Effect of Antidepressant Medication Use on Emotional Information Processing in Major Depression
Abstract
Objective
Acute administration of antidepressant medication increases emotional information processing for positive information in both depressed and healthy persons. This effect is likely relevant to the therapeutic actions of these medications, but it has not been studied in patients with major depressive disorder taking antidepressants as typically prescribed in the community.
Method
The authors used eye tracking to examine the effects of antidepressant medication on selective attention for emotional stimuli in a sample of 47 patients with major depressive disorder (21 medicated and 26 unmedicated) and 47 matched comparison subjects without depression. Participants completed a passive-viewing eye-tracking task assessing selective attention for positive, dysphoric, threatening, and neutral stimuli in addition to providing medication information and self-report measures of depression and anxiety severity.
Results
Depressed participants currently taking antidepressants and nondepressed comparison subjects demonstrated greater total gaze duration and more fixations for positive stimuli compared with unmedicated depressed participants. Depressed participants on medication also had fewer fixations for dysphoric stimuli compared with depressed participants not on medication.
Conclusions
Antidepressants, as prescribed in the community to patients with depression, appear to modify emotional information processing in the absence of differences in depression severity. These results are consistent with previous work and indicate a robust effect for antidepressants on positive information processing. They also provide further evidence for modification of information processing as a potential mechanism of action for antidepressant medication.
There is now substantial theoretical and empirical support for the importance of information processing biases in the maintenance, and perhaps the etiology, of major depression (1–3). Specifically, biased attention to depression-relevant material and avoidance of positive information are hypothesized to maintain the disorder. Recent research has also demonstrated that modifying these biases reduces symptoms of depression (4–6).
According to a recent cognitive neuropsychological model of depression (7), antidepressant medications—particularly those that target serotonin and norepinephrine—may act by modifying emotional information processing. Modified emotional information processing, in turn, is thought to lead to downstream antidepressant effects. This model helps explain why antidepressant medication use is not immediately associated with amelioration of depressed mood. As Harmer and colleagues note (7), “Rather than acting as direct ‘mood enhancers,’ antidepressants may re-tune how we process personal and socially relevant affective information” (p. 107).
This model follows from a series of studies suggesting that 1) antidepressants influence emotional information processing early in treatment; 2) changes in emotional information processing occur earlier than and in the absence of changes in subjective mood; and 3) early changes in information processing are associated with eventual therapeutic improvement (see the review by Harmer et al. [7]). Thus far, much of this research has been conducted with healthy subjects. For example, Browning et al. (8) randomly assigned 32 healthy volunteers to receive either one dose of citalopram or a placebo pill. Consistent with the cognitive neuropsychological hypothesis, individuals who received the antidepressant demonstrated greater attention to positive stimuli, as assessed with a visual probe task.
More recently, researchers have begun to evaluate this phenomenon in individuals with depression. For instance, Harmer et al. (9) conducted a double-blind placebo-controlled study evaluating patients with depression and healthy subjects. In line with the cognitive neuropsychological model, depressed patients who received placebo exhibited lower recognition of positive facial expressions and lower memory for positive information, as well as slower speed to respond to positive personality adjectives, compared with healthy subjects. Notably, these information processing effects in the depressed patients were reversed with the administration of just a single dose of an antidepressant (reboxetine). However, there were not corresponding reductions in subjective ratings of mood or anxiety after this initial administration.
In this study, we built on previous research to focus specifically on the relationship between antidepressant medication use and selective attention to emotional visual stimuli, using eye-tracking technology, in a sample of participants with major depressive disorder as well as a nondepressed comparison group. This research adds to the small number of studies that have empirically examined the cognitive neuropsychological model of depression in a clinical sample. The inclusion of a nondepressed comparison group is also valuable for comparative purposes insofar as it allows us to more clearly delineate “normal” attention for emotional information.
