Association of Predeployment Gaze Bias for Emotion Stimuli With Later Symptoms of PTSD and Depression in Soldiers Deployed in Iraq
Abstract
Objective:
Biased processing of emotion stimuli is thought to confer vulnerability to psychopathology, but few longitudinal studies of this link have been conducted. The authors examined the relationship between predeployment gaze bias for emotion stimuli and later symptoms of posttraumatic stress disorder (PTSD) and depression in soldiers deployed to Iraq.
Method:
An eye-tracking paradigm was used to assess line of gaze in 139 soldiers while they viewed a two-by-two matrix of fearful, sad, happy, and neutral facial expressions before they were deployed to Iraq. Once they were deployed, the soldiers periodically reported on their levels of war zone stress exposure and symptoms of PTSD and depression.
Results:
War zone stress exposure predicted higher scores on PTSD and depression symptom measures; however, eye gaze bias moderated this relationship. In soldiers with war zone stress exposure, shorter mean fixation time when viewing fearful faces predicted higher PTSD symptom scores, and greater total fixation time and longer mean fixation time for sad faces predicted higher depressive symptom scores.
Conclusions:
Biased processing of emotion stimuli, as measured by gaze bias, appears to confer vulnerability to symptoms of PTSD and depression in soldiers who experience war zone stress.
Although the psychological impact of war-related trauma was recognized as early as the American Civil War, empirical investigation of war zone trauma did not emerge until the Vietnam War (1). More recently, a survey of 1,965 U.S. veterans of the wars in Iraq and Afghanistan (2) found that 14% screened positive for posttraumatic stress disorder (PTSD) and 14% for major depressive disorder in the past 30 days. In another study (3), after controlling for baseline characteristics, symptoms were three times as likely to develop in deployed soldiers with exposure to combat stressors than in deployed soldiers with no combat stress exposure.
However, exposure to war zone stressors does not affect all soldiers equally; war zone stress leads to symptoms of anxiety and depression for some soldiers but not others (3). This is not unique to military populations. Epidemiologic data indicate that many Americans (60.7%) have been exposed to a traumatic stressor, yet only a small minority (8%) develop PTSD (4). Likewise, life stress often precedes an increase in depressive symptoms, but only a minority of individuals exposed to stressful life events become depressed (5).
These data suggest that individual differences may influence the impact of stress exposure on the development of symptoms of PTSD and depression. Previous work has identified several risk factors for PTSD, such as lower education levels, nonwhite race, female gender, previous trauma, and catastrophic thinking (6, 7). Similarly, subthreshold symptoms, rumination, and a history of depression increase the risk of depression (8, 9). In this study, we examined whether biased processing of emotion stimuli might help explain why some soldiers experience stronger symptomatic reactions to war zone stress exposure than others.
Selective processing and avoidance of threat-related stimuli are believed to increase vulnerability to PTSD symptoms (10, 11). A tendency to quickly process and attend to threat information may have adaptive significance. However, subsequent cognitive avoidance of threat-related material is posited to maintain anxiety, as it may reduce opportunities to habituate or reappraise stimuli as nonthreatening (12). Consistent with this conceptualization, highly anxious individuals initially orient to threat stimuli and then avoid those stimuli (13, 14).
In contrast, maintained processing of dysphoric information is thought to characterize vulnerability to depression. This bias occurs relatively late in postconscious processing compared to biases associated with PTSD. Depressed and dysphoric individuals display maintained attention for sad facial expressions when they are presented for at least 1,000 msec (15, 16). Research has shown that people who have recovered from depression, as well as adolescent girls with a maternal history of depression, display a similar bias for sad stimuli (17, 18). Selective attention for dysphoric information can be observed up to 30 seconds after stimulus onset in depressed individuals (19, 20).
Although biased processing of emotion stimuli has been posited as having a causal role in the onset and maintenance of anxiety and depression, few studies have examined whether these biases moderate effects of environmental stress and contribute to future exacerbation of symptoms of PTSD and depression (for exceptions, see references 21, 22). In this study, we used a brief eye-tracking paradigm to measure gaze bias (total time, mean fixation time, number of fixations) while a sample of soldiers viewed a 2×2 matrix of fearful, sad, happy, and neutral facial expressions prior to deployment. Once deployed to Iraq, the soldiers periodically reported war zone stressor exposure and symptom severity. We examined whether predeployment gaze bias moderated the effect of war zone stressor exposure on symptoms of PTSD and depression reported during deployment. We hypothesized that a gaze bias to avoid fear stimuli would be associated with greater PTSD symptoms in soldiers exposed to war zone stress. We expected that a gaze bias toward sad stimuli would predict greater symptoms of depression in soldiers exposed to war zone stress.
