preserved behavioral performance, suggests an adaptive response to the at-risk mental state. While the between-group similarities in performance may reflect a reduced psychometric sensitivity of the task in this population, it is noteworthy that neuroimaging contrasts were sensitive to the at-risk condition when performance measures were not.
Alternatively, the positive correlation between PANSS symptom severity and manipulation-related activation in the right dorsolateral prefrontal cortex may simply denote greater difficulty in engaging task-driven cognition in at-risk participants. Symptomatic individuals may have to cope with intrusive thoughts or auditory hallucinations. This explanation is supported by studies of patients as well as first-degree relatives that found increased prefrontal cortex activity to be correlated with higher symptom severity (24, 78).
Limitations
While this study suggests altered fMRI activation patterns in at-risk persons without their manifesting overt working memory impairment, these remain baseline results. Six of our 60 at-risk participants presented with a first episode of psychosis after at least 1 year (see Figure S3 in the online data supplement). This low conversion rate is one of the inherent difficulties in prodromal psychosis research. At-risk individuals are heterogeneous, presenting with a range of symptoms, including anxiety and depression (58% of at-risk participants in our sample were receiving antidepressants), and often have functional deficits. Therefore, the group differences could be related to general psychopathology rather than specifically psychosis risk; continued follow-up to identify individuals who convert will be required to relate neuroimaging findings to specific risk of psychosis.
The healthy comparison subjects are likely higher functioning than the at-risk participants based on education level. However, neither task performance nor brain activity was correlated with education, and when education was included as a covariate in the analyses, the results remained significant. Furthermore, the vocabulary scores of the Wechsler Abbreviated Scale of Intelligence, often used as a proxy for IQ, were not significantly different between the two groups.
Conclusions
We found that while not apparent at the behavioral level, individuals classified as being at risk for psychosis manifested functional neuroimaging changes consistent with altered processing of salient stimuli. These may affect the manipulation of working memory contents in a manner resulting in overactivation of the dorsolateral prefrontal cortex, as well as abnormal default-mode network function. Of functional significance, dorsolateral prefrontal cortex signal alteration was associated with symptom severity. Together, these findings highlight the potential utility of detecting early brain changes to facilitate early treatment for at-risk persons.
1 : Working memory. Science 1992; 255:556–559Crossref, Medline, Google Scholar
2 : Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci 1994; 6:348–357Crossref, Medline, Google Scholar
3 : Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry 1992; 49:975–982Crossref, Medline, Google Scholar
4 : Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry 1999; 45:1128–1137Crossref, Medline, Google Scholar
5 : Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry 2001; 158:1105–1113Link, Google Scholar
6 : Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Arch Gen Psychiatry 2005; 62:1071–1080Crossref, Medline, Google Scholar
7 : fMRI study of maintenance and manipulation processes within working memory in first-episode schizophrenia. Am J Psychiatry 2005; 162:1849–1858Link, Google Scholar
8 : Neural correlates of working memory dysfunction in first-episode schizophrenia patients: an fMRI multi-center study. Schizophr Res 2007; 89:198–210Crossref, Medline, Google Scholar
9 : Changes in distributed neural circuitry function in patients with first-episode schizophrenia. Br J Psychiatry 2004; 185:205–214Crossref, Medline, Google Scholar
10 : Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment, and their interactions. Schizophr Bull 2003; 29:653–669Crossref, Medline, Google Scholar
11 : The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry 2001; 50:884–897Crossref, Medline, Google Scholar
12 : The neurodevelopmental hypothesis of schizophrenia: a review of recent developments. Ann Med 2003; 35:86–93Crossref, Medline, Google Scholar
13 : A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res 2004; 71:405–416Crossref, Medline, Google Scholar