Our use of an eye-tracking paradigm is particularly valuable because it allows for multiple dynamic measures of selective attention (10). This is critical because it enables us to capture the more elaborative stages of attention that are particularly relevant for patients with major depression (11). Eye tracking also specifically provides an assessment of overt attention, since eye movements are necessarily associated with shifts in attention, whereas the dot-probe task used in previous studies does not always elicit eye movements and may measure both overt and covert shifts in attention (12, 13). Ours is also the first study to examine the effects of antidepressant medication, as prescribed in the community, on emotional information processing.
We used eye tracking to measure selective attention (total gaze duration, mean number of fixations, mean fixation duration) for dysphoric, threatening, positive, and neutral emotional scenes in a sample of community participants with major depression (both medicated and unmedicated) and a never-depressed comparison group. Consistent with previous work (8, 9), we hypothesized that antidepressant medication use, compared with nonuse, would be associated with greater selective attention for positive stimuli. We further predicted that there would not be significant group differences between the medicated major depression group and the nondepressed comparison group on selective attention for positive information, consistent with the idea that antidepressant medication use normalizes emotional information processing.
Method
Participants
We recruited 49 patients with major depression and 47 never-depressed participants through Internet, television, and radio advertisements. Two participants in the depression group were excluded from the analyses because of inadequate eye-tracking data, resulting in a group size of 47 patients with major depression, of whom 21 were currently taking antidepressant medication and 26 were not. The 47 never-depressed comparison subjects were not taking any antidepressant medications. Comparison subjects were matched as closely as possible with participants in the major depression group for age and years of education.
Inclusion criteria for depressed participants were a DSM-IV diagnosis of major depressive disorder and a score >20 on the Beck Depression Inventory-II (BDI-II) (14). Inclusion criteria for the nondepressed comparison subjects were no history of major depression and a score <13 on the BDI-II. Inclusion criteria for all participants were age between 22 and 55 years, normal or corrected-to-normal vision, and ability to speak, read, and understand English. Exclusion criteria for all participants were a current or past DSM-IV diagnosis of substance abuse in the past 6 months; a current or past DSM-IV diagnosis of substance dependence, bipolar disorder, psychotic disorder, obsessive-compulsive disorder, social phobia, panic disorder, posttraumatic stress disorder, or generalized anxiety disorder; and a history of epilepsy or head trauma. Participants provided written informed consent after receiving a complete description of the study. All procedures were approved by the institutional review board of the University of Texas at Austin.
Assessments
The electronic version of the Mini International Neuropsychiatric Interview (15), a short structured screening interview that provides DSM-IV and ICD-10 diagnoses, was used to screen for study eligibility. The patient version of the Structured Clinical Interview for DSM-IV (SCID) (16) was administered on the day of study participation to provide psychiatric diagnoses for the inclusion and exclusion criteria. Two assessors were doctoral students with master’s degrees in clinical psychology and at least 2 years of clinical training and assessment experience, and a third assessor was a full-time research assistant with a bachelor’s degree in psychology who had completed more than 40 hours of training in the administration of the SCID. Twenty percent of all interviews were rated by an independent assessor who was a doctoral student with a master’s degree in clinical psychology and 4 years of assessment experience. Agreement for diagnoses of major depressive disorder between study interviewers and the independent assessor was excellent (k=1.00, p<0.0001).
The BDI-II, a 21-item self-report questionnaire with demonstrated validity in psychiatric outpatient and inpatient samples (17), was used to assess depression severity. The Beck Anxiety Inventory (BAI) (18), a 21-item self-report questionnaire with good internal and test-retest reliability and convergent validity with other measures of anxiety (18, 19), was used to assess symptoms of anxiety.