Method
Participants
Participants were 178 U.S. Army soldiers with no prior war zone experience scheduled for deployment to Iraq from Fort Hood, Texas. Participants were required to complete at least seven of eight eye-tracking trials with 60% or more data points available. Nineteen (11%) soldiers were excluded because of difficulty obtaining eye-tracking data (e.g., excessive blinking, droopy eyelids due to sleepiness); this proportion is typical for eye-tracking studies. Twenty (11%) soldiers were excluded because they did not provide stressor exposure and symptom reports during deployment in Iraq. There were no significant differences in age, gender, ethnicity, race, education, or predeployment symptoms of PTSD or depression between soldiers who provided data during deployment and those who did not.
Demographic information for the 139 participants for whom we had adequate data is summarized in Table 1 . The mean age of the sample was 23.3 years (SD=5.74). Military rank for the majority of participants was private (E-2) (37%) or private first class (E-3) (32%); only 6% were officers. The mean duration of deployment was 384 days. Twenty-three participants met criteria for one or more current axis I diagnoses (substance use disorder, N=10; anxiety disorder, N=9; mood disorder, N=6; adjustment disorder, N=2), as determined by the Structured Clinical Interview for DSM-IV (23). Covarying for presence (yes/no) of a current axis I disorder did not alter findings, so this variable was not examined further.
| Characteristic | N | % |
|---|---|---|
| Male | 125 | 90 |
| Race | ||
| African American or black | 15 | 11 |
| American Indian or Alaska Native | 17 | 12 |
| Asian or Pacific Islander | 4 | 3 |
| Other | 3 | 2 |
| Caucasian | 100 | 72 |
| Hispanic ethnicity | 25 | 18 |
| Education level | ||
| <High school diploma | 14 | 10 |
| High school diploma | 57 | 41 |
| Some college | 54 | 39 |
| College diploma | 9 | 7 |
| Some graduate school or higher | 5 | 4 |
TABLE 1. Demographic Characteristics of Soldiers Who Participated in a Predeployment Eye-Tracking Study (N=139)
Self-Report Assessments
During deployment, soldiers were prompted by e-mail every 30 days to complete self-report assessments through a web-based survey. Participants were asked to complete these assessments throughout their deployment.
War zone stressors.
Soldiers identified stressors from a list of 18 validated war zone stressors from the Deployment Risk and Resilience Inventory (24). Soldiers were allowed to record up to two additional combat-related stressors not included in the list of 18. Soldiers indicated which stressors they had experienced since their most recent assessment (or, for the first assessment, since deployment). The number of combat stressors was summed to estimate level of war zone stress exposure.
Symptoms of PTSD and depression.
The 4-item PTSD Checklist, a brief version of the original 17-item version (25), was used to assess three PTSD symptom clusters: reexperiencing (two items), avoidance (one item), and increased arousal (one item). A 5-point Likert response format is used to indicate severity of each symptom, and the range of possible scores is 0–20. A total score of 7 is a sensitive cutoff for predicting PTSD diagnosis (26). For this sample, internal consistency (Cronbach's alpha=0.72) and test-retest reliability (separated on average by 60 days, r=0.57) were good. In addition, 111 soldiers (79.9%) completed the 17-item PTSD Checklist during the predeployment assessment. The 17-item and 4-item versions at predeployment were highly correlated (r=0.93, p<0.001).
For depressive symptoms, soldiers indicated the extent to which they experienced each of 19 symptoms (e.g., feeling sad, loss of energy, loss of appetite, feeling like a failure) using a 5-point drop-down scale (ranging from 1, not at all, to 5, extremely). Internal consistency (Cronbach's alpha=0.86) and test-retest reliability (separated on average by 72 days, r=0.60) were good. A subsample (N=71) also completed the 10-item version of the Center for Epidemiologic Studies Depression Scale (CES-D Scale) during deployment (27). The depression scale was highly correlated with CES-D Scale total score (r=0.74). These data suggest that our assessment of depression had adequate internal consistency, test-retest reliability, and convergent validity. All 139 soldiers completed the 20-item CES-D Scale (28) during the predeployment assessment.
