Semantic Distance Abnormalities in Mild Cognitive Impairment: Their Nature and Relationship to Function
Abstract
Objective
The authors sought to directly examine compromises in the semantic system in mild cognitive impairment and their possible relationship to everyday functional competencies.
Method
Study participants were 25 patients who met criteria for amnestic mild cognitive impairment, 27 patients with mild or moderate Alzheimer’s disease, and 70 healthy comparison subjects. The authors administered a novel semantic distance task in which participants make decisions about word or image stimuli that correspond to real-world entities that differ in physical size. The authors also administered a performance-based measure of everyday functional competence.
Results
Participants in the mild cognitive impairment and Alzheimer’s groups were consistently less accurate and slower than healthy comparison subjects in semantic decisions in which words were used as stimuli. When these participants had to make more fine-grained decisions about the semantic attribute of size, their performance in accuracy and reaction time disproportionately worsened relative to that of comparison subjects. In image-based conditions in which line drawings were used as stimuli, sensory-perceptual information (i.e., the size of the drawings themselves) had undue influence over semantic knowledge judgments in the mild cognitive impairment and Alzheimer’s groups. Performance in the semantic distance task was a strong and significant predictor of everyday functional competence in the mild cognitive impairment and Alzheimer’s groups.
Conclusions
This study synthesized several distinct strands in the mild cognitive impairment literature by providing evidence for 1) compromises in the semantic system in mild cognitive impairment, not confounded by overt retrieval or refractory access; 2) intrusion of perceptual information on semantic processing; and 3) a robust relation between semantic corruption and difficulties in everyday functioning.
Semantic memory refers to a stored representational knowledge base that includes information about, and enables interpretation of, entities and language (1, 2) (Figure 1). Impaired semantic memory is considered a critical feature of Alzheimer’s disease (3, 4). In amnestic mild cognitive impairment, a disorder often thought to be a transitional phase between cognitive health and Alzheimer’s disease, there is increasing evidence that the range of cognitive abnormalities in mild cognitive impairment extends well beyond episodic memory (5). Few studies, however, have directly addressed compromises of semantic memory. Moreover, findings, when positive, have been confounded by use of paradigms involving overt word retrieval, which would make it ambiguous whether patients simply had word-finding problems (i.e., were logopenic) or had corrupted semantic knowledge; several studies were also limited by small sample sizes (6–10). From a neurobiological perspective, our research was further motivated by the finding of in vitro molecular and in vivo volumetric abnormalities in regions of the lateral temporal cortex known to be engaged during semantic processing in at-risk samples with the APOE4 gene and in samples with mild cognitive impairment (5, 11, 12). From a clinical perspective, we had observed that patients with mild cognitive impairment sometimes appeared perplexed or confused in interviews, as if they did not have the knowledge to comprehend or describe a point. Thus, we sought to determine the relationship between semantic memory compromises and everyday functional competencies, which has only rarely been examined (but see Perry and Hodges [10] for a negative result).

In this study, we examined semantic processing in large groups of healthy comparison subjects, patients with mild cognitive impairment, and patients with Alzheimer’s disease by employing a novel paradigm developed by Cohen et al. (13) that does not require overt word retrieval. The task capitalizes on well-known “distance effects” in the semantic system, such that size judgments of entities similar in size in the real world have longer response times and are less accurate than size judgments about entities that are very dissimilar in size in the real world. These effects correspond to well-known quantity processing effects using numbers (14). We included both word (in which the stimuli were orthographic) and image (in which the stimuli were line drawings of real-world entities) conditions so that we could differentiate between abstract semantic knowledge and semantic knowledge based on visual perception. Furthermore, the image condition depicted the entities as two different sizes that were either congruent or incongruent with real-world size differences. For example, an ant could be depicted as smaller than a house (image-congruent) or as larger than a house (image-incongruent). We predicted that in the word condition, in which the stimuli themselves did not carry information about physical size, any differences between healthy subjects and the mild cognitive impairment and Alzheimer’s groups should reflect impairments in the semantic system above and beyond impairments in word retrieval. Furthermore, because the physical size of the words shown on the monitor did not differ, decisions could only be made after access to a central semantic knowledge store. We further predicted that in the image condition, participants with mild cognitive impairment or Alzheimer’s disease would be prone to undue influence of depicted size and thus show relative processing advantages for congruent stimuli or disadvantages for incongruent stimuli, a situation that might reflect abnormally increased “permeability” between the perceptual and the semantic systems.