Eye-Tracking Task
On each trial in this task, four images selected from the International Affective Picture System (IAPS) (20) were presented simultaneously, with one image appearing in each quadrant of a 20-inch LCD computer monitor. On every trial, one image was selected from each of four stimulus categories: dysphoric, threat, positive, or neutral (see Figure S1 in the data supplement that accompanies the online edition of this article). The method for selecting and categorizing these images has been described previously (11). The location of each image was randomly assigned for each participant by the stimulus presentation software (E-Prime 2.0; Psychology Software Tools, Sharpsburg, Pa.) with the constraint that each stimulus category must appear in each of the four positions three times across 12 trials. In addition to the 12 study trials, four filler trials comprising all neutral images were presented to obscure the nature of the task, resulting in a total of 16 trials. Presentation order of stimuli was randomized for each participant. Each trial lasted 30 seconds. Trials were preceded by a central fixation cross that remained on-screen until the participant fixated within approximately 1° of visual angle of the cross for 1 second.
Participants sat approximately 60 cm from the screen. Each image measured 14.2×10.7 cm (13.5°×10.2° of visual angle). The horizontal distance between the centers of the images was 20.7 cm (19.6° of visual angle) and the vertical distance between the centers of images was 15.5 cm (14.8° of visual angle).
Eye-Tracking System
Line of visual gaze was assessed using a Model R6 remote optics eye-tracking system from Applied Science Laboratories (Bedford, Mass.). Head location was fixed using a chin rest and forehead bar. The location of gaze was sampled every 16.7 ms (60 Hz). Eye movements that were stable for more than 100 ms within a 1° visual angle were classified as a fixation. The total area of each stimulus on a trial was identified as an area of interest. For each area of interest, three selective attention indices were calculated with the GazeTracker software program (Applied Science Laboratories): total gaze time per trial, number of fixations per trial, and mean fixation duration. Greater gaze time represents greater sustained attention. Greater number of fixations represents repeated attentional engagement, whereas greater fixation duration represents greater attentional capture or difficulty disengaging attention.
Procedure
Participants were interviewed with the SCID to determine study eligibility. Eligible participants completed a demographic form, the BDI-II, and the BAI and provided the following information regarding antidepressant use: current use (yes/no), name of medication, medication dosage, and length of time on medication. For eye tracking, participants were seated in a height-adjustable chair, which was adjusted to minimize discomfort with the participant’s head location fixed with the chinrest and forehead bar. Camera adjustments were made to best capture pupil and corneal reflection of the participant’s right eye. A 9-point calibration was conducted to confirm recording line of visual gaze within 1° of visual angle for each calibration point. Calibration was repeated until this criterion was met.
Participants were instructed verbally and via computer screen to view the images naturally, as if watching television or viewing a photo album. The only constraint was that they view the images at all times during each trial. To minimize demand effects, instructions emphasized that the study measured processing of emotional information without specifically mentioning the measurement of eye movements. Participants were instructed to look at the fixation cross preceding each trial to standardize the starting location of their gaze. An experimenter located in an adjacent room monitored the stimulus presentation and eye-tracking quality throughout the task.
Results
Participant Characteristics
Descriptive statistics for the sample are presented in Table 1. There were no significant differences between groups in age or years of education. The medicated and unmedicated depression groups did not differ in severity of depression, severity of anxiety, number of depressive episodes, or length of current depressive episode. The medicated and unmedicated depression groups both reported greater severity of depression and anxiety than the comparison group. The majority of participants were women, and there were no significant differences between the comparison group, the medicated depression group, and the unmedicated depression group in proportion of women (81%, 81%, and 85%, respectively). There was substantial variability in length of time medicated depression participants had been on their primary antidepressant medication (range, 1.5–520 weeks). (Medication information provided by participants in the medicated depression group is presented in Table S1 in the online data supplement.) A majority of participants in the medicated depression group (N=16) reported taking a selective serotonin reuptake inhibitor (SSRI) or a serotonin-norepinephrine reuptake inhibitor (SNRI) as their primary medication. Three participants reported that bupropion was their primary medication, and two participants endorsed currently taking an antidepressant medication but did not provide any information about their medication. Analyses of data only from participants reporting an SSRI or SNRI as their primary medication were nearly identical to those of the full sample, so results from the full sample are reported below.