During deployment in Iraq, 139 soldiers provided 829 reports of stress exposure and depression (mean=5.96 reports per soldier), and 132 soldiers provided 537 reports of PTSD symptoms (mean=4.06 reports per soldier). Fewer reports were obtained for PTSD symptoms because the 4-item PTSD Checklist was added to the assessment battery later on during the study.
Eye-Tracking Paradigm
The eye-tracking task involved simultaneous presentation of four images selected from the Pictures of Facial Affect collection (29). Stimuli were faces from 12 different actors divided evenly by gender. Four facial expressions from the same actor were presented simultaneously in each quadrant of the visual field (Figure 1). For each participant, happy, sad, fearful, and neutral facial expressions were randomly assigned to each quadrant with the constraint that facial expressions from each emotion category were presented in each quadrant with equal frequency.
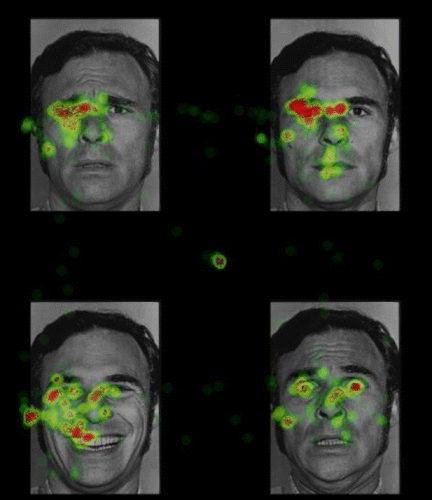
FIGURE 1. Example of Stimuli Used in a Predeployment Eye-Tracking Studya
a Participants' gaze locations from this trial are superimposed on the image. Color intensity reflects higher gaze density. Images are from the Pictures of Facial Affect database, copyright 1976 by the Paul Ekman Group LLC. Reproduced with permission.
Eight critical trials presented happy, sad, fearful, and neutral facial expressions in a new random order for each participant. Four filler trials with faces from other emotion categories were also presented. Each trial began with a centrally presented fixation cross, followed by presentation of face stimuli for 30 seconds. Line of visual gaze was assessed using a remote optics eye-tracking system Model R6 from Applied Science Laboratories (Bedford, Mass.). Direction of gaze, measured with x and y coordinates, was sampled every 16.7 msec (60 Hz), producing 1,796 gaze location measurements for each 30-second trial. Eye movements that were stable for more than 100 msec within 1° of visual angle were classified as a fixation.
Procedure
Participants typically reported for study participation at the University of Texas by 8:00 a.m., usually in groups of four to six soldiers, and were monitored by study personnel until dismissal approximately 7 hours later. Participants completed a number of study assessments, including several that are not a focus of this report. After providing informed consent, participants provided demographic information, completed questionnaires, and were interviewed to assess for presence of DSM-IV diagnoses. Eye tracking typically occurred between 1 p.m. and 3 p.m. After eye-tracking calibration, participants were instructed to view images naturally, as if they were watching television or viewing images in a photo album, and to view whatever seemed interesting. They were also instructed to look at the fixation cross prior to each trial in order to standardize starting location of gaze.
Soldiers were deployed to Iraq approximately 3 months after the predeployment assessment. Self-report assessments before and during deployment were obtained through a web-based system developed by study personnel.
Analytic Plan
We used mixed-effects regression analyses (30). Initial models tested associations between symptom scores and time (in days) since deployment. However, after entering war zone stress exposure score, time was not a significant predictor of symptom scores in any model. Subsequent models examined whether total fixation time, mean fixation time, or number of fixations for each emotion category moderated the effect of war zone stress exposure on symptoms reported during deployment.