Beyond determining the presence and nature of semantic abnormalities in mild cognitive impairment, we also sought to investigate whether semantic processing and everyday functional competence were associated in patients with mild cognitive impairment and Alzheimer’s disease. We hypothesized that even subtle corruption of the semantic system would result in inefficiency or failure on real-world tasks. To explore this question, we used the UCSD Performance-Based Skills Assessment (UPSA) (15), a performance-based measure of everyday functional competence that we recently showed (16) is sensitive to failures in instrumental activities of daily living in patients with mild cognitive impairment. In particular, we hypothesized that abnormalities in semantic distance effects (in accuracy or response time) would be a robust predictor of everyday functional competence.
Method
Staging Instruments
The Mini-Mental State Examination (MMSE) (17) was used for screening of cognitive level. The Clinical Dementia Rating scale (18) was used to assess dementia; this instrument consists of items related to memory, orientation, problem solving, personal care, functioning at home and in hobbies, and functioning in community affairs. Domain scores were integrated in an algorithm in order to yield the global value.
Participants
The study included 122 participants—25 patients with mild cognitive impairment, 27 patients with Alzheimer’s disease, and 70 healthy comparison subjects. All participants were between the ages of 55 and 90. The diagnostic procedures for patients with mild cognitive impairment and Alzheimer’s disease were identical to those employed in Goldberg et al. (16). Basic demographic characteristics and data on global cognition and functioning are presented in Table 1. Participants were assessed at the Litwin-Zucker Center for Research in Alzheimer’s Disease and Memory Disorders. After the consent/assent process, all participants underwent a basic medical examination by a neurologist or a geriatric psychiatrist to determine study eligibility. Exclusion criteria included evidence of clinically significant and active pulmonary, gastrointestinal, renal, hepatic, endocrine, or cardiovascular disease; clinically significant folate or vitamin B12 deficiency; current cancer treatment; a Hachinski Ischemic Scale score >4; evidence of other neurological disorders, including but not limited to stroke, Parkinson’s disease, seizure disorder, hydrocephalus, and head injury with loss of consciousness lasting >30 minutes within the past 5 years; and any current DSM-IV axis I disorder other than Alzheimer’s disease (including substance use disorders within the previous year). Controlled diabetes, hypertension, and hypothyroidism were not among the exclusion criteria.
| Variable | Healthy Comparison Group (N=70) | Mild Cognitive Impairment Group (N=25) | Alzheimer’s Disease Group (N=27) | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Malea | 22 | 31 | 8 | 32 | 17 | 63 |
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 75.3 | 8.7 | 76.0 | 6.1 | 78.2 | 7.1 |
| Education (years) | 15.1 | 3.3 | 14.8 | 3.6 | 14.6 | 3.7 |
| Mini-Mental State Examination scoreb | 28.4 | 1.6 | 26.3 | 1.8 | 21.7 | 3.4 |
| UPSA scorec (percent) | 84.9 | 9.8 | 74.5 | 10.2 | 57.0 | 13.0 |
Mild cognitive impairment.
Twenty-five individuals met criteria for amnestic mild cognitive impairment according to the criteria of Petersen et al. (19). These participants had memory impairment of greater than 1.5 standard deviations on either selective reminding or logical memory and had preserved activities of daily living (i.e., were “functioning well”). Individuals who had additional impairments in other nonmnemonic domains of cognition were included as long as activities of daily living were preserved (i.e., “amnestic plus”). All participants with mild cognitive impairment had MMSE scores ≥24 and global Clinical Dementia Ratings of 0.5 (as specified by the National Institute of Aging Working Group on Study Design).
Alzheimer’s disease.
Twenty-seven individuals met National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria for probable Alzheimer’s disease. Diagnostic criteria include memory impairment (as defined above for mild cognitive impairment) and at least one other area of impaired cognition, including speed of processing, executive ability, and/or semantic processing/language; report of decline in memory and other areas of cognition; and impairments in activities of daily living. Patients in the Alzheimer’s group had MMSE scores <24 and >18 (i.e., in the mild to mild moderate range) and Clinical Dementia Ratings ≥1.0 on the global scale, with two exceptions, who had MMSE scores <24 and Clinical Dementia Ratings=0.5 but were determined to have experienced significant declines in everyday functional capacity.