| Comparison Group (N=47) | Medicated Depression Group (N=21) | Unmedicated Depression Group (N=26) | ||||
|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | Mean | SD |
| Age (years) | 33.6a | 11.2 | 37.2a | 12.8 | 31.3a | 8.7 |
| Education (years) | 14.3a | 1.3 | 14.3a | 1.3 | 14.0a | 1.7 |
| Beck Depression Inventory–II score | 2.0a | 3.2 | 27.6b | 6.6 | 28.9b | 9.7 |
| Beck Anxiety Inventory score | 3.2a | 5.5 | 11.0b | 5.2 | 14.2b | 6.5 |
| Depressive episodes | 6.5a | 8.5 | 6.2a | 4.9 | ||
| Length of current depressive episode (weeks) | 29.1a | 37.4 | 25.0a | 24.1 | ||
| Time on current medication (weeks) | 139.8 | 141.2 | ||||
Effects of Medication Status on Eye-Tracking Indices
Total mean gaze duration.
A 3×4 (group [comparison, medicated depression, unmedicated depression] by stimulus type [dysphoric, threat, positive, neutral]) analysis of variance (ANOVA) revealed a significant main effect for stimulus type on mean gaze time (F=18.22, df=3, 376, p<0.001; ηp2=0.13) as well as a significant interaction between group and stimulus type (F=3.24, df=6, 376, p=0.004; ηp2=0.05). There was no main effect for group on mean gaze time. As predicted, planned comparisons revealed that the group-by-stimulus type interaction was driven by longer total gaze duration for positive images in the medicated depression group and in the comparison group compared with the unmedicated depression group. There was no difference between the comparison and medicated depression groups in total gaze duration for positive images. Group differences for dysphoric, threat, and neutral images were not significant (Table 2).
| Comparison Group (N=47) | Medicated Depression Group (N=21)b | Unmedicated Depression Group (N=26) | ||||
|---|---|---|---|---|---|---|
| Measure and Stimulus Type | Mean | SD | Mean | SD | Mean | SD |
| Total mean gaze duration (seconds) | ||||||
| Neutral | 5.05a | 1.46 | 4.77a | 1.54 | 5.03a | 1.85 |
| Dysphoric | 5.99a | 1.47 | 5.84a | 1.95 | 6.60a | 1.74 |
| Positive | 7.46a | 2.63 | 7.84a | 2.64 | 5.79b | 2.04 |
| Threat | 5.33a | 1.60 | 5.17a | 2.28 | 5.72a | 1.93 |
| Mean number of fixations | ||||||
| Neutral | 20.97a | 4.58 | 20.03a | 4.36 | 21.12a | 6.30 |
| Dysphoric | 24.55ab | 4.43 | 22.47a | 5.39 | 26.30b | 5.62 |
| Positive | 27.49a | 5.91 | 31.68a | 10.14 | 23.64b | 6.79 |
| Threat | 21.48a | 4.99 | 19.32a | 5.22 | 22.53a | 6.35 |
To explore potential effects of time course on total gaze duration, the 30-second trial was divided into six 5-second epochs, and total gaze time was calculated for each epoch. A 3×4×6 (group by stimulus type by time [six epochs]) repeated-measures ANOVA revealed a significant main effect for time on total gaze duration (F=17.31, df=5, 360, p<0.001; ηp2=0.19) and a significant interaction between stimulus type and time (F=2.40, df=15, 1086, p=0.002; ηp2=0.03). The stimulus type-by-time interaction is illustrated in Figure 1. The group-by-stimulus type interaction was identical to the group-by-stimulus type interaction reported above for the full 30-second trial. The time-by-group interaction and the three-way interaction on total gaze time were not significant.