Results
Tables 2 and 3 summarize eye-tracking data and measures of symptoms and stress exposure before and after deployment.
| Total Fixation Time (seconds) | Mean Fixation Time (msec) | Number of Fixations | ||||
|---|---|---|---|---|---|---|
| Stimulus | Mean | SD | Mean | SD | Mean | SD |
| Sad | 4.53 | 2.04 | 423 | 11 | 10.23 | 4.08 |
| Fearful | 5.51 | 3.47 | 465 | 25 | 11.51 | 5.23 |
| Happy | 7.53 | 4.83 | 526 | 25 | 14.08 | 6.00 |
| Neutral | 5.37 | 2.42 | 466 | 13 | 11.10 | 4.36 |
TABLE 2. Results of Predeployment Eye-Tracking Study for 139 Soldiers
| Measure | Mean | SD | Range |
|---|---|---|---|
| Before deployment | |||
| 17-item PTSD Checklist | 18.05 | 2.90 | 17–53 |
| 20-item Center for Epidemiologic Studies Depression Scale | 9.42 | 7.58 | 0–44 |
| During deploymenta | |||
| War zone stress exposure (list of 18 war zone stressors) | 2.34 | 2.58 | 0–18 |
| 4-item PTSD Checklist | 5.22 | 2.11 | 4–16 |
| Depressive symptoms (19-item scale) | 18.17 | 7.08 | 0–50 |
TABLE 3. Symptom and Stress Exposure Measures Before and During Deployment for 139 Soldiers
PTSD Symptoms
War zone stress exposure was significantly associated with PTSD symptoms (β=0.19, SE=0.04; t=5.10, df=131, p<0.001); higher war zone stress exposure was associated with elevated PTSD symptom score. Total fixation time for sad, happy, fearful, and neutral faces did not moderate the association between war zone stress exposure and PTSD symptom score.
In contrast, mean fixation time for fearful faces significantly moderated the effect of war zone stress exposure on PTSD symptom score (β=–0.31, SE=0.07; t=–4.71, df=130, p<0.001). This association remained significant when controlling for predeployment PTSD symptom score (β=–0.32, SE=0.02; t=–5.26, df=109, p<0.001). Shorter mean fixation time for fearful faces was associated with higher PTSD symptom score as war zone stress exposure increased. Soldiers with shorter mean fixation time (one standard deviation below the mean) for fearful faces required nine war zone stressors to reach a clinical cutoff score of 7, whereas soldiers with longer mean fixation time (one standard deviation above the mean) required 17 war zone stressors to reach clinical threshold (see Figure 2A).
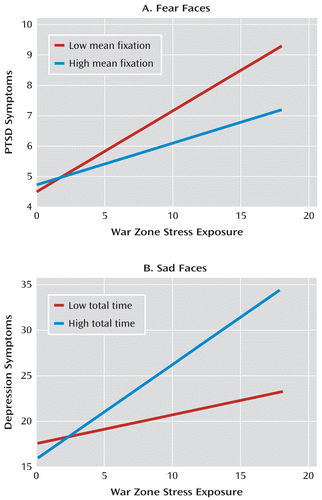
FIGURE 2. Total and Mean Fixation Time for Viewing Fearful and Sad Facial Expressions in a Predeployment Eye-Tracking Paradigm, by War Zone Stress Exposurea
a In panel A, mean fixation time for fearful faces moderates effect of war zone stress exposure on PTSD symptom score (t=–4.21, df=130, p<0.001). In panel B, total time viewing sad faces moderates effect of combat war zone stress exposure on depressive symptom score (t=2.57, df=137, p=0.01). High and low gaze biases are defined as one standard deviation above and below the group mean, respectively.
Mean fixation time for sad, happy, and neutral faces did not moderate the effect of war zone stress exposure on PTSD symptom score. When simultaneously entering mean fixation time for sad, happy, neutral, and fearful faces, mean fixation time for fearful faces continued to moderate the association between war zone stress exposure and PTSD symptom score (β=–0.37, SE=0.07; t=–4.18, df=127, p<0.001; all other coefficients, p>0.31).
Number of fixations for fearful, sad, happy, and neutral faces did not significantly moderate the effect of war zone stress exposure on PTSD symptom score.