Cognitively healthy comparison subjects.
Seventy individuals had MMSE scores ≥24 and did not meet psychometric criteria for mild cognitive impairment or Alzheimer’s disease. All formal neurocognitive test scores for these participants were within 1.5 standard deviations of normative data in published studies or manuals.
Measures
Neurocognitive Battery.
From a larger battery of neuropsychological measures that was administered, we used the following tests in this study (for regression analyses, see below): the Boston Naming Test (confrontation naming), verbal fluency for letters F, A, and S; semantic fluency (for animals); the Buschke Selective Reminding Test (verbal list learning); the Trail Making Test, parts A and B (speed and executive function); letter number span (executive function); digit span (working memory); digit symbol coding (speed); the clock drawing test (semantic knowledge and visual motor ability); and the Wechsler Memory Scale logical memory subtest (memory for stories). See Goldberg et al. (16) for more detailed descriptions of each measure, and see the data supplement that accompanies the online edition of this article for group means.
Semantic distance task.
We used a paradigm developed by Cohen et al. (13) in which participants respond to stimuli corresponding to real-world entities that differ in physical size. Subjects were instructed to indicate as quickly and as accurately as possible, by pressing one of two buttons, which of the two entities is larger (or smaller) in the real world. Small semantic distances (contrasts between entities that are relatively similar in real-world size) are more difficult to resolve than larger semantic distances (contrasts between entities that are relatively dissimilar in real-world size) and hence result in lower accuracy and longer response times (which we measure as reaction time). In contrast, large semantic distances result in higher accuracy and shorter reaction times.
Three conditions were used: word, image-congruent, and image-incongruent. For all conditions, participants saw two words or images and were asked which was larger in the real word (or smaller in the real world). For the word condition, two words representing real-world entities of different sizes were presented. The font size of the print was equal for the two words. In the image condition, stimuli were line drawings depicting each of the entities drawn in two different sizes. Images were congruent if real-world size and stimulus presentation size (i.e., size of drawings on the monitor) were consistent (e.g., a small line drawing of “ant,” a large line drawing of “house”) and incongruent if the two parameters were not consistent (e.g., large “ant,” small “house”). These conditions and stimulus examples are illustrated schematically in Figure 2. Further details about task stimuli, trials, timing, and instructions are presented in the online data supplement.

a The subject views two words or two illustrations representing real-world entities and makes a decision as to which is larger or smaller based on their respective sizes in the real world. Accuracy and reaction times were computed. Comparisons of entities that are dissimilar in size in the real world result in more accurate and faster reaction times than comparisons in which the entities are similar in size.
For each condition, we calculated accuracy as percentage correct. For reaction time, we included correct responses only. Median reaction times were calculated for individual subjects, and means of the medians were used for group data. The word condition was administered first; word pairs were presented in fixed random order. The word condition was followed by the image conditions, in which congruent and incongruent trials were intermixed in a fixed random order.
The computer recorded subject response data (median reaction time and accuracy) for each trial. For the purposes of data reduction and clarity of interpretation, we collapsed data from contrasts for semantic distances 1 and 2 (which reflect entities whose real-world sizes were similar and hence more difficult) and contrasts for distances 3 to 8 (which reflected entities whose sizes were relatively dissimilar and hence easier), such that each subject had two data points for each condition for both median reaction time and accuracy. Our rationale for collapsing contrasts is further detailed in the online data supplement. In sum, when size differences between entities are relatively large, as in contrasts 3 through 8, accuracy increases and reaction time is shorter. We consider these contrasts to reflect “large” semantic distances and, as noted, collapsed them by averaging. When size differences between entities are small (as in contrasts 1 and 2), accuracy declines and reaction time is slowed. We consider these contrasts to reflect “small” semantic distances and, as noted, collapsed them by averaging.
Test of everyday functional competence.
The UPSA is a performance-based measure of functional abilities using proxy tasks and scenarios that have ecological validity. It has recently been validated in mild cognitive impairment and Alzheimer’s populations (16). It includes measures of real-world activities such as planning a trip to the beach, determining a route, dialing a telephone number, and writing a check. In the present study, we used a recently validated (20) short form of the UPSA consisting of the communication and comprehension/planning subtests. The composite score (percent correct) was our primary dependent measure.