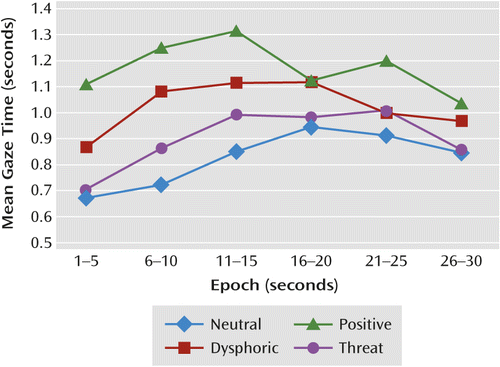
Despite the nonsignificant interactions between time and group, given our significant results of group on total gaze time for positive stimuli, we conducted exploratory post hoc tests examining time course effects of group on total gaze time for positive stimuli. There were significant differences between groups for total gaze time for positive stimuli at the fifth epoch (seconds 21–25; F=7.25, df=2, 93, p=0.001) and at the sixth epoch (seconds 26–30; F=3.53, df=2, 93, p=0.034) and approaching significance at the fourth epoch (F=2.39, df=2, 93, p=0.097). These results were driven by longer gaze times for positive stimuli by the comparison and medicated depression groups compared with the unmedicated depression group. (These results are illustrated in Figure S2 in the online data supplement.) There were no significant differences for total gaze time for positive stimuli between groups for the first, second, or third epochs.
Mean number of fixations.
Because of a programming error, mean number of fixations and mean fixation duration (see below) could not be calculated for eight of the medicated depression participants. A 3×4 (group by stimulus type) ANOVA revealed a pattern of results similar to the mean gaze time findings. There was a significant main effect for stimulus type on number of fixations (F=21.27, df=3, 343, p<0.001; ηp2=0.16) as well as a significant interaction between group and stimulus type (F=4.23, df=6, 343, p<0.001; ηp2=0.07). There was no main effect for group on number of fixations.
Similar to the total gaze duration results and consistent with hypotheses, planned contrasts for number of fixations revealed that the group-by-stimulus type interaction was driven by a greater mean number of fixations on positive images in the medicated depression group and in the comparison group relative to the unmedicated depression group. There was no difference between the comparison and medicated depression groups in number of fixations for positive images. The medicated depression group also made fewer fixations on dysphoric images compared with the unmedicated depression group. There were no significant differences between the comparison group and either the medicated or the unmedicated depression groups on number of fixations for dysphoric images. Again, differences for threat and neutral images were nonsignificant (Table 2).
Mean fixation duration.
A 3×4 (group by stimulus type) ANOVA revealed no significant main effects for group or stimulus type and no significant interaction between group and stimulus type on mean fixation duration. Because of the lack of significant main effects or interaction, post hoc analyses were not performed for mean fixation duration.
Discussion
In this study, we tested the cognitive neuropsychological model of depression by evaluating the association between antidepressant medication use and selective attention for emotional information among individuals with major depressive disorder and nondepressed comparison subjects. As hypothesized, among depressed participants, we found an association between antidepressant medication use (compared with nonuse) and greater selective attention for positive stimuli. Individuals in the medicated depression group had longer total gaze duration and more fixations for positive images compared with the unmedicated depression group. The nondepressed comparison group also demonstrated longer total gaze duration compared with the unmedicated depression group. Notably, the medicated depression group did not differ from the comparison group in selective attention for positive stimuli, suggesting that antidepressant medication use normalizes information processing. We did not find any significant differences between our groups in gaze duration for dysphoric stimuli, but we did find that the medicated depression group had fewer fixations for dysphoric stimuli compared with the unmedicated depression group.
Our results are consistent with previous research demonstrating an association between antidepressant medication use and changes in information processing for positive emotional stimuli (7–9). While previous studies have investigated the effects of a single dose of antidepressant medication compared with placebo on information processing, this is the first study to examine the effects of antidepressant medication use as prescribed in the community on information processing in participants with major depression. The association between antidepressant use and emotional information processing for positive stimuli has now been observed across healthy and depressed samples and across several different information processing tasks. The effect has been observed after a single administration of an antidepressant in previous studies and, in our study, with depressed individuals on a consistent regimen of antidepressant medication. The fact that the effect of antidepressant medication use on emotional information processing for positive stimuli has now been observed across samples, tasks, and methods of medication administration, in combination with the large effect sizes observed in the present study, suggests that this effect is robust.