14 : Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry 2006; 59:863–871Crossref, Medline, Google Scholar
15 : Cognitive functioning in the schizophrenia prodrome. Schizophr Bull 2007; 33:761–771Crossref, Medline, Google Scholar
16 : Neuropsychological deficits in individuals with an at risk mental state for psychosis - working memory as a potential trait marker. Schizophr Res 2007; 97:14–24Crossref, Medline, Google Scholar
17 : Neurocognitive deficits in the (putative) prodrome and first episode of psychosis. Schizophr Res 2007; 93:266–277Crossref, Medline, Google Scholar
18 : Generalized and specific cognitive performance in clinical high-risk cohorts: a review highlighting potential vulnerability markers for psychosis. Schizophr Bull 2006; 32:538–555Crossref, Medline, Google Scholar
19 : Neuroimaging predictors of transition to psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev 2010; 34:1207–1222Crossref, Medline, Google Scholar
20 : Spatial working memory ability is a marker of risk-for-psychosis. Psychol Med 2003; 33:1239–1247Crossref, Medline, Google Scholar
21 : Spatial working memory deficits in adolescents at clinical high risk for schizophrenia. Schizophr Res 2006; 81:211–215Crossref, Medline, Google Scholar
22 : The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophr Res 2007; 89:191–197Crossref, Medline, Google Scholar
23 : Altered brain activation in dorsolateral prefrontal cortex in adolescents and young adults at genetic risk for schizophrenia: an fMRI study of working memory. Schizophr Res 2006; 85:58–72Crossref, Medline, Google Scholar
24 : Functional magnetic resonance imaging during auditory verbal working memory in nonpsychotic relatives of persons with schizophrenia: a pilot study. Biol Psychiatry 2004; 55:490–500Crossref, Medline, Google Scholar
25 : Transient and sustained activity in a distributed neural system for human working memory. Nature 1997; 386:608–611Crossref, Medline, Google Scholar
26 : A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 1997; 5:49–62Crossref, Medline, Google Scholar
27 : Altered brain function directly related to structural abnormalities in people at ultra high risk of psychosis: longitudinal VBM-fMRI study. J Psychiatr Res 2011; 45:190–198Crossref, Medline, Google Scholar
28 : An fMRI study of working memory in first-degree unaffected relatives of schizophrenia patients. Schizophr Res 2008; 104:85–95Crossref, Medline, Google Scholar
29 : Neural correlates of executive function and working memory in the “at-risk mental state.” Br J Psychiatry 2008; 194:25–33Crossref, Google Scholar
30 : Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res 2003; 60:285–298Crossref, Medline, Google Scholar
31 : Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry 2003; 160:709–719Link, Google Scholar
32 : Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry 2005; 39:964–971Crossref, Medline, Google Scholar
33 : Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci 2012; 16:27–34Crossref, Medline, Google Scholar
34 : Working memory and prefrontal cortex dysfunction: specificity to schizophrenia compared with major depression. Biol Psychiatry 2003; 53:376–384Crossref, Medline, Google Scholar
35 : Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 2000; 10:1078–1092Crossref, Medline, Google Scholar
36 : Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry 2005; 162:475–484Link, Google Scholar
37 : Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry 2007; 164:450–457Link, Google Scholar
38 : Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA 2009; 106:1279–1284Crossref, Medline, Google Scholar
39 : Resting state and task-induced deactivation: A methodological comparison in patients with schizophrenia and healthy controls. Hum Brain Mapp 2010; 31:424–437Medline, Google Scholar
40 : The role of default network deactivation in cognition and disease. Trends Cogn Sci 2012; 16:584–592Crossref, Medline, Google Scholar
41 : Working memory encoding and maintenance deficits in schizophrenia: neural evidence for activation and deactivation abnormalities. Schizophr Bull 201139:168–178Crossref, Medline, Google Scholar
42 : Positive-negative symptom assessment in schizophrenia: psychometric issues and scale comparison. Psychiatr Q 1990; 61:163–178Crossref, Medline, Google Scholar
43 : The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res 2004; 68:283–297Crossref, Medline, Google Scholar
44 : Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, Tex, Harcourt Assessment, 1999Google Scholar