Procedure
The neurocognitive test battery, the UPSA, and the semantic distance task were administered to each participant in one or two testing sessions.
Results
Word Condition
Word accuracy.
Data from the word accuracy condition were subjected to a 3×2 repeated-measures analysis of variance (ANOVA) using group as the between-subject variable and semantic distance (small or large) as the within-subject variable. We observed a significant main effect for diagnosis (Table 2). A series of post hoc t tests demonstrated that the mild cognitive impairment group and the Alzheimer’s group both had consistently lower accuracy than the healthy comparison group (see also Table 2). We also observed a diagnosis-by-distance interaction, such that the mild cognitive impairment and Alzheimer’s groups had disproportionately more difficulty when making accurate responses in contrasts involving small semantic distances compared with the healthy comparison group (Figure 3A). As expected, a main effect of distance indicated that the large distance condition was performed more accurately than the small distance condition.
| Reaction Time | Proportion Correct | ||||
|---|---|---|---|---|---|
| Task | df | F | p | F | p |
| Words | |||||
| Diagnosis | 2, 119 | 9.88 | 0.0001a | 5.55 | 0.005a |
| Distance | 1, 119 | 556.74 | 0.0001 | 198.01 | 0.0001 |
| Diagnosis-by-distance interaction | 2, 119 | 6.69 | 0.002 | 3.86 | 0.02 |
| Images (congruent) | |||||
| Diagnosis | 2, 119 | 4.84 | 0.001b | 5.64 | 0.005a |
| Distance | 1, 119 | 200.50 | 0.0001 | 75.60 | 0.0001 |
| Diagnosis-by-distance interaction | 2, 119 | 1.78 | 0.17 | 4.71 | 0.01 |
| Images (incongruent) | |||||
| Diagnosis | 2, 119 | 4.12 | 0.02a | 6.84 | 0.002a |
| Distance | 1, 119 | 98.01 | 0.0001 | 109.08 | 0.0001 |
| Diagnosis-by-distance interaction | 2, 119 | 0.42 | 0.66 | 3.63 | 0.03 |
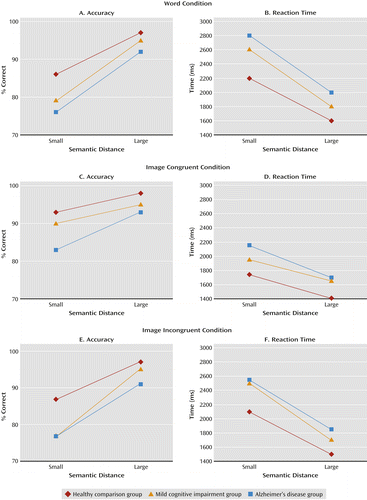
Word reaction time.
The results for reaction time were similar to those for accuracy. We observed a significant main effect for diagnosis, such that the mild cognitive impairment and Alzheimer’s groups both had significantly longer reaction times than the healthy comparison group (by post hoc t test; see also Table 2). We also observed a diagnosis-by-distance interaction, such that the mild cognitive impairment and Alzheimer’s groups were disproportionately slower in making responses in contrasts involving small semantic distances (Figure 3B). As expected, a main effect of distance indicated that the large distance condition was performed more rapidly than the small distance condition.
Image Conditions
Image accuracy.
Accuracy data from the image conditions were first subjected to a 3×2×2 repeated-measures ANOVA using group as a between-subject variable and image (congruent and incongruent) and semantic distance (small and large) as within-subject variables. First, a main effect for diagnosis was observed (F=7.54, df=2, 119, p<0.001), such that healthy comparison subjects outperformed the mild cognitive impairment and Alzheimer’s groups (significant by post hoc t test). A two-way interaction involving diagnosis and semantic distance was also observed (F=5.02, p=0.008). Thus, collapsing across congruency conditions, the mild cognitive impairment and Alzheimer’s groups demonstrated steeper declines in performance from the (easier) large to the (harder) small distance conditions. (Declines were 8 percentage points for the healthy comparison group, 11 points for the mild cognitive impairment group, and 12 points for the Alzheimer’s group). A three-way interaction was also observed, such that the mild cognitive impairment and Alzheimer’s groups demonstrated disproportionate changes in accuracy in the incongruent and congruent conditions depending on semantic distances (F=2.93, df=2, 119, p=0.04).