These findings add to the growing evidence that antidepressant medications exert their antidepressant effects through modification of emotional information processing rather than direct elevation of mood (7). It will be necessary for future research to clarify the extent to which changes in gaze bias mediate the relationship between antidepressant use and subsequent reductions in depressed mood.
Our results must be interpreted in light of some limitations. One key limitation of this study is the exclusion of anxiety disorders from our sample of depressed patients, which limits the external generalizability of the study findings. Other important limitations are the nonrandom assignment to medication condition and the inclusion of multiple classes of antidepressant medications, which limit the internal validity of the findings. While there are clear limitations to these design choices, our more naturalistic approach does enhance the ecological validity of the study. Indeed, participants were being treated with a variety of antidepressants (as opposed to being given a single study medication, sometimes at narrowly specified dosages), which better matches the reality of antidepressant medication use in the general population. Moreover, eye-tracking technology is a more ecologically valid tool than the indicators of selective attention that have been used in the past (e.g., probe detection), insofar as it provides critical information about dynamic stages of attention. Along with previous experimental research (8, 9), the results of this study provide further evidence for modification of information processing as a potential mechanism of action for antidepressant medication.
1 : Cognitive Therapy of Depression. New York, Guilford, 1979Google Scholar
2 : Association of predeployment gaze bias for emotion stimuli with later symptoms of PTSD and depression in soldiers deployed in Iraq. Am J Psychiatry 2011; 168:735–741Link, Google Scholar
3 : Biased processing of emotional information in girls at risk for depression. J Abnorm Psychol 2007; 116:135–143Crossref, Medline, Google Scholar
4 : Biased attention and dysphoria: manipulating selective attention reduces subsequent depressive symptoms. Cogn Emotion 2010; 24:719–728Crossref, Google Scholar
5 : Attentional bias training in depression: therapeutic effects depend on depression severity. J Behav Ther Exp Psychiatry 2010; 41:265–274Crossref, Medline, Google Scholar
6 : Too much of a good thing: testing the efficacy of a cognitive bias modification task for cognitively vulnerable individuals. Cognit Ther Res 2012; 36:493–501Crossref, Google Scholar
7 : Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br J Psychiatry 2009; 195:102–108Crossref, Medline, Google Scholar
8 : A single dose of citalopram increases fear recognition in healthy subjects. J Psychopharmacol 2007; 21:684–690Crossref, Medline, Google Scholar
9 : Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry 2009; 166:1178–1184Link, Google Scholar
10 : Eye movement registration as a continuous index of attention deployment: data from a group of spider anxious students. Cogn Emotion 1999; 13:419–435Crossref, Google Scholar
11 : Time course of selective attention in clinically depressed young adults: an eye tracking study. Behav Res Ther 2008; 46:1238–1243Crossref, Medline, Google Scholar
12 : The role of visual attention in saccadic eye movements. Percept Psychophys 1995; 57:787–795Crossref, Medline, Google Scholar
13 : Covert and overt orienting of attention to emotional faces in anxiety. Cogn Emotion 2000; 14:789–808Crossref, Google Scholar
14 : Manual for the Beck Depression Inventory–II. San Antonio, Tex, Psychological Corp, 1996Google Scholar
15 : The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(suppl 20):22–33Medline, Google Scholar
16 : Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York, New York State Psychiatric Institute, Biometrics Research, 2002Google Scholar
17 : Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 1988; 8:77–100Crossref, Google Scholar
18 : An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988; 56:893–897Crossref, Medline, Google Scholar
19 Wilson KA, de Beurs E, Palmer CA, Chambless DL: Beck Anxiety Inventory, in The Use of Psychological Testing for Treatment Planning and Outcomes Assessment, 2nd ed. Edited by Maruish ME. Mahwah, NJ, Lawrence Erlbaum Associates, 1999, pp 971–992Google Scholar
20 Lang PJ, Bradley MM, Cuthbert BN: International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual (Technical Report A-6). Gainesville, University of Florida, 2005Google Scholar