Depressive Symptoms
Higher war zone stress exposure was associated with higher depressive symptom score (β=0.61, SE=0.15; t=4.00, df=138, p<0.001). Total fixation time for sad faces significantly moderated the effect of war zone stress exposure on depressive symptom score (β=0.20, SE=0.07; t=2.57, df=137, p=0.01) and remained significant when controlling for predeployment depressive symptom score (β=0.17, SE=0.08; t=2.20, df=136, p=0.03). In soldiers with longer total fixation time for sad stimuli, depressive symptom scores increased more as number of war zone stressors increased than it did in soldiers with shorter total fixation time for sad stimuli (see Figure 2B).
Total fixation time for happy, fearful, and neutral faces did not moderate the association between war zone stress exposure and depressive symptom score. When simultaneously entering total fixation time for sad, happy, neutral, and fearful faces, total fixation time for sad faces continued to moderate the effect of war zone stress exposure on depressive symptom score (β=0.21, SE=0.10; t=1.99, df=134, p=0.049; all other coefficients, p>0.52).
Mean fixation time for sad faces approached statistical significance for moderating the effect of war zone stress exposure on depressive symptom score (β=2.65, SE=1.39; t=1.91, df=137, p=0.06) and was significant when controlling for predeployment depressive symptom score (β=2.44, SE=1.25; t=1.94, df=136, p=0.05). Longer mean fixation time for sad faces was associated with higher depressive symptom score in soldiers with higher levels of war zone stress exposure. Mean fixation time for happy, fearful, and neutral faces did not moderate the effect of war zone stress exposure on depressive symptom score. When simultaneously entering mean fixation time for sad, happy, neutral, and fearful faces, mean fixation time for sad faces significantly moderated the effect of war zone stress exposure on depressive symptom score (β=4.13, SE=1.92; t=2.15, df=134, p=0.049). Mean fixation for fearful faces also approached significance in this analysis (β=–1.53, SE=0.77; t=1.95, df=134, p=0.06; all other coefficients p>0.35).
Number of fixations for sad faces significantly moderated the effect of war zone stress exposure on depressive symptom score (β=0.08, SE=0.04; t=2.04, df=137, p=0.04) and approached significance when controlling for predeployment depression (β=0.06, SE=0.04; t=1.69, df=136, p=0.09). In soldiers with longer total fixation time for sad stimuli, depressive symptom score increased more as number of war zone stressors increased than it did in soldiers with shorter total fixation time for sad stimuli. Furthermore, number of fixations for happy, fearful, and neutral faces did not significantly moderate the effect of war zone stress exposure on depressive symptom score. When simultaneously entering number of fixations for sad, happy, neutral, and fearful faces, number of fixations for sad faces no longer moderated the effect of war zone stress exposure on depressive symptom score.
Prediction of Nonoverlapping Symptoms of PTSD and Depression
The final analysis examined whether eye gaze bias predicted PTSD symptoms when controlling for concurrent depression, and vice versa. These analyses examine whether gaze bias predicts variability in symptoms that are unique to each condition. Notably, the average correlation between PTSD symptoms and depressive symptoms reported during deployment was relatively strong (r=0.43, p<0.001). For PTSD symptoms, mean fixation time for fearful faces continued to moderate the effect of war zone stress exposure on PTSD symptom score when controlling for concurrent depressive symptom score (β=–0.22, SE=0.06; t=–3.41, df=130, p=0.001). In contrast, total time, mean fixation time, and number of fixations for sad faces did not significantly predict depressive symptom score when controlling for concurrent PTSD symptom score.
Discussion
Gaze bias for emotion stimuli predicted symptoms of PTSD and depression during deployment to a war zone. Soldiers with shorter mean fixation time when viewing faces depicting fear prior to deployment were at greater risk of experiencing PTSD symptoms in the context of war zone stress than were those with longer mean fixation time. Put differently, less war zone stress exposure was required to elicit PTSD symptoms in individuals with brief fixation times for fearful faces prior to deployment. In contrast, total fixation time, longer mean fixation time, and number of fixations for sad faces significantly potentiated the impact of war zone stress exposure on depressive symptoms. Individuals with longer and more frequent fixations on sad faces prior to deployment reported higher levels of depressive symptoms in response to war zone stress. These data provide new evidence that biased processing of emotion stimuli predicts future symptoms of PTSD and depression.