To further understand these accuracy results, we next analyzed image-congruent and image-incongruent accuracy scores separately (see Table 2). In the image-congruent condition (Figure 3C), there was a main effect of diagnostic group for accuracy, such that the healthy comparison group was more accurate than the mild cognitive impairment and Alzheimer’s groups. A diagnosis-by-distance interaction was also noted, such that the Alzheimer’s group demonstrated a disproportionate gain in performance on the easier large-distance contrasts. For accuracy scores in the incongruent condition (Figure 3E), a main effect of diagnosis was observed, such that the healthy comparison group was significantly more accurate than the mild cognitive impairment and Alzheimer’s groups (see Table 2). A diagnosis-by-distance interaction was also observed, such that the mild cognitive impairment group showed a disproportionate reduction in accuracy in the more difficult small-distance condition.
Image reaction time.
In the image conditions, a main effect of diagnosis for reaction time was observed (F=4.98, df=2, 119, p=0.008) (Figures 3D and 3F). The healthy comparison group had faster reaction times than the mild cognitive impairment and Alzheimer’s groups, by post hoc t test. For reaction time, neither a two-way nor a three-way interaction was observed. Further subanalyses were not conducted, given the nonsignificant interactions.
Prediction of Function
We sought to determine whether any of the 12 accuracy and reaction time variables from the semantic distance paradigm, other semantic measures (semantic fluency, Boston Naming Test), and other cognitive measures in multiple domains (episodic memory in the selective reminding test; speed in Trails A, digit symbol coding, and letter fluency; executive function in Trails B and letter number span; working memory in digit span; and clock test) could predict functional competence, as indicated by the UPSA score. In these analyses, we combined the Alzheimer’s and mild cognitive impairment groups because they were similar in profile and did not demonstrate restriction of range. Additionally, demographic variables (age, sex, and education) were forced to enter the model and accounted for an R2=0.30.
For the combined mild cognitive impairment and Alzheimer’s group, we found that four variables entered our regression model significantly and independently at p<0.05 above and beyond demographics. These were selective reminding memory total score (ΔR2=0.13, F=6.31, p=0.0007, standardized beta=0.48), followed by three semantic distance variables: word accuracy for large semantic distances (ΔR2=0.04, F=5.58, p=0.03, beta=0.76), image-congruent reaction time for large distances (ΔR2=0.12, F=7.29, p=0.004, beta=0.46), and word accuracy for small semantic distances (ΔR2=0.06, F=5.35, p=0.03, beta=0.39). In this model, the image-congruent reaction time variable acted as a suppressor variable, as upon entering, it unsuppressed the relationship of word accuracy with UPSA, such that its beta parameter increased from 0.34 to 0.76. Thus, together these four predictors accounted for nearly 35% of the variance in function (overall model, F=7.89, df=7, 30, p=0.0001). None of the cognitive variables other than selective reminding entered in the combined mild cognitive impairment and Alzheimer’s group.
In the healthy comparison group, selective reminding memory was a significant predictor (ΔR2=0.03, F=3.81, p=0.05; beta= 0.29; overall model, F=2.65, df=5, 59, p=0.03). No other variable reached entry significance.
Discussion
Semantic Processing
Although it is well established that impairment in semantic memory disrupts the cognitive processes of language comprehension/production and object recognition in the Alzheimer’s disease population, there is little direct evidence about the presence and character of semantic abnormalities in mild cognitive impairment. This study has several important findings that bear on the issue. It demonstrated that semantic processing abnormalities in mild cognitive impairment, as determined in a novel paradigm, are not the result of retrieval problems but rather of refractory access to or corruption of the attributes of semantic representations. Several elements of the pattern of performance of the groups are worth noting. First, patients in the mild cognitive impairment and Alzheimer’s disease groups were consistently less accurate and slower than healthy comparison subjects in the word condition. Second, when these patients had to make more fine-grained decisions about semantic attributes, their performance disproportionately worsened relative to that of comparison subjects.