45 : The binomial distribution of right, mixed and left handedness. Q J Exp Psychol 1967; 19:327–333Crossref, Medline, Google Scholar
46 : Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci 2004; 24:4560–4567Crossref, Medline, Google Scholar
47 : Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron 2008; 60:378–389Crossref, Medline, Google Scholar
48 : Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci 2009; 34:418–432Medline, Google Scholar
49 : Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 2001; 21:RC159Crossref, Medline, Google Scholar
50 : Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage 2012; 60:830–846Crossref, Medline, Google Scholar
51 : A longitudinal functional magnetic resonance imaging study of verbal working memory in depression after antidepressant therapy. Biol Psychiatry 2007; 62:1236–1243Crossref, Medline, Google Scholar
52 : Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. J Affect Disord 2011; 130:66–74Crossref, Medline, Google Scholar
53 : Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry 2004; 61:877–889Crossref, Medline, Google Scholar
54 : Fronto-parietal hypo-activation during working memory independent of structural abnormalities: conjoint fMRI and sMRI analyses in adolescent offspring of schizophrenia patients. Neuroimage 2011; 58:234–241Crossref, Medline, Google Scholar
55 : Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27:2349–2356Crossref, Medline, Google Scholar
56 : Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 2010; 214:655–667Crossref, Medline, Google Scholar
57 : Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci 2012; 37:17–27Crossref, Medline, Google Scholar
58 : The role of the insula in schizophrenia. Schizophr Res 2010; 123:93–104Crossref, Medline, Google Scholar
59 : Fronto-striatal hypoactivation during correct information retrieval in patients with schizophrenia: an fMRI study. Neuroscience 2008; 153:54–62Crossref, Medline, Google Scholar
60 : Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 2001; 98:4259–4264Crossref, Medline, Google Scholar
61 : Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2001; 2:685–694Crossref, Medline, Google Scholar
62 : A default mode of brain function. Proc Natl Acad Sci USA 2001; 98:676–682Crossref, Medline, Google Scholar
63 : Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophr Res 2007; 91:82–86Crossref, Medline, Google Scholar
64 : Altered engagement of attention and default networks during target detection in schizophrenia. Schizophr Res 2011; 125:169–173Crossref, Medline, Google Scholar
65 : Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol Med 2008; 38:1185–1193Crossref, Medline, Google Scholar
66 : Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA 2003; 100:14504–14509Crossref, Medline, Google Scholar
67 : The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry 2004; 161:398–413Link, Google Scholar
68 : Prefrontal, parietal and basal activation associated with the reordering of a two-element list held in working memory. Biol Psychol 2010; 85:143–148Crossref, Medline, Google Scholar
69 : Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn 1999; 41:66–86Crossref, Medline, Google Scholar
70 : Activity in human frontal cortex associated with spatial working memory and saccadic behavior. J Cogn Neurosci 2000; 12(Suppl 2):2–14Crossref, Medline, Google Scholar
71 : Maintenance and manipulation of information in schizophrenia: further evidence for impairment in the central executive component of working memory. Schizophr Res 2004; 68:173–187Crossref, Medline, Google Scholar
72 : Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry 2000; 48:99–109Crossref, Medline, Google Scholar
73 : Functional magnetic resonance imaging of a parametric working memory task in schizophrenia: relationship with performance and effects of antipsychotic treatment. Psychopharmacology (Berl) 2011; 216:17–27Crossref, Medline, Google Scholar
74 : Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophr Res 2009; 108:143–150Crossref, Medline, Google Scholar
75 : Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry 2003; 160:2209–2215Link, Google Scholar
76 : When less is more and when more is more: The mediating roles of capacity and speed in brain-behavior efficiency. Intelligence 2009; 37:207–222Crossref, Medline, Google Scholar
77 : Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage 2002; 17:1394–1402Crossref, Medline, Google Scholar
78 : A systematic fMRI investigation of the brain systems subserving different working memory components in schizophrenia. Eur J Neurosci 2009; 30:693–702Crossref, Medline, Google Scholar