Shorter fixation time for fearful faces specifically moderated the effect of war zone stress exposure on PTSD symptoms, whereas total fixation time and number of fixations for fearful faces did not. This bias was specific to PTSD symptoms, as it continued to predict symptoms even after controlling for concurrent depression. Cognitive avoidance via rapid disengagement of attention from fear stimuli may reflect the tendency to avoid processing fear-related information (31) and interfere with naturally occurring habituation or reappraisal (32).
Attentional avoidance of threat-related stimuli has been associated with greater distress in people exposed to acute stressors, such as rocket attacks (11). However, other cross-sectional research finds that veterans with elevated PTSD symptoms show an attentional bias toward negative images compared to veterans with low levels of PTSD symptoms (33, 34). One possible explanation for these divergent findings is that attentional avoidance may be particularly relevant for predicting anxiety responses to acute stressors, whereas attentional vigilance may occur after the onset of PTSD. Additional research into the development of biased attention may help identify conditions that produce attentional avoidance rather than vigilance in PTSD.
In contrast, soldiers who viewed sad faces for longer durations (total time, mean fixation time, and number of fixations) before deployment were at greater risk for experiencing depressive symptoms if they also experienced war zone stress exposure. These biases did not predict depressive symptoms when covarying for concurrent PTSD symptoms, suggesting that gaze bias for sad faces may confer vulnerability to negative affect (35) rather than to depression per se. Other biases, such as blunted reward sensitivity (36), may be better predictors of symptoms specific to depression. Nevertheless, although a number of studies have documented information processing biases in currently depressed individuals (15, 20), this is one of the first studies to demonstrate that such a bias is associated with vulnerability to future depressive symptoms in the context of stress (37).
Our results should be interpreted in light of the study's limitations. We used a validated but brief (4-item) assessment of PTSD symptoms (26) when soldiers were deployed in Iraq. Although the 4-item scale was strongly correlated with the 17-item version at the predeployment assessment, it measures a relatively restricted set of symptoms. Future research should use the 17-item version to comprehensively assess PTSD and measure other important symptoms, such as reliving of past experiences, hypervigilance for threats, and sleep difficulties.
An important alternative explanation to consider is that gaze bias predicted future symptoms because it was a proxy for symptoms prior to deployment. However, when statistically controlling for predeployment axis I psychopathology and symptoms of PTSD and depression, results were largely unchanged. Other risk factors not measured in this study could have influenced results. For instance, high trait anxiety has been associated with biased attention for threat (32), and neuroticism has been associated with a greater risk for PTSD (38, 39). Future work should measure these risk factors and examine whether they contribute to a greater risk for PTSD and depression, perhaps via their influence on biased attention for emotion stimuli.
In summary, war zone stress exposure clearly increases vulnerability to symptoms of PTSD and depression, although its impact varies. Gaze bias when viewing emotion stimuli approximately 3 months prior to deployment predicted greater psychopathology symptoms in response to war zone stress during deployment. Soldiers with shorter fixations on facial expressions depicting fear reported higher PTSD symptom levels as war zone stress increased. Soldiers who spent more total time, had longer mean fixations, and had more total fixations directed toward sad faces reported higher depressive symptom levels as war zone stressors increased. Thus, gaze biases for emotional stimuli may identify individuals who are vulnerable to psychopathology in the context of stressful life experiences.