In the image condition, sensory-perceptual information (i.e., the size of the stimuli themselves) had undue influence over semantic knowledge judgments in the mild cognitive impairment and Alzheimer’s groups, albeit in subtly different ways. For Alzheimer’s patients, when the images were visually congruent with their semantic representations in contrasts in which relative size differences of the entities in the real world were large, normalization of performance accuracy was observed. When the stimuli were visually incongruent, patients with mild cognitive impairment demonstrated disproportionately more difficulty responding accurately, especially when the relative size of the objects was similar. Broadly, this leakage of perceptual information into semantic decision making might reflect selective attention impairments (i.e., a compromised filtering mechanism resulting in increased permeability between perceptual and semantic processes). Similar interpretations of Stroop-type data have been made (7, 21). It might also reflect weakened or noisy semantic representation, such that stored knowledge could not “override” immediate perceptual information. Finally, it might reflect reduced executive ability to allocate cognitive resources under demanding conditions (perhaps especially relevant to the mild cognitive impairment group in the image-incongruent condition).
Everyday Functional Competence and Semantic Processing
Critically, abnormalities in semantic processing were specifically related to compromises in everyday functional competence as measured by the UPSA, a performance based instrument that assays ecologically relevant proxy tasks. Abnormalities in semantic distance performance accounted for unique variance above and beyond that accounted for by episodic memory. Tests from other domains of cognition, such as speed and executive function, did not enter our regression models. Interestingly, clinical tests of language that relied on overt retrieval (fluency, naming) also did not enter our final regression model as predictors of functional capacity on the UPSA, possibly suggesting that UPSA scores are driven more by compromises in semantic knowledge than by failure to retrieve the verbal labels of things.
Limitations
It is important to appreciate that we are using the semantic distance paradigm as a model system that can be manipulated with a relatively high degree of precision. Thus, we are not proposing that patients with mild cognitive impairment or Alzheimer’s disease have specific deficits in making judgments based on size representations. In our view, it is likely that these patients have corruption of multiple attributes or properties of semantic entities. Nevertheless, it remains to be experimentally determined whether our results generalize to a range of semantic attributes. We also note that while it is possible that the interaction in accuracy in the word condition could reflect a ceiling effect in the healthy comparison group, we think that this is unlikely because we found parallel results for word reaction time in which no such ceiling effect was present. It could be argued that word accuracy represents a decision-making failure, rather than a failure in semantic processing. To test this notion, we excluded the two word-accuracy variables and found that word reaction time for short and long distances both entered our regression model significantly and accounted for similar amounts of variance in the UPSA (see the online data supplement). Because reaction time was derived from correct responses only, this also suggests that decision making per se does not play a major role. Finally, we do not think that our findings were driven by the Alzheimer’s group, as post hoc contrasts demonstrated that the healthy comparison group performed significantly better than both the mild cognitive impairment and Alzheimer’s groups.
Implications
The cognitive and cortical architectures of the semantic memory system are complex (22–24). It is known, however, that loss of finer distinctions during attribute-based semantic decision making is sensitive to semantic breakdowns in dementias. Furthermore, loss of knowledge about physical attributes has long been viewed as a hallmark of various neurodegenerative conditions (25). Both of these phenomena were carefully measured by our paradigm.
We also tentatively suggest that our data are consistent with a degraded semantic store, rather than refractory access to representations. We base this on findings that patients had reliably and disproportionately more difficulty when size distinctions were small in semantic space and hence relatively difficult, despite the fact that the stimuli were the same in the easier condition (i.e., only the pairwise contrasts differed). It would thus be unlikely for access to be consistently gained in one context but not the other. Nevertheless, we acknowledge that our paradigm was not designed a priori to adjudicate between the alternatives. Perhaps more importantly, the distinction is not critical for our central argument that semantic compromises are independent of overt retrieval in mild cognitive impairment and are related to everyday functional impairments.
Most studies of intact and damaged semantic systems implicate anterior and lateral temporal lobe regions as playing an important role in multiple types of semantic processing. This also suggests that neuropathology in mild cognitive impairment may not be restricted to the medial temporal lobe, but extends to lateral temporal cortices that are engaged during semantic tasks (12, 26). It was recently observed in the Alzheimer’s Disease Neuroimaging Initiative cohort (5) that lateral temporal cortical thickness was significantly reduced in mild cognitive impairment.
Summary
This study usefully synthesized several distinct strands in the mild cognitive impairment literature by providing evidence for 1) corruption in the semantic system in mild cognitive impairment, not confounded by overt retrieval of words in expressive language tasks; 2) intrusion of perceptual information on semantic knowledge, due perhaps to failures in filtering; and 3) a robust relation between semantic processing compromises and difficulties in everyday functional competence.
1 : The organisation of conceptual knowledge in the brain: the future’s past and some future directions. Cogn Neuropsychol 2006; 23:13–38Crossref, Medline, Google Scholar
2 : Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci 2007; 8:976–987Crossref, Medline, Google Scholar
3 : Naming and knowing in dementia of Alzheimer’s type. Brain Lang 1996; 54:302–325Crossref, Medline, Google Scholar
4 : The underlying mechanisms of semantic memory loss in Alzheimer’s disease and semantic dementia. Neuropsychologia 2008; 46:12–21Crossref, Medline, Google Scholar
5 : Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer’s Disease Neuroimaging Initiative. Arch Gen Psychiatry 2011; 68:961–969Crossref, Medline, Google Scholar
6 : Episodic and semantic memory in mild cognitive impairment. Neuropsychologia 2005; 43:1266–1276Crossref, Medline, Google Scholar
7 : The cognitive and neural expression of semantic memory impairment in mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia 2010; 48:978–988Crossref, Medline, Google Scholar
8 : The nature of lexico-semantic processing deficits in mild cognitive impairment. Neuropsychologia 2006; 44:1928–1935Crossref, Medline, Google Scholar
9 : Nonepisodic memory deficits in amnestic MCI. Cogn Behav Neurol 2007; 20:99–106Crossref, Medline, Google Scholar
10 : Relationship between functional and neuropsychological performance in early Alzheimer disease. Alzheimer Dis Assoc Disord 2000; 14:1–10Crossref, Medline, Google Scholar
11 : Molecular signatures in post-mortem brain tissue of younger individuals at high risk for Alzheimer’s disease as based on APOE genotype. Mol Psychiatry 2011; 16:836–847Crossref, Medline, Google Scholar
12 : Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 2011; 24:547–557Crossref, Medline, Google Scholar
13 : Cognitive control and semantics in schizophrenia: an integrated approach. Am J Psychiatry 2005; 162:1969–1971Link, Google Scholar
14 : Representation of number in the brain. Annu Rev Neurosci 2009; 32:185–208Crossref, Medline, Google Scholar
15 Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV: UCSD Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull 2001; 27:235–245Crossref, Medline, Google Scholar
16 : Performance-based measures of everyday function in mild cognitive impairment. Am J Psychiatry 2010; 167:845–853Link, Google Scholar
17 : Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993; 269:2386–2391Crossref, Medline, Google Scholar
18 : The Clinical Dementia Rating (CDR): current vision and scoring rules. Neurology 1993; 43:2412–2414Crossref, Medline, Google Scholar
19 : Current concepts in mild cognitive impairment. Arch Neurol 2001; 58:1985–1992Crossref, Medline, Google Scholar
20 : Development and cross-validation of the UPSA short form for the performance-based functional assessment of patients with mild cognitive impairment and Alzheimer disease. Am J Geriatr Psychiatry 2011; 19:915–922Crossref, Medline, Google Scholar
21 : The “benefits” of distractibility: mechanisms underlying increased Stroop effects in schizophrenia. Schizophr Bull 1999; 25:749–762Crossref, Medline, Google Scholar
22 : Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex 2009; 19:2767–2796Crossref, Medline, Google Scholar
23 : Concepts and categories: a cognitive neuropsychological perspective. Annu Rev Psychol 2009; 60:27–51Crossref, Medline, Google Scholar
24 : Generalization and differentiation in semantic memory: insights from semantic dementia. Ann N Y Acad Sci 2008; 1124:61–76Crossref, Medline, Google Scholar
25 : The selective impairment of semantic memory. Q J Exp Psychol 1975; 27:635–657Crossref, Medline, Google Scholar
26 : With or without FUS, it is the anatomy that dictates the dementia phenotype. Brain 2009; 132:2906–2908Crossref, Medline, Google Scholar