1. : Trauma and the Vietnam War Generation: Report of Findings From the National Vietnam Veterans Readjustment Study. Philadelphia, Brunner/Mazel, 1990Google Scholar
2. : Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, Calif, RAND Corporation, 2008Google Scholar
3. : New onset and persistent symptoms of post-traumatic stress disorder self reported after deployment and combat exposures: prospective population based US military cohort study. BMJ 2008; 336:366–371Crossref, Medline, Google Scholar
4. : Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995; 52:1048–1060Crossref, Medline, Google Scholar
5. : The effects of stressful life events on depression. Annu Rev Psychol 1997; 48:191–214Crossref, Medline, Google Scholar
6. : Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol 2000; 68:748–766Crossref, Medline, Google Scholar
7. : Maladaptive appraisals as a risk factor for posttraumatic stress: a study of trainee firefighters. Psychol Sci 2005; 16:749–752Crossref, Medline, Google Scholar
8. : The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol 2000; 109:504–511Crossref, Medline, Google Scholar
9. : Offspring of depressed parents: 20 years later. Am J Psychiatry 2006; 163:1001–1008Link, Google Scholar
10. : Cognitive aspects of posttraumatic stress disorder, in Behavior and Cognitive Therapy Today: Essays in Honor of Hans J Eysenck. Edited by Sanavio E. Oxford, England, Elsevier, 1998, pp 181–187Crossref, Google Scholar
11. : Life-threatening danger and suppression of attention bias to threat. Am J Psychiatry 2010; 167:694–698Link, Google Scholar
12. : Time course of attentional bias for threat information in non-clinical anxiety. Behav Res Ther 1997; 35:297–303Crossref, Medline, Google Scholar
13. : Spider fearful individuals attend to threat, then quickly avoid it: evidence from eye movements. J Abnorm Psychol 2006; 115:231–238Crossref, Medline, Google Scholar
14. : Anxiety and orienting of gaze to angry and fearful faces. Biol Psychol 2007; 76:163–169Crossref, Medline, Google Scholar
15. : Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol 2004; 113:127–135Crossref, Google Scholar
16. : Mood-congruent attentional bias in dysphoria: maintained attention to and impaired disengagement from negative information. Emotion 2005; 5:446–455Crossref, Medline, Google Scholar
17. : Selective attention to emotional faces following recovery from depression. J Abnorm Psychol 2007; 116:80–85Crossref, Medline, Google Scholar
18. : Biased processing of emotional information in girls at risk for depression. J Abnorm Psychol 2007; 116:135–143Crossref, Medline, Google Scholar
19. : A naturalistic visual scanning approach to assess selective attention in major depressive disorder. Psychiatry Res 2003; 118:117–128Crossref, Medline, Google Scholar
20. : Time course of selective attention in clinically depressed young adults: an eye tracking study. Behav Res Ther 2008; 46:1238–1243Crossref, Medline, Google Scholar
21. : Plasticity in attention: implications for stress response in children. Behav Res Ther 2008; 46:450–461Crossref, Medline, Google Scholar
22. : Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. J Abnorm Psychol 2002; 111:107–123Crossref, Medline, Google Scholar
23. : Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
24. : Deployment Risk and Resilience Inventory: a collection of measures for studying deployment-related experiences of military personnel and veterans. Mil Psychol 2006; 18:89–120Crossref, Google Scholar
25. : The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Paper presented at the
26. : Validating the Primary Care Posttraumatic Stress Disorder Screen and the Posttraumatic Stress Disorder Checklist with soldiers returning from combat. J Consult Clin Psychol 2008; 76:272–281Crossref, Medline, Google Scholar
27. : Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med 1994; 10:77–84Crossref, Medline, Google Scholar
28. : The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1:385–401Crossref, Google Scholar
29. : Pictures of Facial Affect. Palo Alto, Calif, Consulting Psychologists Press, 1976Google Scholar
30. : HLM 5: Hierarchical Linear and Nonlinear Modeling. Chicago, Scientific Software International, 2001Google Scholar
31. : Emotional processing of fear: exposure to corrective information. Psychol Bull 1986; 99:20–35Crossref, Medline, Google Scholar
32. : Threat-related attentional bias in anxious and non-anxious individuals: a meta-analytic study. Psychol Bull 2007; 133:1–24Crossref, Medline, Google Scholar
33. : Eye tracking and visual attention to threatening stimuli in veterans of the Iraq War. J Anxiety Disord 2010; 24:293–299Crossref, Medline, Google Scholar
34. : Eye movement and electrodermal responses to threat stimuli in post-traumatic stress disorder. Int J Psychophysiol 1995; 20:209–213Crossref, Medline, Google Scholar
35. : Testing a tripartite model, I: evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol 1995; 104:3–14Crossref, Medline, Google Scholar
36. : Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 2009; 166:702–710Link, Google Scholar
37. : Attentional bias and mood persistence as prospective predictors of dysphoria. Cognit Ther Res 2003; 27:619–637Crossref, Google Scholar
38. : Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry 1991; 48:216–222Crossref, Medline, Google Scholar
39. : Neuroticism and low educational level predict the risk of posttraumatic stress disorder in women after miscarriage or stillbirth. Gen Hosp Psychiatry 2006; 28:414–417Crossref, Medline, Google Scholar



